Abstract
OBJECTIVE--To evaluate whether quantitative or qualitative IgA deficiencies in cervicovaginal secretions can be identified in patients with recurrent vulvovaginal candidiasis. DESIGN--Prospective and controlled study. SETTING--Department of Dermatology, University of Vienna. SUBJECTS--30 patients with symptomatic and recurrent vulvovaginal candidiasis at the time of their presentation. 30 healthy women as a control group. INTERVENTION--Blood samples were drawn for measurement of serum IgA levels. Smears of the cervix and vagina were taken for direct microscopy and microbiological culture. Lavage of the vagina and ectocervix was performed with sterile saline solution for measurement of cervicovaginal IgA levels. MAIN OUTCOME MEASURES--IgA levels of serum and cervicovaginal secretion evaluated by Single Radial Immunodiffusion. IgA labelling was demonstrated on fungal elements in vaginal smears and subcultured blastospores after incubation with vaginal secretions by immunohistochemistry. RESULTS--We could not find any significant difference of IgA levels in serum and cervicovaginal secretions between the symptomatic group and healthy controls (p value for serum = 0.5796, p value for secretion = 0.2381). In vaginal smears yeasts revealed IgA coating on their surfaces, whereas three of the 61 subcultures were negative. Negative subcultures were assigned to three patients with recurrent candidiasis. No correlation was found between IgA levels of cervicovaginal secretions and staining intensity of subcultured blastospores after incubation with vaginal secretions (r = -0.0578). IgA levels of serum and vaginal secretion showed no correlation (r = -0.00012). CONCLUSION--Recurrent vulvovaginal candidiasis cannot be attributed to IgA deficiency. In some cases an IgA coating defect of yeasts might be involved. In addition inactivation of the IgA molecule by candida proteases might be of pathogenetic importance.
Full text
PDF

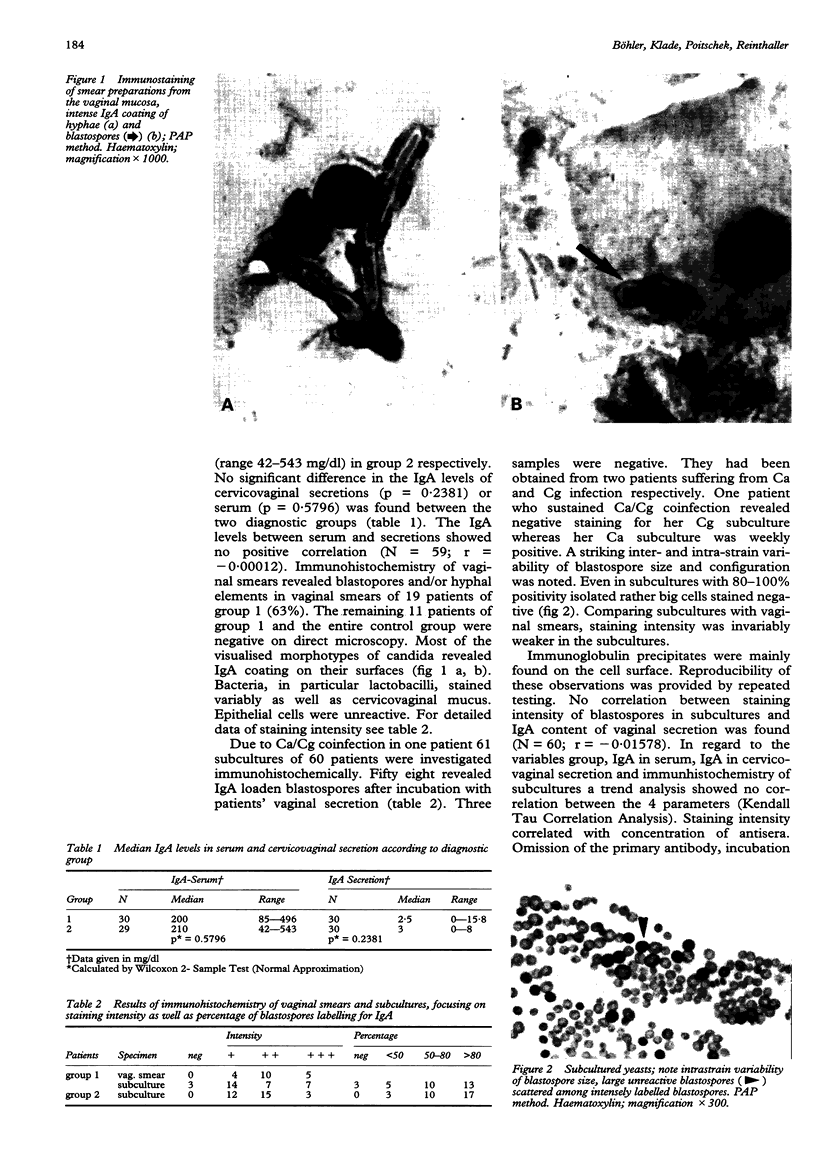


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertholf M. E., Stafford M. J. Colonization of Candida albicans in vagina, rectum, and mouth. J Fam Pract. 1983 May;16(5):919–924. [PubMed] [Google Scholar]
- Brandtzaeg P., Fjellanger I., Gjeruldsen S. T. Adsorption of immunolgobulin A onto oral bacteria in vivo. J Bacteriol. 1968 Jul;96(1):242–249. doi: 10.1128/jb.96.1.242-249.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone R. A. Host-parasite relationships in candidosis. Mycoses. 1989;32 (Suppl 2):12–17. doi: 10.1111/j.1439-0507.1989.tb02303.x. [DOI] [PubMed] [Google Scholar]
- Cassone A., De Bernardis F., Mondello F., Ceddia T., Agatensi L. Evidence for a correlation between proteinase secretion and vulvovaginal candidosis. J Infect Dis. 1987 Nov;156(5):777–783. doi: 10.1093/infdis/156.5.777. [DOI] [PubMed] [Google Scholar]
- Chipperfield E. J., Evans B. A. Effect of local infection and oral contraception on immunoglobulin levels in cervical mucus. Infect Immun. 1975 Feb;11(2):215–221. doi: 10.1128/iai.11.2.215-221.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipperfield E. J., Evans B. A. The influence of local infection on immunoglobulin formation in the human endocervix. Clin Exp Immunol. 1972 Jun;11(2):219–233. [PMC free article] [PubMed] [Google Scholar]
- Gough P. M., Warnock D. W., Richardson M. D., Mansell N. J., King J. M. IgA and IgG antibodies to Candida albicans in the genital tract secretions of women with or without vaginal candidosis. Sabouraudia. 1984;22(4):265–271. [PubMed] [Google Scholar]
- Mathur S., Koistinen G. V., Horger E. O., 3rd, Mahvi T. A., Fudenberg H. H. Humoral immunity in vaginal candidiasis. Infect Immun. 1977 Jan;15(1):287–294. doi: 10.1128/iai.15.1.287-294.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNabb P. C., Tomasi T. B. Host defense mechanisms at mucosal surfaces. Annu Rev Microbiol. 1981;35:477–496. doi: 10.1146/annurev.mi.35.100181.002401. [DOI] [PubMed] [Google Scholar]
- Metze D., Kersten A., Jurecka W., Gebhart W. Immunoglobulins coat microorganisms of skin surface: a comparative immunohistochemical and ultrastructural study of cutaneous and oral microbial symbionts. J Invest Dermatol. 1991 Apr;96(4):439–445. doi: 10.1111/1523-1747.ep12469908. [DOI] [PubMed] [Google Scholar]
- Milne J. D., Warnock D. W. Effect of simultaneous oral and vaginal treatment on the rate of cure and relapse in vaginal candidosis. Br J Vener Dis. 1979 Oct;55(5):362–365. doi: 10.1136/sti.55.5.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebello R., Green F. H., Fox H. A study of the secretory immune system of the female genital tract. Br J Obstet Gynaecol. 1975 Oct;82(10):812–816. [PubMed] [Google Scholar]
- Rüchel R. A variety of Candida proteinases and their possible targets of proteolytic attack in the host. Zentralbl Bakteriol Mikrobiol Hyg A. 1984 Jul;257(2):266–274. [PubMed] [Google Scholar]
- Rüchel R. Cleavage of immunoglobulins by pathogenic yeasts of the genus Candida. Microbiol Sci. 1986 Oct;3(10):316–319. [PubMed] [Google Scholar]
- Slutsky B., Buffo J., Soll D. R. High-frequency switching of colony morphology in Candida albicans. Science. 1985 Nov 8;230(4726):666–669. doi: 10.1126/science.3901258. [DOI] [PubMed] [Google Scholar]
- Sobel J. D. Pathophysiology of vulvovaginal candidiasis. J Reprod Med. 1989 Aug;34(8 Suppl):572–580. [PubMed] [Google Scholar]
- Soll D. R. High-frequency switching in Candida albicans and its relations to vaginal candidiasis. Am J Obstet Gynecol. 1988 Apr;158(4):997–1001. doi: 10.1016/0002-9378(88)90113-5. [DOI] [PubMed] [Google Scholar]
- Staebell M., Soll D. R. Temporal and spatial differences in cell wall expansion during bud and mycelium formation in Candida albicans. J Gen Microbiol. 1985 Jun;131(6):1467–1480. doi: 10.1099/00221287-131-6-1467. [DOI] [PubMed] [Google Scholar]
- White D. J., Emens M., Shahmanesh M. Recurrent vulvovaginal candidosis. Int J STD AIDS. 1991 Jul-Aug;2(4):235–239. doi: 10.1177/095646249100200401. [DOI] [PubMed] [Google Scholar]
- Witkin S. S., Hirsch J., Ledger W. J. A macrophage defect in women with recurrent Candida vaginitis and its reversal in vitro by prostaglandin inhibitors. Am J Obstet Gynecol. 1986 Oct;155(4):790–795. doi: 10.1016/s0002-9378(86)80022-9. [DOI] [PubMed] [Google Scholar]
- Witkin S. S., Yu I. R., Ledger W. J. Inhibition of Candida albicans--induced lymphocyte proliferation by lymphocytes and sera from women with recurrent vaginitis. Am J Obstet Gynecol. 1983 Dec 1;147(7):809–811. doi: 10.1016/0002-9378(83)90044-3. [DOI] [PubMed] [Google Scholar]