Abstract
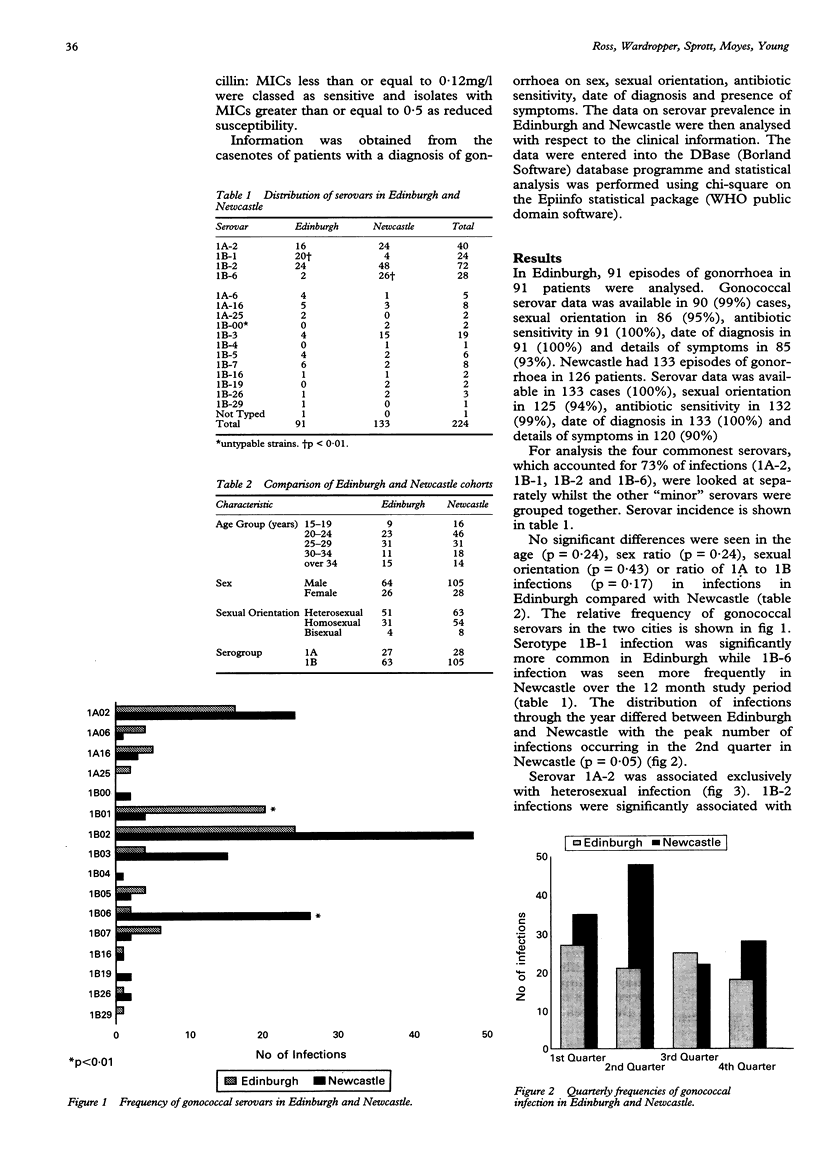
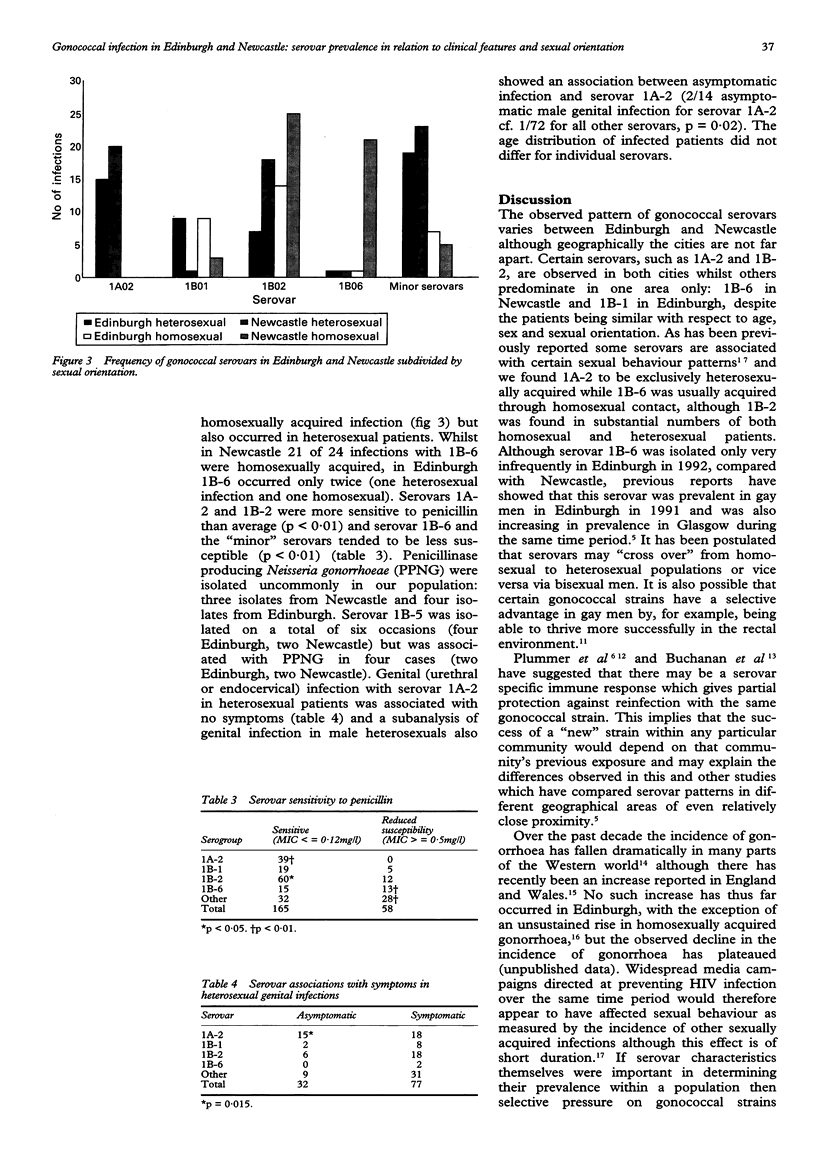
AIMS--The variable distribution of gonococcal serovars in different areas is well recognised but the factors that are important determinants of serovar prevalence are less clear. The aim of this study was to identify relevant clinical variables by comparing serovar prevalence in two cities over the same time period. METHODS--A prospective analysis of serovar prevalence was made between January and December 1992 in Edinburgh and Newcastle with respect to age, sex, sexual orientation, antibiotic sensitivity and presence of symptoms. RESULTS--224 infective episodes of gonorrhoea were studied. The serovar distribution varied between the two cities with serovar 1B-1 being more common in Edinburgh (20/91 cf. 4/133, p < 0.01) and serovar 1B-6 more common in Newcastle (26/133 cf. 2/91, p < 0.01). Serovar 1A-2 was associated with heterosexual infection (35/114 in heterosexuals cf. 0/85 in homosexuals, p < 0.01) and was more sensitive to penicillin than average (39/39 1A-2 strains highly penicillin sensitive cf. 98/184 for all other strains, p < 0.01) whilst 1B-6 was mostly acquired through homosexual contact (22/26 cf. 63/142 for all other strains, p < 0.01) and tended to show reduced penicillin susceptibility (13/28 1B-6 strains less penicillin sensitive cf. 45/195 for all other strains, p < 0.01). Infection with serovar 1A-2 was significantly less often symptomatic in heterosexuals than average (15/33 asymptomatic 1A-2 infections cf. 17/59 for all other serovars, p = 0.015). Subgroup analysis of male heterosexual infections confirms an association between asymptomatic infection and serovar 1A-2 (2/14 asymptomatic 1A-2 infections cf. 1/72 for all other serovars, p = 0.02). The distribution of infections over the year differed between the cities. CONCLUSIONS--A variety of factors including penicillin sensitivity and virulence may be important in determining the prevalence of gonococcal serovars within a given area.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeck D., Johnson A. P., Korting H. C. Characterisation of penicillinase producing gonococci isolated in Munich, 1981-6. Genitourin Med. 1988 Feb;64(1):3–6. doi: 10.1136/sti.64.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M. S., Davies H. G., Blockley J. R., Heffernan H. M. Antibiotic susceptibilities, serotypes and auxotypes of Neisseria gonorrhoeae isolated in New Zealand. Genitourin Med. 1992 Oct;68(5):321–324. doi: 10.1136/sti.68.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan T. M., Eschenbach D. A., Knapp J. S., Holmes K. K. Gonococcal salpingitis is less likely to recur with Neisseria gonorrhoeae of the same principal outer membrane protein antigenic type. Am J Obstet Gynecol. 1980 Dec 1;138(7 Pt 2):978–980. doi: 10.1016/0002-9378(80)91091-1. [DOI] [PubMed] [Google Scholar]
- Crawford C., Knapp J. S., Hale J., Holmes K. K. Asymptomatic gonorrhea in men: caused by gonococci with unique nutritional requirements. Science. 1977 Jun 17;196(4296):1352–1353. doi: 10.1126/science.405742. [DOI] [PubMed] [Google Scholar]
- Danielsson D., Bygdeman S., Kallings I. Epidemiology of gonorrhoea: serogroup, antibiotic susceptibility and auxotype patterns of consecutive gonococcal isolates from ten different areas of Sweden. Scand J Infect Dis. 1983;15(1):33–42. doi: 10.3109/inf.1983.15.issue-1.07. [DOI] [PubMed] [Google Scholar]
- Dillon J. R., Bygdeman S. M., Sandström E. G. Serological ecology of Neisseria gonorrhoeae (PPNG and non-PPNG) strains: Canadian perspective. Genitourin Med. 1987 Jun;63(3):160–168. doi: 10.1136/sti.63.3.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan B., Bek M. D., Pethebridge A. M., Nelson M. J. Heterosexual gonorrhoea in central Sydney: implications for HIV control. Med J Aust. 1991 Feb 4;154(3):175–180. doi: 10.5694/j.1326-5377.1991.tb121024.x. [DOI] [PubMed] [Google Scholar]
- Evans B. G., Catchpole M. A., Heptonstall J., Mortimer J. Y., McCarrigle C. A., Nicoll A. G., Waight P., Gill O. N., Swan A. V. Sexually transmitted diseases and HIV-1 infection among homosexual men in England and Wales. BMJ. 1993 Feb 13;306(6875):426–428. doi: 10.1136/bmj.306.6875.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill M. J. Serotyping Neisseria gonorrhoeae: a report of the Fourth International Workshop. Genitourin Med. 1991 Feb;67(1):53–57. doi: 10.1136/sti.67.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner P. J., Coker R. J., Turner A., Shafi M. S., Murphy S. M. Gonorrhoea: signs, symptoms and serogroups. Int J STD AIDS. 1992 Nov-Dec;3(6):430–433. doi: 10.1177/095646249200300606. [DOI] [PubMed] [Google Scholar]
- Ison C. A., Easmon C. S. Epidemiology of penicillin resistant Neisseria gonorrhoeae. Genitourin Med. 1991 Aug;67(4):307–311. doi: 10.1136/sti.67.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ison C. A., Whitaker L., Renton A. Concordance of auxotype/serovar classes of Neisseria gonorrhoeae between sexual contacts. Epidemiol Infect. 1992 Oct;109(2):265–271. doi: 10.1017/s0950268800050214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp J. S., Holmes K. K., Bonin P., Hook E. W., 3rd Epidemiology of gonorrhea: distribution and temporal changes in auxotype/serovar classes of Neisseria gonorrhoeae. Sex Transm Dis. 1987 Jan-Mar;14(1):26–32. [PubMed] [Google Scholar]
- Kohl P. K., Knapp J. S., Hofmann H., Gruender K., Petzoldt D., Tams M. R., Holmes K. K. Epidemiological analysis of Neisseria gonorrhoeae in the Federal Republic of Germany by auxotyping and serological classification using monoclonal antibodies. Genitourin Med. 1986 Jun;62(3):145–150. doi: 10.1136/sti.62.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Lysko P. G., McFarland L., Knapp J. S., Sandstrom E., Critchlow C., Holmes K. K. Gonococcal strains from homosexual men have outer membranes with reduced permeability to hydrophobic molecules. Infect Immun. 1982 Aug;37(2):432–438. doi: 10.1128/iai.37.2.432-438.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyes A., Young H. Epidemiological typing of Neisseria gonorrhoeae: a comparative analysis of three monoclonal antibody serotyping panels. Eur J Epidemiol. 1991 Jul;7(4):311–319. doi: 10.1007/BF00144994. [DOI] [PubMed] [Google Scholar]
- Plummer F. A., Brunham R. C. Gonococcal recidivism, diversity, and ecology. Rev Infect Dis. 1987 Jul-Aug;9(4):846–850. doi: 10.1093/clinids/9.4.846. [DOI] [PubMed] [Google Scholar]
- Plummer F. A., Simonsen J. N., Chubb H., Slaney L., Kimata J., Bosire M., Ndinya-Achola J. O., Ngugi E. N. Epidemiologic evidence for the development of serovar-specific immunity after gonococcal infection. J Clin Invest. 1989 May;83(5):1472–1476. doi: 10.1172/JCI114040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice R. J., Biddle J. W., JeanLouis Y. A., DeWitt W. E., Blount J. H., Morse S. A. Chromosomally mediated resistance in Neisseria gonorrhoeae in the United States: results of surveillance and reporting, 1983-1984. J Infect Dis. 1986 Feb;153(2):340–345. doi: 10.1093/infdis/153.2.340. [DOI] [PubMed] [Google Scholar]
- Ross J. D., McMillan A., Young H. Changing trends of gonococcal infection in homosexual men in Edinburgh. Epidemiol Infect. 1991 Dec;107(3):585–590. doi: 10.1017/s0950268800049281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J. D., Scott G. R. Seasonal variation in gonorrhoea. Eur J Epidemiol. 1992 Mar;8(2):252–255. doi: 10.1007/BF00144809. [DOI] [PubMed] [Google Scholar]
- Ross J. D., Scott G. R. The association between HIV media campaigns and number of patients coming forward for HIV antibody testing. Genitourin Med. 1993 Jun;69(3):193–195. doi: 10.1136/sti.69.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrard J., Barlow D. Gonorrhoea in men. Lancet. 1993 Jan 23;341(8839):245–245. doi: 10.1016/0140-6736(93)90110-3. [DOI] [PubMed] [Google Scholar]
- Woodford N., Bindayna K. M., Easmon C. S., Ison C. A. Associations between serotype and susceptibility to antibiotics of Neisseria gonorrhoeae. Genitourin Med. 1989 Apr;65(2):86–91. doi: 10.1136/sti.65.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young H., Moyes A., Robertson D. H., McCartney A. C., Lindsay G., Gallacher G., Tait I. B., Brogan O., Fox C., Kohiyar G. A. Gonococcal infection within Scotland: antigenic heterogeneity and antibiotic susceptibility of infecting strains. Eur J Epidemiol. 1990 Mar;6(1):1–8. doi: 10.1007/BF00155541. [DOI] [PubMed] [Google Scholar]
- Young H., Moyes A., Ross J. D., McMillan A. Patterns of homosexually acquired gonococcal serovars in Edinburgh 1986-90. Genitourin Med. 1991 Aug;67(4):312–316. doi: 10.1136/sti.67.4.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young H., Moyes A., Ross J. D., McMillan A., Robertson D. H. Serotype patterns of gonococcal infection in contact pairs. Eur J Epidemiol. 1993 Mar;9(2):195–198. doi: 10.1007/BF00158791. [DOI] [PubMed] [Google Scholar]
- Young H., Moyes A., Ross J., McMillan A. A serovar analysis of heterosexual gonorrhoea in Edinburgh 1986-90. Genitourin Med. 1992 Feb;68(1):16–19. doi: 10.1136/sti.68.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]


