Abstract
Purpose:
Female sex has been implicated with higher stage at diagnosis and as a negative prognostic factor amongst patients with non-muscle invasive bladder cancer(NMIBC). Whether this holds true with contemporary management paradigms is unknown. We analyzed a cohort of patients treated with adequate bacillus Calmette-Guerin(BCG) for NMIBC in an effort to identify sex-specific influence on BCG response.
Methods:
An IRB-approved review of patients with NMIBC treated at our institution with at least ‘adequate BCG’, as defined by the US FDA and EAU, from 2000–2018 was performed. Patients were then stratified by sex and response to BCG. Non-parametric tests were used to summarize the data overall and by groups. The Kaplan-Meier product limit method was used to calculate median survival endpoints.
Results:
Of the 541 patients treated with adequate BCG, 111(20.5%) were female and 430(79.5%) were male. Female patients were younger (median 66 vs. 69, p=0.071), had a lower BMI (median 27.3 vs. 28.8, p=0.010) and were more likely to have no smoking history (49.5% vs 27.0%, p<0.001). Tumor characteristics with respect to stage, size, multifocality, presence of carcinoma in situ, and presence of variant histology were similar between sexes. While rates of recurrence were higher in females than in males this, was not statistically significant (44.1% vs. 34.7%, p=0.064) and Kaplan-Meier estimates of recurrence-free, progression-free and overall survival demonstrated no significant difference between sexes (p=0.409, p=0.253, p=0.171, respectively).
Conclusion:
In a contemporary cohort of patients with NMIBC treated with adequate BCG, female sex was not associated with adverse oncologic outcomes.
Keywords: gender, non-muscle invasive bladder cancer, bladder cancer, BCG
INTRODUCTION
Bladder cancer (BCa) is the second most common genitourinary malignancy with 81,4000 new diagnoses of BCa expected in the United States in 20201. Of these, 19,3000 (23.7%) will occur in females with 4,930 expected female BCa-related deaths. Amongst those diagnosed with BCa, approximately 75% will present with non-muscle invasive bladder cancer (NMIBC)2.
Despite women representing a minority of new BCa diagnoses, significant sex-related disparities in presentation and outcomes have been reported with women often presenting with more aggressive disease and having worse disease-specific mortality3–5. Research efforts in recent years have focus on sex-related differences in patients with muscle invasive bladder cancer (MIBC) with a paucity of data specifically focused on sex-related outcomes in NMIBC. Currently published literature is conflicting on the sex-specific differences in disease recurrence after treatment of NMIBC; some studies have found females are at increased risk of recurrence6,7 and progression7, while others have found female sex to be protective for recurrence8. Further complicating interpretation of the data is the lack of standard bacillus Calmette-Guerin (BCG) utilization and outcomes reporting in many of these retrospective studies. Palou et al. evaluated 146 patients with high-grade(HG) T1 disease, however, none received maintenance BCG therapy7. Similarly, in the study by Jancke and colleagues only 80 (21.3%) of 374 intermediate and high-risk patients received BCG8.
In this study, we sought to evaluate the impact of sex on BCG response of NMIBC in a contemporary cohort at a tertiary care center.
METHODS
We used an institutional review board approved retrospectively collected database of patients diagnosed with NMIBC to select those who underwent at least one complete induction course (five of six planned instillations) of intravesical BCG at the University of Texas MD Anderson Cancer Center between 2000–2018. Analysis was further restricted to only those patients who received ‘adequate’ BCG therapy according to the definition proposed by the US Food and Drug Administration (FDA), the European Association of Urology (EAU), and the International Bladder Cancer Group (IBCG), defined as at least five of six induction instillations plus at least two additional instillations as a component of either maintenance or re-induction within a 6-month time period9,10. Surveillance schedules were standardized among all providers and based on available NMIBC guidelines. The use of peri-operative chemotherapy, restaging transurethral resection (TUR), BCG maintenance or re-induction were left to the discretion of individual providers. Peri-operative chemotherapy was defined as a one-time instillation of gemcitabine or mitomycin-C.
Clinicopathologic data was collected for all patients and included age, sex, body mass index (BMI), smoking history, prior BCG exposure, tumor stage, tumor size and multifocality, presence of variant histology, presence of carcinoma in situ (CIS), performance of re-staging TURBT, peri-operative intravesical chemotherapy, pathologic staging, number of BCG instillations, response to BCG, subsequent treatments, and disease outcomes.
T-tests (or Wilcoxon’s rank sum) and Pearson’s chi-square (or Fisher’s exact test) were used to compare variables by groups. Recurrence-free survival (RFS) and progression-free survival (PFS) were calculated as the number of months from the start of first BCG instillation to event date or death date. Patients without an event at their last follow-up were censored on that date. Overall survival (OS) was calculated as the number of months from start of first BCG instillation to death or last follow-up date. Patients who were alive at their last follow-up were censored on that date. The Kaplan-Meier product limit method was used to estimate the median survival endpoints. Cox proportional hazards models were used to determine the association between RFS and potential risk factors. The full model multivariate analysis included all the variables with p<0.25 in the univariate analyses or important clinical variables such as gender. Backward elimination selection methods were used the final reduced model. For all comparisons, a two-sided p-value of <0.05 was considered statistically significant. Statistical analysis was performed using Stata/SE version 16.1 statistical software (State Corp. LP, College Station, TX).
RESULTS
Table 1 shows the clinicopathologic characteristic of the 111 female patients (20.5%) and 430 male patients (79.5%). Median follow-up was 45.2 months for female patients (interquartile range [IQR] 25.7–95.0) and 45.5 months for males (IQR 23.6–74.3). Female patients were younger at diagnosis (66 vs. 69 years, p=0.071), had lower BMI (27.3 vs. 28.8, p=0.010) and were more likely to have no smoking history (49.5% vs. 28.9%, p<0.001). Rates of prior BCG therapy were similar between sexes (6.3% vs. 8.6%, p=0.430). There were no differences between sexes in tumor characteristics with respect to initial tumor stage, grade, size, presence of carcinoma in situ (CIS) or variant histology. Additionally, males and females demonstrated similar EAU risk group distribution. 86% of females and 84.5% of males with T1 tumors underwent guideline-recommended re-staging TURBT (p=0.791). Receipt of peri-operative chemotherapy was similar between the sexes (13.6% vs. 14.0%, p=0.912).
Table 1:
Patient demographics and tumor characteristics in all NMIBC patients stratified by sex
| Variable | Female (n=111) | Male (n=430) | P |
|---|---|---|---|
| Age, median (IQR) | 66 (59–74) | 69 (62–76) | 0.071 |
| BMI, median (IQR) | 27.3 (23.3–30.9) | 28.8 (25.4–32.6) | 0.010 |
| Smoking Status, n(%) | <0.001 | ||
| Never | 55 (49.5) | 116 (27.0) | |
| Current/Former | 56 (50.5) | 314 (73.0) | |
| Prior BCG, n(%) | 0.430 | ||
| No | 104 (93.7) | 393 (91.4) | |
| Yes | 7 (6.3) | 37 (8.6) | |
| Tumor Stage | 0.872 | ||
| Ta | 52 (46.8) | 201 (46.7) | |
| Tis | 9 (8.1) | 29 (6.7) | |
| T1 | 50 (45.0) | 200 (46.5) | |
| Tumor Grade | 0.140 | ||
| Low | 15 (13.5) | 38 (8.8) | |
| High | 96 (86.5) | 392 (91.2) | |
| Tumor Size, n(%) | 0.717 | ||
| ≤3 cm | 69 (65.1) | 261 (63.2) | |
| >3 cm | 37 (34.9) | 152 (36.8) | |
| Focality, n(%) | 0.161 | ||
| Solitary | 48 (43.2) | 217 (50.7) | |
| Multifocal | 63 (56.8) | 211 (49.3) | |
| Variant Histology, n(%) | 0.592 | ||
| No | 107 (97.3) | 400 (95.5) | |
| Yes | 3 (2.7) | 19 (4.5) | |
| CIS, n(%) | 0.733 | ||
| Absent | 77 (69.4) | 291 (67.7) | |
| Present | 34 (30.6) | 139 (32.3) | |
| T1 with Re-staging TURBT, n(%) | 0.791 | ||
| No | 7 (14.0) | 31 (15.5) | |
| Yes | 43 (86.0) | 169 (84.5) | |
| Peri-operative chemotherapy, n(%) | 0.912 | ||
| No | 95 (86.4) | 361 (86.0) | |
| Yes | 15 (13.6) | 59 (14.0) | |
| EAU Risk Stratification | 0.801 | ||
| Low | 7 (6.3) | 18 (4.2) | |
| Intermediate | 23 (20.7) | 97 (22.6) | |
| High | 58 (52.3) | 224 (52.1) | |
| Very High | 23 (20.7) | 91 (21.2) |
Time from TURBT to initiation of induction BCG (median 45 days in females vs. 52 days in males, p=0.222) and mean number of BCG doses administered (19.7 doses vs 19.5 doses, p=0.854) were the same between sexes (Table 2). Recurrence rates were higher in females than males (44.1% vs 34.7%, p=0.064) but not statistically significant and rates of any stage progression and progression to muscle invasive or metastatic disease were also similar between sexes (9.0% vs 11.6%, p=0.433 and 5.4% vs. 7.9, p=0.366, respectively). Rates of radical cystectomy were also similar between groups (11.7% vs. 12.3%, p=0.860). When we further stratified female patients by history of smoking there were no differences between the two groups, no smoking history (55 patients, 49.5%) vs. former or current smokers (56 patients, 50.5%), with respect to recurrence, treatment failure or progression (Supplementary Table 1). There were no significant differences in RFS, PFS, and OS when patients were stratified by sex. (Figure 1).
Table 2:
Response to BCG by sex
| Variable | Female | Male | P |
|---|---|---|---|
| Total # of BCG Doses (mean, SD) | 19.7 (8.4) | 19.5 (7.4) | 0.854 |
| Time from Diagnosis to Induction BCG (median, IQR) | 45 (27–71) | 52 (32–71) | 0.222 |
| Recurrence, n(%) | 0.064 | ||
| No | 62 (55.9) | 281 (65.3) | |
| Yes | 49 (44.1) | 149 (34.7) | |
| BCG Relapse, n(%) | 0.832 | ||
| No | 92 (82.9) | 360 (83.7) | |
| Yes | 19 (17.1) | 70 (16.3) | |
| BCG Unresponsive, n(%) | 0.264 | ||
| No | 87 (79.1) | 357 (83.6) | |
| Yes | 23 (20.9) | 70 (16.4) | |
| Progression on BCG (any stage), n(%) | 0.433 | ||
| No | 101 (91.0) | 380 (88.4) | |
| Yes | 10 (9.0) | 50 (11.6) | |
| Progression to MIBC or distant metastatic disease, n(%) | 0.366 | ||
| No | 105 (94.6) | 395 (92.1) | |
| Yes | 6 (5.4) | 34 (7.9) | |
| Systemic chemotherapy, n(%) | 0.852 | ||
| No | 101 (91.0) | 390 (91.5) | |
| Yes | 10 (9.0) | 36 (8.5) | |
| Radical cystectomy, n(%) | 0.860 | ||
| No | 98 (88.3) | 377 (87.7) | |
| Yes | 13 (11.7) | 53 (12.3) |
Figure 1:

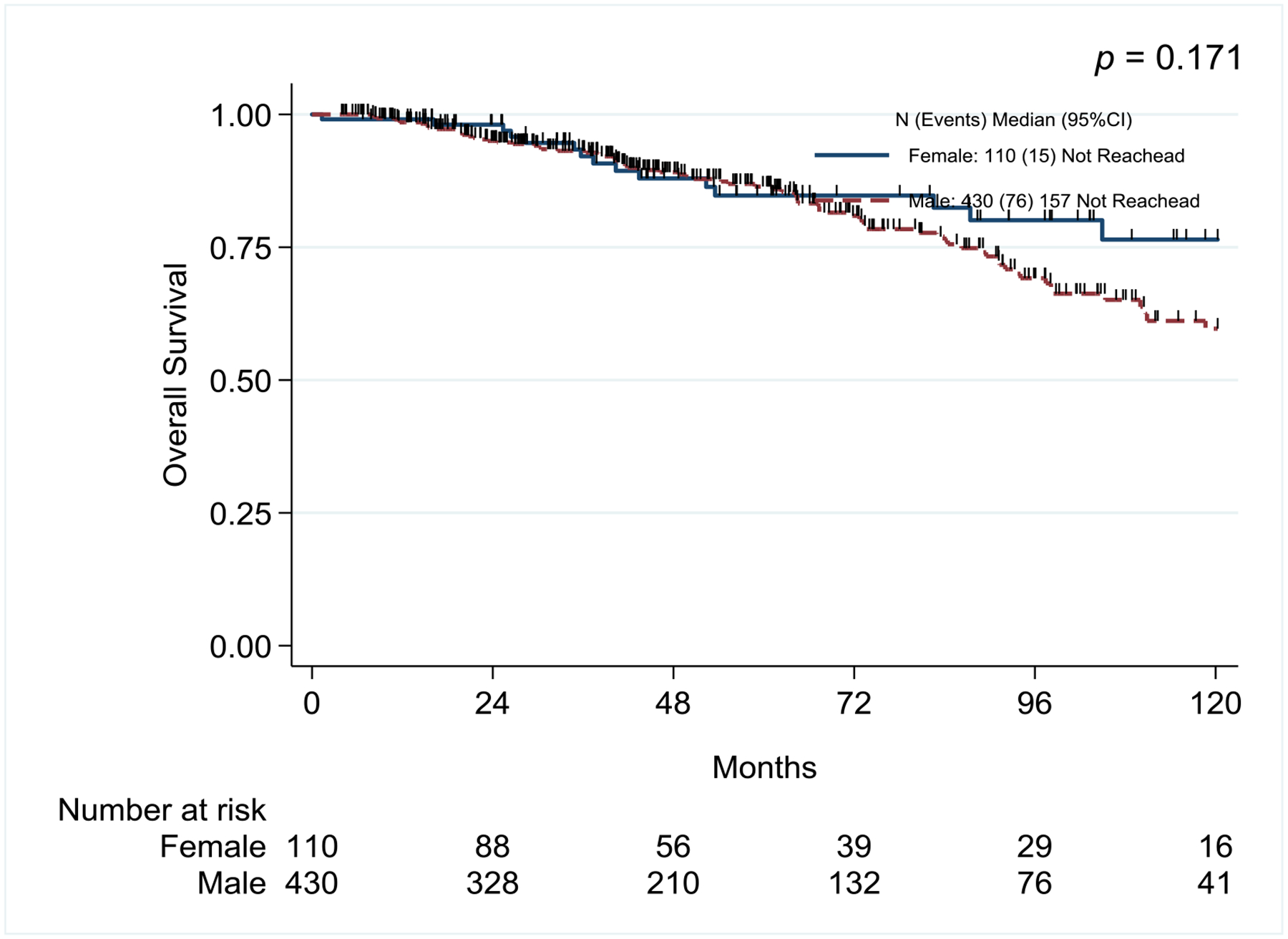
Kaplan-Meier survival curves for Recurrence-Free Survival, Progression-Free Survival, and Overall Survival
On univariate and multivariate analysis, sex was not associated with recurrence-free survival (Hazard ratio [HR] 1.14, 95% Confidence Interval [CI] 0.84–1.55, p=0.409 and HR 1.11, 95% CI 0.80–1.53, p=0.532, respectively). Patient characteristics that were significantly associated with recurrence on univariate analysis were advanced age, receipt of prior BCG and lack of re-staging TURBT. On multivariate analysis, prior BCG exposure and lack of re-staging TURBT were the main prognostic factors for recurrence (Table 3).
Table 3:
Univariate and Multivariate Models for Recurrence-Free Survival
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | P | HR | 95% CI | P |
| Sex (male/female) | 1.14 | 0.84–1.55 | 0.409 | 1.12 | 0.83–1.53 | 0.457 |
| BMI* | 0.98 | 0.96–1.00 | 0.129 | |||
| Prior BCG | 2.28 | 1.55–3.35 | <0.001 | 2.19 | 1.48–3.21 | <0.001 |
| Re-staging TURBT | 0.69 | 0.53–0.90 | 0.006 | 0.72 | 0.56–0.94 | 0.016 |
| Smoking history (current/former, never)** | 1.04 | 0.79–1.38 | 0.771 | |||
| EAU Risk Stratification | ||||||
| Low | ||||||
| Intermediate | 1.39 | 0.63–3.07 | 0.420 | |||
| High | 1.59 | 0.74–3.41 | 0.232 | |||
| Very High | 1.50 | 0.68–3.32 | 0.314 | |||
Cox regression with continuous covariate
This variable was not included in the multivariate analysis.
DISCUSSION
In our study of patients with NMIBC treated with adequate BCG in a contemporary fashion, we found that sex does not influence response to BCG therapy.
There are discordant findings in the literature surrounding the impact of female sex on recurrence following treatment for NMIBC. Female sex has previously been identified as a risk factor for disease recurrence and is one of the prognostic factors incorporated into the Spanish Urological Club for Oncological Treatment (CUETO) scoring model derived from 4 CUETO trials in which the majority, but not all, received adequate BCG therapy11. A recent meta-analysis by Uhlig et al. confirmed these findings indicating that females had an 11% higher risk of disease recurrence compared to male patients12. Subgroup analyses demonstrated that sex-specific disparities were most pronounced in studies administering BCG (HR 1.64); conversely, there were no sex-related differences in studies where patients did not receive BCG. A proposed mechanism for the potential sex-related discrepancies in response to BCG includes the role of estrogen on the downregulation of IL-6 and other key cytokines responsible for BCG’s antitumor activity13.
Despite the aforementioned studies, there are also numerous studies suggesting that outcomes of males and females with NMIBC treated with BCG are similar. Sex was not a variable included in the European Organization for Research and Treatment of Cancers (EORTC) risk tables as it was not associated with recurrence or progression in the combined analysis of 2596 NMIBC patients in prior EORTC trials14. Additionally, a retrospective review by Boorjian et al. of 1021 patients (756 men, 265 women) treated with induction BCG found that on multivariate analysis sex was not associated with increased risk of recurrence (HR 0.94, 95% CI 0.79–1.11, p=0.44) or progression (HR 1.18, 95% CI 0.85–1.63, p=0.33)15. Similar results have also been demonstrated in a multicenter retrospective analysis of 2,451 patients (2,012 men, 439 women) with high-grade T1 NMIBC in which sex was not identified as a prognostic indicator of recurrence or progression16. Drawing accurate conclusion from the literature is difficult as there is significant heterogenicity in treatments utilized, variable utilization of restaging TUR, and the majority of studies included patients who underwent induction BCG only rather than the current standard of induction followed by maintenance therapy.
In our present study of NMIBC patients who have received adequate BCG, there was a non-significant increase in recurrence amongst female patients (44.1% vs 35.0%, p=0.076) but there was no difference in progression rates. Additionally, the majority of patients with T1 tumors underwent guideline recommended re-staging TURBT.
Our study is one of few in which sex differences have been evaluated in patients undergoing current standard of care treatment for NMIBC including re-staging TURBT, when indicated, and adequate BCG, both of which have previously been shown to impact recurrence and progression rates17,18. When receiving the current standard of care treatment for NMIBC at our institution, there were no differences in BCG response rates between sexes. Our analysis did demonstrate a trend towards increased recurrence in females, albeit our sample size was small (111 females received adequate BCG) and the female recurrence rates did not reach statistical significance. Nonetheless, this finding, in combination with the previous meta-analysis by Uhlig et al.12 highlight that there may in fact be a difference in BCG response amongst female patients, especially those receiving long-term maintenance BCG. These findings should be further explored in future prospective studies as they may have implications on patient counseling, surveillance frequency, and treatment in female patients with NMIBC.
Sex disparities in BCa have previously been attributed to carcinogen exposure, delay in diagnosis and treatment, or higher grade/stage at diagnosis amongst females. Similar to a recent large retrospective study of more than 27,000 patients with MIBC, our study of patients with NMIBC did not identify any significant differences in treatment patterns and quality between sexes that would explain differences in outcomes19. However, differences in BCa incidence and outcomes are influenced by sex hormones, sex-specific differences in immune response, tumor genetics and the bladder microbiome20– all of which can influence response to immune-based therapies such as BCG in NMIBC and immune checkpoint inhibitors in the NMIBC and muscle-invasive settings.
Limitations of our study include its retrospective design, however, there were no significant differences in clinicopathologic features including tumor stage, presence of CIS or variant histology between sexes. Additionally, treatment decisions were at the discretion of the urologic oncologist accounting for potential differences in use of repeat induction BCG, use of second line agents after BCG failure, etc.. Finally, there is a trend in our data towards inferior recurrence among female patients, this was not statistically significant in our analysis, however, our small sample may be inadequately powered to detect a difference.
CONCLUSION
In the present study of patients treated uniformly with adequate BCG therapy at a large tertiary care center, sex did not influence response to BCG nor long-term rates of recurrence or progression. Amongst those receiving adequate BCG, there was a higher recurrence rate in female patients, although this was not statistically significant. Prospective studies further elucidating the role of sexual dimorphism in disease recurrence, innate immune differences and response to treatment are needed.
Supplementary Material
Acknowledgements:
This research was supported by the Wayne B. Duddlesten Professorship in Cancer Research, the Raymond and Maria Floyd Bladder Cancer Research Foundation Grant to AMK and NIH/NCI UTMD Anderson SPORE in Genitourinary Cancer (Bladder) (P50CA091846) to CPND and the Cancer Center Support Grant (NCI Grant P30 CA016672).
Conflicts of interest/Competing interests:
KK Bree: Stratify genomics - consultant
CP Dinney: National Cancer Institutes research funding, University of East Finland Faculty of Health Sciences (UEFHS) research funding, grant and personal fees from FKD Therapies, creator of intellectual property owned by UT/MDACC related to the use of genetic alterations as a predictive biomarker for response to Nadofaragene firadenovac.
AM Kamat: personal fees and other from Merck, personal fees from Abbott Molecular, personal fees from Arquer, personal fees from ArTara, personal fees from Asieris, personal fees and other from Photocure, personal fees from Astra Zeneca, personal fees from BioClin Therapeutics, personal fees and other from BMS, personal fees from Cepheid, personal fees from Cold Genesys, other from FKD Industries, personal fees from Eisai, personal fees from Engene, Inc., personal fees from Ferring, personal fees from FerGene, personal fees from Imagin, personal fees from Janssen, personal fees from MDxHealth, personal fees from Medac, personal fees from Pfizer, personal fees from Roviant, personal fees from Sessen Bio, personal fees from ProTara, personal fees from Nucleix, personal fees from Seattle Genetics, grants from CEC Oncology, personal fees from Theralase, personal fees from TMC Innovation, personal fees from US Biotest, other from Adolor, other from Heat Biologics, patent for CyPRIT-Cytokine Panel for Response to Intravesical Immunotherapy pending.
Footnotes
Ethics approval (include appropriate approvals or waivers): This study had been approved by the competent ethics committee (2017–02257) and all applicable institutional and governmental regulations concerning the ethical use of the data were followed. All data were encrypted and kept confidential.
Consent to participate (include appropriate statements): waiver of consent granted given retrospective nature of review
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Kamat AM, Hahn NM, Efstathiou JA, et al. Bladder cancer. Lancet. 2016;388(10061):2796–2810. [DOI] [PubMed] [Google Scholar]
- 3.Dobruch J, Daneshmand S, Fisch M, et al. Gender and Bladder Cancer: A Collaborative Review of Etiology, Biology, and Outcomes. Eur Urol. 2016;69(2):300–310. [DOI] [PubMed] [Google Scholar]
- 4.Donsky H, Coyle S, Scosyrev E, Messing EM. Sex differences in incidence and mortality of bladder and kidney cancers: national estimates from 49 countries. Urol Oncol. 2014;32(1):40 e23–31. [DOI] [PubMed] [Google Scholar]
- 5.Kluth LA, Rieken M, Xylinas E, et al. Gender-specific differences in clinicopathologic outcomes following radical cystectomy: an international multi-institutional study of more than 8000 patients. Eur Urol. 2014;66(5):913–919. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez-Gomez J, Solsona E, Unda M, et al. Prognostic factors in patients with non-muscle-invasive bladder cancer treated with bacillus Calmette-Guerin: multivariate analysis of data from four randomized CUETO trials. Eur Urol. 2008;53(5):992–1001. [DOI] [PubMed] [Google Scholar]
- 7.Palou J, Sylvester RJ, Faba OR, et al. Female gender and carcinoma in situ in the prostatic urethra are prognostic factors for recurrence, progression, and disease-specific mortality in T1G3 bladder cancer patients treated with bacillus Calmette-Guerin. Eur Urol. 2012;62(1):118–125. [DOI] [PubMed] [Google Scholar]
- 8.Jancke G, Damm O, Rosell J, Jahnson S. Risk factors for local recurrence in patients with pTa/pT1 urinary bladder cancer. Scand J Urol Nephrol. 2008;42(5):417–421. [DOI] [PubMed] [Google Scholar]
- 9.Kamat AM, Sylvester RJ, Bohle A, et al. Definitions, End Points, and Clinical Trial Designs for Non-Muscle-Invasive Bladder Cancer: Recommendations From the International Bladder Cancer Group. J Clin Oncol. 2016;34(16):1935–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Babjuk MB M, Compérat E, Gontero P, Liedberg F, Masson-Lecomte A, Mostafid AH, Palou J, van Rhijn BWG, Rouprêt M, Shariat SF, Sylvester R. EAU Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and CIS). 2021. https://uroweb.org/guideline/non-muscle-invasive-bladder-cancer/.
- 11.Fernandez-Gomez J, Madero R, Solsona E, et al. Predicting nonmuscle invasive bladder cancer recurrence and progression in patients treated with bacillus Calmette-Guerin: the CUETO scoring model. J Urol. 2009;182(5):2195–2203. [DOI] [PubMed] [Google Scholar]
- 12.Uhlig A, Strauss A, Seif Amir Hosseini A, et al. Gender-specific Differences in Recurrence of Non-muscle-invasive Bladder Cancer: A Systematic Review and Meta-analysis. Eur Urol Focus. 2018;4(6):924–936. [DOI] [PubMed] [Google Scholar]
- 13.Shang Z, Li Y, Hsu I, et al. Targeting estrogen/estrogen receptor alpha enhances Bacillus Calmette-Guerin efficacy in bladder cancer. Oncotarget. 2016;7(19):27325–27335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49(3):466–465; discussion 475–467. [DOI] [PubMed] [Google Scholar]
- 15.Boorjian SA, Zhu F, Herr HW. The effect of gender on response to bacillus Calmette-Guerin therapy for patients with non-muscle-invasive urothelial carcinoma of the bladder. BJU Int. 2010;106(3):357–361. [DOI] [PubMed] [Google Scholar]
- 16.Gontero P, Sylvester R, Pisano F, et al. Prognostic factors and risk groups in T1G3 non-muscle-invasive bladder cancer patients initially treated with Bacillus Calmette-Guerin: results of a retrospective multicenter study of 2451 patients. Eur Urol. 2015;67(1):74–82. [DOI] [PubMed] [Google Scholar]
- 17.Herr HW. Restaging transurethral resection of high risk superficial bladder cancer improves the initial response to bacillus Calmette-Guerin therapy. J Urol. 2005;174(6):2134–2137. [DOI] [PubMed] [Google Scholar]
- 18.Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol. 2000;163(4):1124–1129. [PubMed] [Google Scholar]
- 19.Krimphove MJ, Szymaniak J, Marchese M, et al. Sex-specific Differences in the Quality of Treatment of Muscle-invasive Bladder Cancer Do Not Explain the Overall Survival Discrepancy. Eur Urol Focus. 2019. [DOI] [PubMed] [Google Scholar]
- 20.Koti M, Ingersoll MA, Gupta S, et al. Sex Differences in Bladder Cancer Immunobiology and Outcomes: A Collaborative Review with Implications for Treatment. Eur Urol Oncol. 2020;3(5):622–630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


