Abstract
Salmonella is a major foodborne pathogen, that poses a serious threat to poultry farm production. Phage-based biocontrol offered a promising alternative strategy to eradicate the persistent and challenging infections caused by Salmonella in this setting. This study isolated and purified the lytic Salmonella phage vB_SenM_BP13076 (simple as BP13076) using its host strain Salmonella Enteritidis ATCC 13076. Its genome was extensively analyzed, and its potential biocontrol application towards eggs was investigated. Morphological analyses revealed that phage BP13076 is characterized by an icosahedral head and a contractile tail, placing it among Caudoviricetes. The phage demonstrated a broad host range, lysing 66 out of 68 tested Salmonella strains, including eight globally prevalent serovars. Moreover, it also exhibited a short latent period of approximately 5 min and a burst size of about 105 PFU/cell. It also demonstrates good thermal stability and a wide pH range tolerance. The genome of phage BP13076 consists of 160, 318 bp of dsDNA with a G + C content of 37.13% with nucleotide homology placing it among phages of the genus Gelderlandvirus. Notably, the genomic analysis revealed no known genes associated with virulence, antibiotic resistance, or lysogeny, making it a safe candidate for biocontrol applications. In vitro, bacteriostatic tests indicated higher MOI (multiplicity of infection), resulting in a more significant reduction in Salmonella counts. When applied to table and breeding eggs, phage BP13076 at MOIs of 100 and 1000 resulted in a significant decrease in Salmonella levels compared to the positive control groups. These findings highlight the efficacy of phage BP13076 as a promising biocontrol agent for managing Salmonella contamination and transmission for table and breeding eggs, offering a foundation for its potential application in the prevention and control of Salmonella in the poultry industry.
Keywords: Salmonella phage, biological characteristics, genome sequencing, biocontrol, breeding eggs
Introduction
Salmonella is a Gram-negative facultative anaerobic bacterium and is a zoonotic pathogen of significant importance to agriculture and the food industry (Medalla et al., 2021). It can cause serious illness in humans and animals, leading to symptoms such as diarrhoea and vomiting, with contraction of the bacterium typically occurring through contaminated foodstuffs (Huang et al., 2022). As one of the most important foodborne pathogens globally (Ortiz-Solà et al., 2022), Salmonella is responsible for many food poisoning cases worldwide. According to statistics, Salmonella is ranked first among bacterial foodborne illnesses reported globally (Frost et al., 2023). Moreover, it is a significant cause of infections among poultry, reducing their overall well-being and potentially contaminating poultry products, which can then affect human health (Lei et al., 2020). Eggs and egg-based products are the primary source of Salmonella infections, followed by poultry meat, pork, and cheese (Popa and Popa, 2021). The U.S. Food and Drug Administration (FDA) has classified eggs as the second highest food risk for foodborne diseases. Moreover, if breeding eggs are contaminated with Salmonella, vertical transmission can infect chicks, leading to illness and significant economic damage in poultry production (Ramasamy et al., 2014). Unsanitary conditions in hatcheries exacerbate this issue, enabling the spread of Salmonella to newly hatched chicks. This dual role as both a foodborne pathogen and a poultry farm pathogen underscored the necessity of effective control measures in food production and animal husbandry to prevent the spread and impact of Salmonella infections.
Currently, antibiotics are the primary method for controlling Salmonella infections. However, the misuse of antibiotics has led to a significant increase in antibiotic-resistant strains of Salmonella (Debroy et al., 2020; He et al., 2020; Ling et al., 2022). The World Health Organization (WHO) identified antibiotic resistance as one of the most severe threats to global health (World Health Organization, 2022). The emergence of multidrug-resistant (MDR) Salmonella strains has further compounded the challenge of infection control. This threat underscores the urgent need for alternative treatments to combat multi-drug-resistant Salmonella.
Phage therapy for preventing and treating pathogen infections has regained prominence in recent years (Hesse and Adhya, 2019). Bacteriophages, or phages, are viruses that specifically target and infect host bacteria (Miernikiewicz and Dąbrowska, 2022). They are the most abundant biological entities in nature, ubiquitous, and found in almost every environment, with an estimated population of about 1031 within the biosphere, ten times the number of their bacterial hosts (Mushegian, 2020). Compared to antibiotics, phage-based biocontrol strategies offer several advantages: they are highly host-specific, self-replicating, easy to prepare, and have no toxic side effects on animals. (Jaroszewicz et al., 2022; Styles et al., 2022). These characteristics enable the use of phages as potential alternatives for combating pathogenic infections.
Studies have demonstrated the successful application of phage therapy for treating Salmonella infections in food, livestock and poultry farm animals (Galtier et al., 2016; Hong et al., 2016; Mhone et al., 2022; Torres-Acosta et al., 2019). For instance, Phothaworn et al. (2020) developed phage cocktails that were found effective in inhibiting the proliferation of Salmonella in food. Additionally, phage LPST94 has been demonstrated to be capable of controlling Salmonella contamination in various food products, including milk, apple juice, chicken breast and lettuce, demonstrating its potential as a biocontrol agent (Islam et al., 2020). However, studies exploring the use of lytic phages as biocontrol agents to overcome Salmonella contamination in eggs are limited (Azari et al., 2023).
In the current study, we isolated and characterized a broad-host-range lytic Salmonella phage BP13076 and conducted an in-depth analysis of its biological characteristics and genomic information. Furthermore, we explored the efficacy of this phage as a biocontrol agent against Salmonella infection in table and breeding eggs. This dual-target strategy offers a comprehensive solution to address Salmonella contamination at its source. Our work not only aims to enhance food safety for consumers but also supports the poultry industry by preventing re-infection, reducing losses, and offering an alternative to antibiotics.
Materials and methods
Bacterial strains and growth conditions
Salmonella Enteritidis ATCC 13076 was used as the host strain to isolate the phage. Other bacteria strains were employed for host range analysis. All strains were routinely cultured at 37°C in Luria-Bertani (LB) broth or Tryptic Soy Broth (TSB) broth with shaking or on agar plates.
Phage isolation, purification and propagation
The poultry fecal samples were centrifuged at 12,000 RPM at 4°C for 10 min following established protocols (Bao et al., 2015). The resulting supernatant was then combined with Salmonella Enteritidis ATCC 13076 and introduced into LB liquid medium shaking overnight at 37°C for phage enrichment. Following another centrifugation at 12,000 RPM for 10 min, the supernatant was filtered through a sterilized 0.22 μm pore size membrane (Millipore, Germany). The filtered supernatant was then subjected to a double-layer agar plate method for phage isolation. Specifically, 100 μL of each serial dilution was mixed with 100 μL of overnight bacterial suspension in 4 mL of 0.6 % [w/v] LB soft agar. This mixture was then poured onto LB agar plates, allowing for even distribution. The plates were incubated to enable the formation of plaques, indicating phage activity against the bacterial host. Then, individual plaques were selected and purified through 5-8 consecutive generations to obtain a purified stock solution. For amplification, the mixture of the phage and its host strain at a multiplicity of infection (MOI) of 1 was added to 10 mL of LB broth supplemented with 2 mM CaCl2 and incubated at 37°C for 5 h. The phage lysate was then centrifugated at 12,000 RPM for 10 min at 4°C, and the supernatant was filtered through a 0.22 μm pore size membrane (Millipore, Germany). The double-layer agar method was subsequently used to determine the phage titers.
Preparation of phage particles
Phage particles were precipitated using PEG8000 (Polyethylene Glycol 8000, Germany). Briefly, phage lysates were treated with DNase I and RNase A, each at a final concentration of 1 μg/mL, for 30 min at room temperature. Subsequently, NaCl was added, and the mixture was incubated at 4°C for 1 h. Following centrifugation at 10,000 RPM for 10 min, phages were precipitated overnight at 4°C with PEG8000. The precipitated phages were then recovered by centrifugation at 10,000 RPM for 10 min and resuspended in SM buffer (100 mM NaCl, 8 mM MgSO4, 50 mM Tris-HCl (pH7.5), 0.01% gelatin). Finally, phages were purified by adding an equal volume of chloroform and harvested from the upper aqueous layer.
Phage particles were diluted in a 10-fold serial gradient and mixed with its host bacteria, Salmonella Enteritidis ATCC 13076. The mixture was then combined with 4 mL of 0.6% LB semi-solid agar and evenly poured on 1.2% LB solid agar plates. The plates were incubated at 37°C for 24 h, after which plaques were enumerated to determine the phage titer per mL in each sample.
Transmission electron microscopy (TEM) observation
Refer to the methodology provided in the literature (Cong et al., 2021). Phage concentrate (108 PFU/mL) was added dropwise onto a copper grid and allowed to settle naturally for 20 min. Excess liquid was removed with the filter paper. A drop of 2% phosphotungstic acid (PTA) solution at pH 7.0 was applied for staining and left for 10 min. After drying, the grid was placed in a transmission electron microscope (H-7650, Hitachi High-Technologies Corporation, Tokyo, Japan). The morphology of the phages was captured in photographs.
Host range determination
The host range of the phage was determined using the spotting method. Individual colonies of each bacterium strain (Supplementary Table S1) were taken and cultured separately to the logarithmic growth phase. 100 μL of the bacterial culture was added to 4 mL of LB semi-solid agar, mixed thoroughly, and poured onto 1.2% LB solid agar plates. After the plates dried, 10 μL of phage suspension (108 PFU/mL) was spotted on the surface of the bacterial lawn. Once the droplets were dried, the plates were incubated at 37°C for 10∼12 h. Results were observed and recorded, and the experiment was repeated three times.
Biological characteristics of phage BP13076
Optimal multiplicity of infection (MOI) Multiplicity of infection (MOI) refers to the ratio of phages to their host in a system. To determine the optimal MOI for phage, we followed a previously described method (Lu et al., 2022) with some modifications. The host bacteria were cultured to the logarithmic phase, and their concentration was adjusted to 1 × 108 PFU/mL, phage and host bacteria were then mixed at various infection ratios of 0.001,0.01, 0.1, 1, and 10. The mixtures were incubated at 37°C for 5 h. Subsequently, the cultures were centrifuged at 12,000 RPM for 10 min. Phage titer was determined using the double-layer agar method. The infection ratio yielding the highest phage titer was identified as the optimal MOI.
Temperature and pH stability To assess temperature stability, the phage stock solution was incubated in a water bath at 30°C, 40°C, 50°C, 60°C, 70°C, and 80°C for 30 min and 60 min, respectively. The titer of phage at various temperatures was measured using the double-layer agar method.
To evaluate pH stability, the pH of peptone water solution was adjusted to 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13 using HCl or NaOH solution. After mixing the adjusted peptone water with phage, the mixture was incubated at 37°C for 2 h. The titer of phage under different pH conditions was then determined using the double-layer plate method. Each experiment was repeated three times.
One-step growth curve The host culture (5 × 108 CFU/mL) and phage lysate were mixed and incubated at 37°C for 15 min to allow sufficient phage adsorption. The mixture was then centrifuged at 12,000 RPM for 1 min at 4°C, the supernatant was discarded. The pellets were washed once with pre-warmed LB broth to remove any unabsorbed free phage. The pellets were resuspended in a pre-warmed LB broth at 37°C and quickly placed in a shaker (180 RPM) at 37°C. The timer was started simultaneously. Samples were collected at 5 min, and then every 10 min until 120 min, and phage potency at each point was measured using the double-layer agar method. The experiment was conducted in triplicate. The latent period was defined as the time interval between the adsorption (not including 15 min preincubation), and the burst size was calculated as the ratio of the final count of liberated phage particles to the initial count of infected bacterial cells during the latent period.
The lytic capacity of phage at different multiplicities of infection (MOI) To evaluate the bactericidal effect of phages at various MOI, Salmonella Enteritidis ATCC 13076 was cultured overnight and diluted to 108 CFU/mL. In the phage-free group, 100 μL of bacteria and 100 μL of LB broth were added. In the bacteria-free group, 100 μL of phage and 100 μL of LB broth were added. For the MOI test groups, phages were added at different MOI (0.01, 0.1, 1, 10, and 100) by mixing 100 μL of phage with 100 μL bacteria, respectively. The mixtures were thoroughly mixed, and each added 3 mL of LB broth, incubated in a shaker at 37°C for 12 h. The OD600 value was measured every hour using a spectrophotometer. The experiment was conducted in triplicate. An inhibition curve was plotted with time on the x-axis and OD600 on the y-axis.
Extraction and sequencing analysis of phage DNA
The phage (∼1010 PFU/mL) DNA was extracted using a phage DNA extraction kit (Norgen Biotek, Canada) following the instructions, and sent to Wuhan Benagen Technology company Limited (Wuhan, China) for whole-genome sequencing on the Illumina HiSeq platform. The sequencing data was assembled by SPAdes (v.3.15.4) (Bankevich et al., 2012). The whole-genome sequence was deposited to GenBank with accession number PQ118624.
Various bioinformatics tools were utilized for genome analysis. Coding genes were predicted from the assembled genome using Prokka software (version 1.14.6) (Seemann, 2014). Genes for tRNAs were determined using tRNAscan-SE (Chan et al., 2021). BLASTp was used to predict the function of protein sequences based on homology to known protein sequences. Genomic information obtained from predictions, such as genome structure annotation information, GC distribution, and genome COG functions annotation, were plotted onto a genome map using the R-package Circles (Gu et al., 2014). Resistance genes were identified using the ARDB (Antibiotic Resistance Genes Database, http://ardb.cbcb.umd.edu/) and CARD databases (Comprehensive Antibiotic Resistance Database, https://card.mcmaster.ca/). Virulence genes were identified using the VFDB database (Virulence Factor Database http://www.mgc.ac.cn/VFs/).
To determine the evolutionary relationships, the gene sequences of the large terminase subunits, were compared to those on GenBank of NCBI, with those of highest homology to phage BP13076 downloaded. Resulting sequences were analyzed, and neighbour-joining phylogenetic evolutionary trees were constructed using MEGA (version 7.0). Easyfig (version 2.2.2) was used for the generation of genomic maps for comparative illustration of phage genomes at the amino acid level (Sullivan et al., 2011).
Application of phage BP13076 on eggs
Pre-treatments of egg samples The table eggs used in this study were purchased from a market in Nanjing, Jiangsu Province, China. The breeding eggs were supplied by a poultry company (Changzhou, China). The two kinds of intact eggs′ surface was washed thoroughly with water to remove any visible dirt and debris. After washing, the eggs were sterilized by immersing them in 75% ethanol. This step effectively decontaminated the eggs′ surfaces by killing a wide range of microorganism. Before bacterial inoculation, all egg samples were exposed to ultraviolet (UV) light for approximately 15 min. This additional step ensured the removal of any remaining bacteria on the eggshells, thereby providing a sterile environment for subsequent experiments. Before initiating the biocontrol experiments, breeding eggs were randomly selected and tested to confirm the absence of Salmonella. Only eggs verified to be Salmonella-free were included in the study.
Immersion-based biological control of Salmonella on table eggs A total of 108 Sterile, intact, Salmonella-free eggs were used in the experiment. These eggs were inoculated with 106 CFU/mL of Salmonella Enteritidis ATCC 13076 for 30 min using an immersion method to ensure uniform bacteria exposure. After air drying, 36 inoculated eggs served as the control group were treated with SM buffer only. The remaining 72 inoculated eggs were assigned into the phage treatment groups and immersed for 5 s in phage BP13076 at two different concentrations: 108 PFU/mL (MOI of 100) or 109 PFU/mL (MOI of 1000). The treated eggs were then incubated for 24 h under two different conditions: room temperature (25°C) and refrigerated temperature (4°C). The bacteria load was measured at 0, 1, 3, 6, 12, and 24 h post-treatment. At each time point, three eggs from each group were aseptically sampled and washed in 20 mL of sterile 0.85 % saline for 10 min. Viable counts of S. Enteritidis ATCC13076 were determined by spread plating 100 μL or 2 mL of the wash solution or its serial 10-fold dilutions onto XLT-4 agar. After 18 h of incubation, Salmonella colonies were counted. The minimum detection limit for this experiment was 10 CFU/egg. This experiment was independently repeated three times.
Spray control test of phage BP13076 on breeding eggs A total of 54 pre-treated breeding eggs were randomly divided into phage and control groups. The phage group was subdivided based on the multiplicity of infection (MOI) into two subgroups: MOI 100 and MOI 1000. Each group contained 18 eggs. The eggshells of all groups were first sprayed with 500 µL of a 1 × 106 CFU/mL suspension of Salmonella Enteritidis ATCC 13076, ensuring even coverage over the eggshell's surface area, which is approximately 80 cm² per egg. After air drying, the breeding eggs in the phage group were sprayed with 500 µL phage BP13076 at concentrations corresponding to MOIs of 100 (108 PFU/mL) and 1000 (109 PFU/mL). The control group eggs were sprayed with an equal amount of SM buffer. All eggs were incubated at room temperature, the optimal temperature for breeding eggs, for 24 h. At designated time points (0, 1, 3, 6, 12, and 24 h) post- treatment, three eggs from each group were sampled. Each egg was placed in a sterile sampling bag containing 20 mL of sterile 0.85 % saline and gently shaken for 10 min to detach the surface bacteria. A 100 µL or 2 mL of aliquot of the liquid or its serial 10-fold dilutions was spread evenly on XLT-4 agar plates, then plates were incubated at 37°C for 18 h. Following incubation, the colonies were counted to assess the effectiveness of the phage treatment. The minimum detection limit for this experiment was 10 CFU/egg. This experiment was independently repeated three times to ensure the reliability and reproducibility of the results.
Statistical analysis
All statistical analyses were conducted using GraphPad Prism 6 software (GraphPad Software Inc., San Diego, CA, USA). Data are presented as mean ± standard error of the mean (SEM). One-way ANOVA was used to determine statistical significance, where P < 0.05 was considered statistically significant.
Results
Morphology of the lytic phage
One lytic phage was isolated against Salmonella Enteritidis ATCC 13076 from a poultry fecal sample. The phage was named vB_SenM_BP13076 (BP13076) and was found capable of producing clear plaques (≈1 mm in diameter) (Fig. 1A). Electron microscopy revealed the phage to be a myovirus, placing it among the class Caudoviricetes, possessing a virion with an icosahedral capsid 60 nm in diameter with a contractile tail with a length of 87.5 nm (Fig. 1B).
Figure 1.
Morphology of phage BP13076. (A) Plaques formed by phage BP13076 on LB agar against its host strain S. Enteritidis ATCC 13076. The diameter of clear and round plaques was approximately 1 mm; (B) Transmission electron microscopy (TEM) image of phage BP13076 stained with 1% (w/v) uranyl acetate. The capsid surface is clearly visible on intact phage particles. Red arrows indicate the thin collar structure located beneath the capsids. Blue arrows highlight the tail fibers extending from the upper region of the baseplate structure, observable in both intact (a) and contracted (b) phage particles.
Phage host range
The host range of phage BP13076 was determined using the spot test. Results demonstrated that the phage could lyse 66 out of 68 tested Salmonella strains, encompassing eight globally prevalent serovars (Supplementary Table S1). Furthermore, no lytic activity was detected against E. coli, indicating the specificity of phage BP13076 towards Salmonella species.
Biological characteristics of phage BP13076
The optimal MOI The optimal MOI test of phage BP13076 was shown in Table 1. When MOI = 0.01, the phage reached its highest titer at 5.4 × 109 PFU/mL. Conversely, at MOI =10, the phage titer was the worst, measured at 7 × 108 PFU/mL. The results indicated that the optimal MOI for phage BP13076 was 0.01.
Table 1.
The optimal multiplicity of infection (MOI) of phage BP13076.
| No | Host strains concentration (CFU/mL) |
Phage titer (PFU/mL) | MOI | Phage titer (PFU/mL) |
|---|---|---|---|---|
| 1 | 1 × 108 | 1 × 105 | 0.001 | 8.6 × 108 |
| 2 | 1 × 108 | 1 × 106 | 0.01 | 5.4 × 109 |
| 3 | 1 × 108 | 1 × 107 | 0.1 | 3.1 × 109 |
| 4 | 1 × 108 | 1 × 108 | 1 | 7.1 × 108 |
| 5 | 1 × 108 | 1 × 108 | 10 | 7 × 108 |
Phage temperature and pH stability The stability of phage BP13076 under various temperature and pH conditions is presented in Fig 2A and B. The data indicated that phage BP13076 retained its activity after exposure to temperatures between 30°C to 50°C for 30 min and 60 min, showing minimal impact on its activity. However, as the temperature increased from 60°C to 70°C, phage activity progressively decreased, with complete inactivation observed at 80°C.
Figure 2.
Characteristics of phage BP13076. A. Resistance to temperature; B. Resistance to pH; C. One-step growth curve, and D. Lytic activity in vitro. The lytic ability of phage BP13076 was evaluated using a turbidity reduction assay in LB broth. A range of multiplicities of infection (MOI) was tested to determine the phage's lytic efficiency against a bacterial host. All data represent the mean ± standard deviation from three independent experiments.
Regarding pH stability, phage BP 13076 maintained its activity within a pH range of 4 to 10, demonstrating substantial stability. A noticeable titer decline was observed at pH below 3 or above 10. High levels of phage inactivation were detected when exposed to extreme pH conditions (pH ≤ 2 or pH ≥ 11).
One-step growth curve The result, shown in Fig. 2C, indicated that phage BP13076 had a very short latent period of approximately 5 min. The titer of phage BP13076 reached its stable status around 40 min post-infection. The average burst sizes were about 105 PFU/cell.
Lytic capacity at different MOI The lysis of Salmonella Enteritidis ATCC 13076 in LB broth by phage BP13076 at five different MOIs is shown in Fig. 2D. Negative controls (bacteria-free) maintained a steady state, while the OD600 value of the positive control group (bacteria without phage) increased over time and remained high throughout the 12.5-h incubation period. In contrast, phage-treated groups exhibited significant bacterial lysis, as indicated by dynamic changes in OD600 values. At all tested MOIs, OD600 values initially rose, followed by a decrease starting at 1 h. By 8 h, the OD600 values stabilized, reflecting effective bacterial lysis. These findings demonstrate the phage's ability to lyse Salmonella effectively across all tested MOIs.
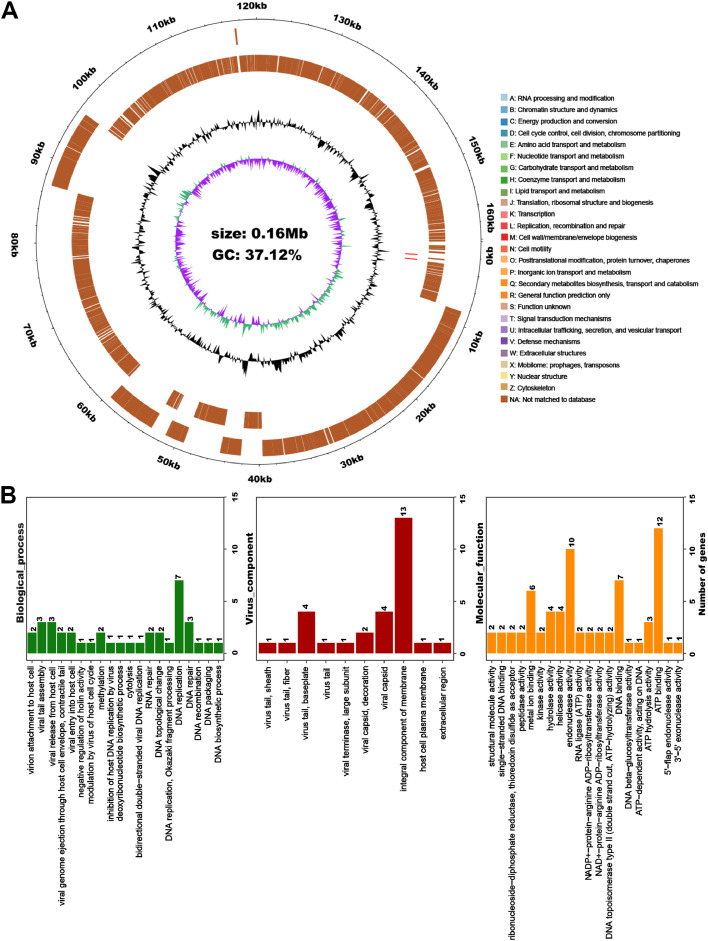
Genome features
The whole genome sequencing of phage BP13076 reveals a double-stranded DNA genome of 160,318 bp with a GC content of 37.13%. The genome contains 257 open reading frames (ORFs), of which 46 are positively orientated, with the remaining 211 ORFs orientated in the opposite direction (Fig. 3A). We could assign 88 (34.78%) of these ORFs with annotation, with the remaining ORFs identified as putative proteins with unknown functions (Fig. 3B, Supplementary Table S2). Our annotation efforts could place ORFs into eight major functional categories: virion assembly (38), host lysis (4), DNA-related (31), RNA-related (5), accessory (3), host takeover (1), transcriptional (5) and translation regulation (1).
Figure 3.
Genome analysis. A. Whole genome map of phage BP13076. Circles display in order from the outside to the inside: (1) genomic coordinates; (2) genes on the positive strand of the genome sequence, with different colors representing different COG classifications; (3) genes on the negative strand of the genome sequence, with different colors representing different COG functional classifications; (4) rRNA (blue) and tRNA (red) genes; (5) a GC content profile, calculated with a 200 bp sliding window, showing the average GC content across the genome; (6) GC skew curves, also using a 200 bp sliding window, where green indicates regions with higher G content than C, and purple indicates regions with lower G content. B. GO database annotation is represented in three sections: Biological processes, Virus components and Molecular functions.
The genome of BP13076 was also identified to contain four tRNA genes for amino acids for methionine, arginine, asparagine and glutamine. It is common to find tRNA genes in phage genomes, and tRNA might be crucial for optimising the translation efficiency during the phage replication cycle (Bailly-Bechet et al., 2007). Phage BP13076 may harbour these tRNA to it to cope better with differences in codon usage between its genome and that of the bacterium it infects.
Phylogenetic and comparative genomic analysis
A BLASTn comparison of these phages at the nucleotide level shows a shared nucleotide identity of >95% with several phages (vB_SnwM_CGG4-1, STML-198, Melville, and CF-SP2) placed in the among the genus Gelderlandvirus of the Tevenvirinae subfamily within Caudoviricetes. Additionally, gene synteny can be observed between their genomes, as in a gene map generated with Easyfig (Fig. 4A). The terminase large subunit, due to its crucial role in DNA packaging, is typically conserved across related phage genomes and is particularly suitable for phylogenetic analyses. Using the large terminase subunit of phage BP13076 as an indicator, we constructed a phylogenetic tree, showing the close relationship between BP13076 and Salmonella phage vB_SnwM_CGG4-1 (Fig. 4B).
Figure 4.
Phylogenetic relationship of phage BP13076. A. Comparison of amino acid of phage BP13076 against the close related Salmonella phages (phage STML-198, vB_SnwM_CGG4-1, Melville and CF-SP2) at the nucleotide level. The arrows represent genes, with orange indicating forward genes and gray lines indicating homology between sequences; B. Construction of evolutionary tree based on the large subunit of terminase of phage BP13076.
Application of phage BP13076
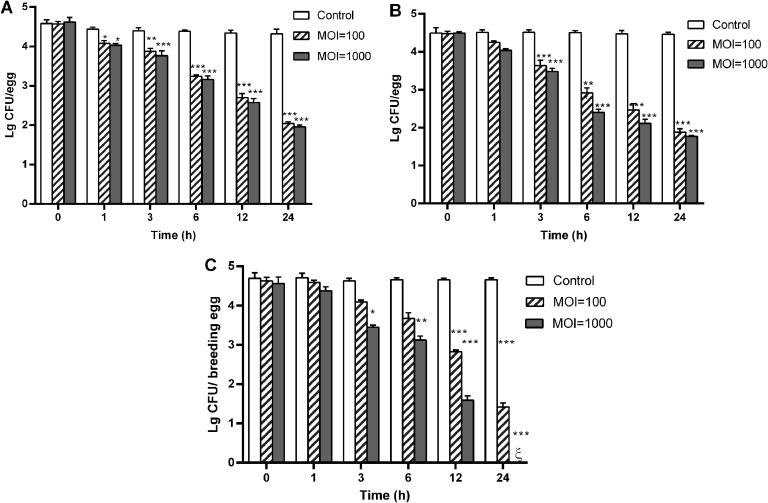
Immersion-based biological control of Salmonella on eggs Surviving bacterial counts were measured at various time points over the duration of the experiment with treated eggs incubated at 4°C and 25°C (Fig. 5A and B). The control groups, inoculated with host bacteria and treated with SM buffer, exhibited minimal changes in bacterial counts at both temperatures over 24 h. In contrast, the phage-treated groups demonstrated significant reductions in bacterial counts. Specifically, the group treated with MOI ∼100 showed 2.27 and 2.58 Log CFU/egg reductions at 4°C and 25°C, respectively. The group treated with MOI ∼1000 showed 2.58 and 2.6 Log CFU/egg reductions at 4°C and 25°C, respectively.
Figure 5.
Application of Salmonella phage BP13076 for the control of Salmonella populations in poultry table and breeding eggs. A. Reduction of Salmonella on eggs T treated by immersion with phage BP13076 at 4°C over time. This subsection evaluates the efficacy of phage BP13076 in reducing Salmonella populations on table eggs stored under refrigerated conditions (4°C).B. Reduction of Salmonella on eggs treated by immersion with phage BP13076 at 25°C over time. This subsection assesses the effect of immersion treatment with phage BP13076 on Salmonella populations on table eggs stored at room temperature (25°C). C. Reduction of Salmonella on breeding eggshells treated by spraying phage BP13076 at room temperature over time. ξ showed value below the detection limit. The values represented the mean and standard deviation of the three independent tests. Significance: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Spray control test of phage BP13076 on breeding eggs As shown in Fig. 5C, the Salmonella levels on the eggshell surface treated with phage BP13076 were significantly lower compared to the control group within 24 h. Specifically, the average bacterial load on the shell surface of breeding eggs treated with phage (at MOI=100 and 1000) decreased by 2.03 and 2.9 Log CFU/breeding eggs, respectively, after 12 h.
Discussion
Salmonella remains one of the most critical pathogens causing human food-borne illnesses. It is a persistent threat to human health and caused significant economic damage to society and the economy. It is also a critical organism responsible for stubborn infections in poultry, which are challenging to eradicate. The high pathogenicity and disease-causing potential of Salmonella underscored the need for effective control measures to protect public health and reduce economic losses associated with outbreaks (Medalla et al., 2021; Rehman et al., 2019). Broad-spectrum antimicrobials such as antibiotics and chemical disinfectants are commonly employed to control foodborne pathogens. However, their extensive use or misuse has led to significant safety issues, including the emergence of multi-resistant strains (Urban-Chmiel et al., 2022). As a result, phages have garnered attention as potential biocontrol agents (Yang et al., 2023).
This study focused on isolating and characterising lytic Salmonella phages from poultry waste. The broad host range was a significant advantage for biocontrol applications (Taslem Mourosi et al., 2022). Phage BP13076 could lyse 97.06% of the tested Salmonella strains, indicating its broad-spectrum activity. This property is particularly critical given the genetic diversity and adaptability of Salmonella, which often complicate control efforts. In commercial production, phages are often grown based on optimal relative infectivity MOI to ensure highly concentrated viral preparations, reducing production costs and improving economic efficiency. However, in practical applications, the number of Salmonella in the environment can vary, necessitating adjustments based on an ideal phage infection ratio determined through in vitro tests to minimize costs (Brathwaite et al., 2015). Our experiments determined the optimal MOI for phage BP13076 to be 0.01, which was advantageous for large-scale applications as it implied a lower phage requirement to achieve effective bacterial lysis.
The latent period of phage BP13076 was about 5 min, with a burst size of approximately 105 PFU/cell. In comparison, Kwon et al. (2020) reported a latent period of 60 min and a burst size of 29.11 for Salmonella phage. This indicated that phage BP13076 had a short latent period and a strong lysis ability. The ability to release many progeny phages quickly allowed for more efficient lysis of pathogens. This is a good characteristic which can significantly save time and costs in future production applications.
Phage BP13076 exhibited a temperature tolerance range of up to 50°C; this suggests that the phage could withstand a broad range of temperatures. Additionally, pH tolerance was a critical factor in assessing the application value of a phage. Phage BP13076 showed pH tolerance ranging from 4.0 to 10.0, indicating stability across various pH environments. This wide pH tolerance enhanced its applicability in different settings, whether in food safety, clinical applications, or biocontrol measures. The tolerance characteristics of phage BP13076 were comparable to those observed in phages analyzed in our previous studies (Bao et al., 2011). The phage's resistance to a wide temperature range, broad pH tolerance, and similarity to other well-studied phages makes it a promising candidate for further development in food safety and clinical settings.
Identify genes that encode proteins implicated in various stages of the phage's development and interaction with its host. Terminase, a key protein encoded by these structural genes, plays a crucial role in DNA packaging. During the phage assembly process, terminase cleaves the phage DNA and translocate it into the preformed capsid, utilising ATP for energy. This step is essential for generating infectious phage particles (Lokareddy et al., 2022). Biological process genes are integral to the phage and host cell interaction. For example, holin creates pores in the bacterial cell membrane at a precise stage of the phage replication cycle. This temporal regulation is critical because it allows endolysins to access and degrade the bacterial cell wall (Pollenz et al., 2022). The highly immunogenic outer capsid protein (ORF54) in phage BP13076 may play a significant role in the host's defense mechanisms against phage infections. Furthermore, genome sequencing and annotation revealed that BP13076 has no genes to encode integrase, excisionase, or repressor proteins indicating that phage BP13076 follows an exclusively lytic lifestyle. Also, there are no genes for antibiotic resistance, virulence factors and lysogeny, which are seen as crucial features of phages intended for use in phage biocontrol/therapy applications (Azari et al., 2023).
The results of this study demonstrated the effectiveness of phage BP13076 as a biocontrol agent against Salmonella on eggs, using both immersion and spray methods. The ability of phage BP13076 to effectively reduce Salmonella enteritidis on eggshells, especially at higher MOIs, highlighted its potential as a biocontrol agent for food safety and for preventing vertical transmission of Salmonella in farmed breeding eggs. The immersion-based method showed that phage application at both 4°C and 25°C led to significant reductions in Salmonella counts compared to the control groups. The effectiveness of the spray method, particularly in breeding eggs, further supported the utility of phage BP13076 in controlling Salmonella contamination in poultry production. The reduction of 2.9 Log CFU/mL after just 12 h highlighted the effective control of phage on S. Eneteritidis, which could be crucial for preventing the spread of Salmonella in hatcheries and farms. These findings were consistent with previous studies showing the efficacy of phage treatments in reducing Salmonella on food products and surfaces. For instance, Akhtar et al. (2021) reported significant reductions in Salmonella on poultry products following phage application. However, this study contributes novel insights into the application of phages for breeding eggs, which has received limited attention compared to table eggs. Despite these promising results, several challenges warrant further investigation. The stability and activity of phages under different environmental conditions, such as temperature fluctuations and varying organic load, must be thoroughly investigated. Furthermore, the potential development of bacterial resistance to phages, though less common than antibiotic resistance, could limit the long-term effectiveness of phage-based biocontrol strategies.
Conclusions
In conclusion, phage BP13076 exhibits significant potential as a biocontrol agent against Salmonella, offering a natural and effective alternative to antibiotics and chemical disinfectants. Its broad host range, stability under diverse conditions, and high lytic activity make it a promising candidate for enhancing food safety and controlling Salmonella-related diseases. Exploring the synergistic effects of phage combinations could enhance efficacy and reduce the potential for resistance development. Additionally, investigating the phage′s interaction with the host immune system will be critical for assessing its safety in clinical applications.
Funding
This study was supported by National Natural Science Foundation of China (32273095), The National Key Research and Development Program of China (2024YFE0198800), Jiangsu Province Science and Technology Plan Special Fund (BZ2023004).
Authors contributions
HDB and YYW - Conceptualization; YYW, HML, LCJ and LY - Data curation; YYW, CB, YZ, and HDB - Formal analysis; HDB and HZ - Funding acquisition; YYW, CB and HDB-Methodology; HDB - Resources; CB and YZ- Software, HZ and RW - Supervision; YYW - Writing original draft; CB and HDB- Review & Editing.
Availability of data and materials The complete genome sequence of phage BP13076 was deposited at GenBank under accession number PQ118624.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors gave their consent for publication.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version.
Declaration of competing interest
The authors declare that they have no competing interests.
Footnotes
Scientific section: Genetics and Molecular Biology
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.psj.2025.104969.
Appendix. Supplementary materials
References
- Akhtar A., Hair-Bejo M., Hussein E.A., Zakaria Z. Inactivation of different Salmonella enteriditis phage types and safety and efficacy of inactivated products in chicken. Vet. Med. Int. 2021;2021 doi: 10.1155/2021/8818308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azari R., Yousefi M.H., Taghipour Z., Wagemans J., Lavigne R., Hosseinzadeh S., Mazloomi S.M., Vallino M., Khalatbari-Limaki S., Berizi E. Application of the lytic bacteriophage Rostam to control enteritidis in eggs. Int. J. Food. Microb. 2023;389 doi: 10.1016/j.ijfoodmicro.2023.110097. [DOI] [PubMed] [Google Scholar]
- Bailly-Bechet M., Vergassola M., Rocha E. Causes for the intriguing presence of tRNAs in phages. Genome Res. 2007;17:1486–1495. doi: 10.1101/gr.6649807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankevich A., Nurk S., Antipov D., Gurevich A.A., Dvorkin M., Kulikov A.S., Lesin V.M., Nikolenko S.I., Pham S., Prjibelski A.D., Pyshkin A.V., Sirotkin A.V., Vyahhi N., Tesler G., Alekseyev M.A., Pevzner P.A. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao H., Zhang H., Wang R. Isolation and characterization of bacteriophages of Salmonella enterica serovar Pullorum. Poult. Sci. 2011;90:2370–2377. doi: 10.3382/ps.2011-01496. [DOI] [PubMed] [Google Scholar]
- Bao H.D., Zhang P..Y., Zhang H., Zhou Y., Zhang L.L., Wang R. Bio-control of Salmonella Enteritidis in foods using bacteriophages. Viruses. 2015;7:4836–4853. doi: 10.3390/v7082847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brathwaite K.J., Siringan P.., Connerton P.L., Connerton I.F. Host adaption to the bacteriophage carrier state of Campylobacter jejuni. Res. Microbiol. 2015;166:504–515. doi: 10.1016/j.resmic.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P.P., Lin B..Y., Mak A.J., Lowe T.M. tRNAscan-SE 2.0: improved detection and functional classification of transfer RNA genes. Nucleic. Acids. Res. 2021;49:9077–9096. doi: 10.1093/nar/gkab688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong C., Wei B., Cui H., Li X., Yuan Y., Wang L., Li S., Xu Y. Isolation, characterization and comparison of lytic epseptimavirus phages targeting Salmonella. Food Res. Int. 2021;147 doi: 10.1016/j.foodres.2021.110480. [DOI] [PubMed] [Google Scholar]
- Debroy R., Miryala S.K., Naha A., Anbarasu A., Ramaiah S. Gene interaction network studies to decipher the multi-drug resistance mechanism in Salmonella enterica serovar Typhi CT18 reveal potential drug targets. Microb. Pathogenesis. 2020;142 doi: 10.1016/j.micpath.2020.104096. [DOI] [PubMed] [Google Scholar]
- Frost I., Sati H., Garcia-Vello P., Hasso-Agopsowicz M., Lienhardt C., Gigante V., Beyer P. The role of bacterial vaccines in the fight against antimicrobial resistance: an analysis of the preclinical and clinical development pipeline. Lancet Microbe. 2023;4:e113–e125. doi: 10.1016/S2666-5247(22)00303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtier M., De Sordi L., Maura D., Arachchi H., Volant S., Dillies M.A., Debarbieux L. Bacteriophages to reduce gut carriage of antibiotic resistant uropathogens with low impact on microbiota composition. Environ. Microb. 2016;18:2237–2245. doi: 10.1111/1462-2920.13284. [DOI] [PubMed] [Google Scholar]
- Gu Z., Gu L., Eils R., Schlesner M., Brors B. Circlize implements and enhances circular visualization in R. Bioinformatics. 2014;30:2811–2812. doi: 10.1093/bioinformatics/btu393. [DOI] [PubMed] [Google Scholar]
- He J., Sun F., Sun D., Wang Z., Jin S., Pan Z., Xu Z., Chen X., Jiao X. Multidrug resistance and prevalence of quinolone resistance genes of Salmonella enterica serotypes 4,[5],12:i:- in China. Intern. J. Food Microb. 2020;330 doi: 10.1016/j.ijfoodmicro.2020.108692. [DOI] [PubMed] [Google Scholar]
- Hesse S., Adhya S. Phage therapy in the Twenty-First century: facing the decline of the antibiotic era; is it finally time for the age of the phage? Annu. Rev. Microbiol. 2019;73:155–174. doi: 10.1146/annurev-micro-090817-062535. [DOI] [PubMed] [Google Scholar]
- Hong Y., Schmidt K., Marks D., Hatter S., Marshall A., Albino L., Ebner P. Treatment of Salmonella-contaminated eggs and pork with a broad-spectrum, single bacteriophage: assessment of efficacy and resistance development. Foodborne Pathog. Dis. 2016;13:679–688. doi: 10.1089/fpd.2016.2172. [DOI] [PubMed] [Google Scholar]
- Huang C., Li J., Wang X., Pan H., Wang J., Chen Y. Phage amplification-based technologies for simultaneous quantification of viable Salmonella in foodstuff and rapid antibiotic susceptibility testing. Food Res. Intern. 2022;156 doi: 10.1016/j.foodres.2022.111279. [DOI] [PubMed] [Google Scholar]
- Islam M.S., Zhou Y.., Liang L., Nime I., Yan T., Willias S.P., Mia M.Z., Bei W., Connerton I.F., Fischetti V.A., Li J. Application of a broad range lytic phage LPST94 for biological control of Salmonella in foods. Microorganisms. 2020;8:247. doi: 10.3390/microorganisms8020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaroszewicz W., Morcinek-Orłowska J., Pierzynowska K., Gaffke L., Węgrzyn G. Phage display and other peptide display technologies. FEMS Microbiol. Rev. 2022;46 doi: 10.1093/femsre/fuab052. fuab052. [DOI] [PubMed] [Google Scholar]
- Kwon J., Kim S.G., Kim H.J., Giri S.S., Kim S.W., Lee S.B., Park S.C. Isolation and characterization of Salmonella jumbo-phage pSal-SNUABM-04. Viruses. 2020;13:27. doi: 10.3390/v13010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei C.W., Zhang Y.., Kang Z.Z., Kong L.H., Tang Y.Z., Zhang A.Y., Yang X., Wang H.N. Vertical transmission of Salmonella Enteritidis with heterogeneous antimicrobial resistance from breeding chickens to commercial chickens in China. Vet. Microbiol. 2020;240 doi: 10.1016/j.vetmic.2019.108538. [DOI] [PubMed] [Google Scholar]
- Ling H., Lou X., Luo Q., He Z., Sun M., Sun J. Recent advances in bacteriophage-based therapeutics: insight into the post-antibiotic era. Acta. Pharma. Sin. B. 2022;12:4348–4364. doi: 10.1016/j.apsb.2022.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokareddy R.K., Hou C.F.D., Doll S.G., Li F.L., Gillilan R.E., Forti F., Horner D.S., Briani F., Cingolani G. Terminase subunits from the Pseudomonas-phage E217. J. Mol. Biol. 2022;434 doi: 10.1016/j.jmb.2022.167799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Xiong W., Li Z., Yan P., Liu R., Liu X. Isolation and characterization of SGF3, a novel Microviridae phage infecting Shigella flexneri. Mol. Genet. Genomics. 2022;297:935–945. doi: 10.1007/s00438-022-01883-5. [DOI] [PubMed] [Google Scholar]
- Medalla F., Gu W., Friedman C.R., Judd M., Folster J., Griffin P.M., Hoekstra R.M. Increased incidence of antimicrobial-resistant nontyphoidal Salmonella infections, United States, 2004-2016. Emerg. Infect. Dis. 2021;27:1662–1672. doi: 10.3201/eid2706.204486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhone A.L., Makumi A.., Odaba J., Guantai L., Gunathilake K.M.D., Loignon S., Ngugi C.W., Akhwale J.K., Moineau S., Svitek N. Salmonella Enteritidis bacteriophages isolated from Kenyan poultry farms demonstrate time-dependent stability in environments mimicking the chicken gastrointestinal tract. Viruses. 2022;14:1788. doi: 10.3390/v14081788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miernikiewicz P., Dąbrowska K. Endocytosis of bacteriophages. Curr. Opin. Virol. 2022;52:229–235. doi: 10.1016/j.coviro.2021.12.009. [DOI] [PubMed] [Google Scholar]
- Mushegian A.R. Are there 10(31) virus particles on Earth, or more, or fewer? J. Bacteriol. 2020;202 doi: 10.1128/JB.00052-20. e00052-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Solà J., Colás-Medà P., Nicolau-Lapeña I., Alegre I., Abadias M., Viñas I. Pathogenic potential of the surviving Salmonella Enteritidis on strawberries after disinfection treatments based on ultraviolet-C light and peracetic acid. Int. J. Food Microbiol. 2022;364 doi: 10.1016/j.ijfoodmicro.2022.109536. [DOI] [PubMed] [Google Scholar]
- Phothaworn P., Supokaivanich R., Lim J., Klumpp J., Imam M., Kutter E., Galyov E.E., Dunne M., Korbsrisate S. Development of a broad-spectrum Salmonella phage cocktail containing Viunalike and jerseylike viruses isolated from Thailand. Food Microbiol. 2020;92 doi: 10.1016/j.fm.2020.103586. [DOI] [PubMed] [Google Scholar]
- Pollenz R.S., Bland J.., Pope W.H. Bioinformatic characterization of endolysins and holin-like membrane proteins in the lysis cassette of phages that infect. PLoS. One. 2022;17 doi: 10.1371/journal.pone.0276603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa G.L., Popa M.I. Salmonella spp. Infection - a continuous threat worldwide. Germs. 2021;11:88–96. doi: 10.18683/germs.2021.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy K.T., Verma P.., Reddy M.R. Toll-like receptors gene expression in the gastrointestinal tract of Salmonella serovar Pullorum-infected broiler chicken. Appl. Biochem. Biotechnol. 2014;173:356–364. doi: 10.1007/s12010-014-0864-8. [DOI] [PubMed] [Google Scholar]
- Rehman T., Yin L., Latif M.B., Chen J., Wang K., Geng Y., Huang X., Abaidullah M., Guo H., Ouyang P. Adhesive mechanism of different Salmonella fimbrial adhesins. Microb. Pathog. 2019;137 doi: 10.1016/j.micpath.2019.103748. [DOI] [PubMed] [Google Scholar]
- Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- Styles K.M., Locke R..K., Cowley L.A., Brown A.T., Sagona A.P. Transposable element insertions into the Escherichia coli polysialic acid gene cluster result in resistance to the K1F bacteriophage. Microb. Spectr. 2022;10 doi: 10.1128/spectrum.02112-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan M.J., Petty N..K., Beatson S.A. Easyfig: a genome comparison visualizer. Bioinformatics. 2011;27:1009–1010. doi: 10.1093/bioinformatics/btr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taslem Mourosi J., Awe A., Guo W., Batra H., Ganesh H., Wu X., Zhu J. Understanding bacteriophage tail fiber interaction with host surface receptor: the key "blueprint" for reprogramming phage host range. Int. J. Mol. Sci. 2022;23:12146. doi: 10.3390/ijms232012146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Acosta M.A., Clavijo V.., Vaglio C., Gonzalez-Barrios A.F., Vives-Florez M.J., Rito-Palomares M. Economic evaluation of the development of a phage therapy product for the control of Salmonella in poultry. Biotechnol. Prog. 2019;35:e2852. doi: 10.1002/btpr.2852. [DOI] [PubMed] [Google Scholar]
- Urban-Chmiel R., Marek A., Stępień-Pyśniak D., Wieczorek K., Dec M., Nowaczek A., Osek J. Antibiotic resistance in bacteria-a review. Antibiotics. (Basel) 2022;11:1079. doi: 10.3390/antibiotics11081079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . WHO; 2022. Antimicrobial Resistance; Fact Sheet.https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance 2021. Accessed June 20. [Google Scholar]
- Yang J., Zhu X., Xu X., Sun Q. Recent knowledge in phages, phage-encoded endolysin, and phage encapsulation against foodborne pathogens. Crit. Rev. Food Sci. Nutr. 2023;64:12040–12060. doi: 10.1080/10408398.2023.2246554. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.