Abstract
OBJECTIVE--To study risk factors for hepatitis C virus (HCV) infection in injecting drug users (IDUs) from central Sydney. SETTING AND SUBJECTS--All IDUs attending a primary health care facility in central Sydney between December 1991 and November 1992 who underwent HCV antibody testing. METHODS--Information was obtained retrospectively from client forms routinely completed at the time of medical consultation. Additional information on injecting history and practice was obtained from the registration forms of subjects who also attended the needle syringe exchange programme at the same health care facility. RESULTS--Of the 201 IDUs tested, 118 (59%) had HCV antibodies, which did not differ significantly between males and females. HCV prevalence increased significantly with age, being highest in IDUs who were aged 35 years or more (93%) and lowest in IDUs aged under 20 years (17%). HCV prevalence increased significantly with time since first injecting, from 26% for IDUs who had injected for less than 3 years to 94% for those who had injected for more than 10 years. HCV prevalence was also significantly higher in heterosexual IDUs as compared with homosexual male IDUs, and in opiate users as compared with stimulant users, even after adjustment for age and duration of injecting. HCV prevalence was strongly associated with exposure to hepatitis B virus, but was not associated with exposure to HIV. CONCLUSION--Recent HCV transmission indicates ongoing injecting risk behaviour despite HIV prevention efforts, and underlies the potential for increased transmission of HIV through the sharing of injecting equipment. Within the population of IDUs, those who are heterosexual or inject heroin appear to be at increased risk of HCV infection.
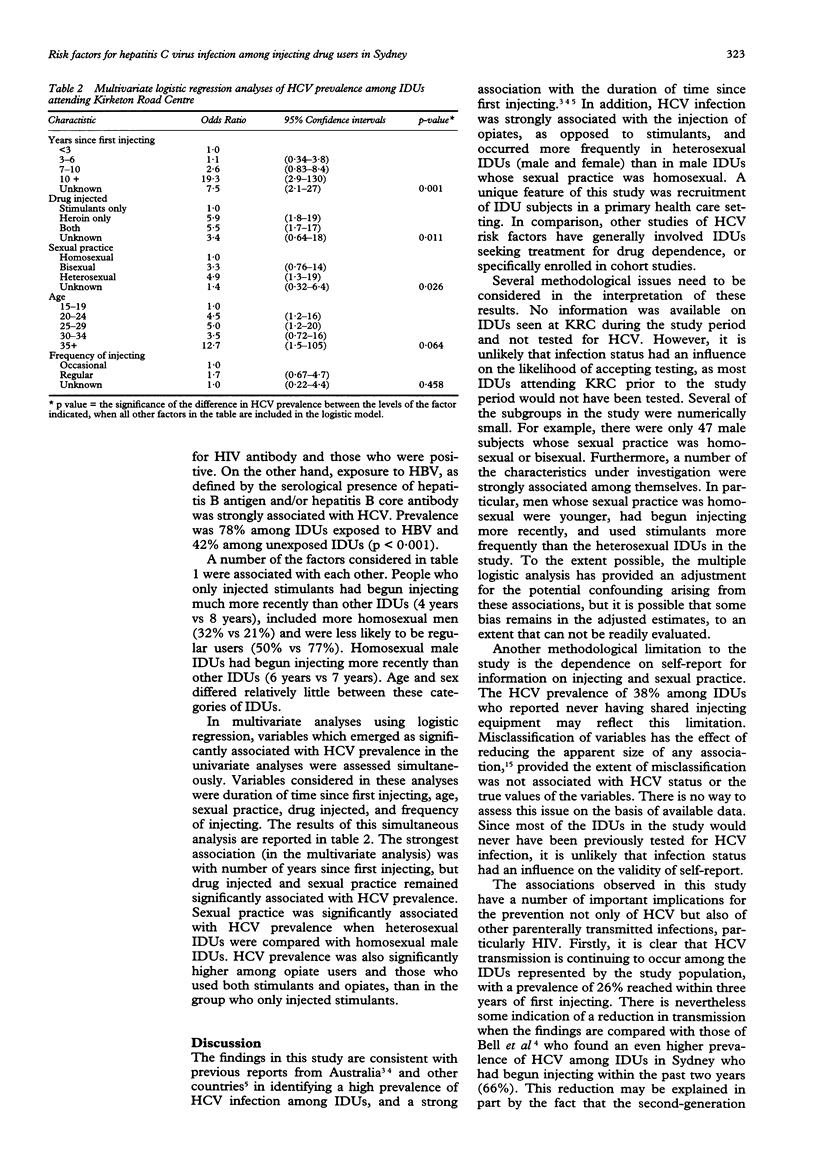
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aach R. D., Stevens C. E., Hollinger F. B., Mosley J. W., Peterson D. A., Taylor P. E., Johnson R. G., Barbosa L. H., Nemo G. J. Hepatitis C virus infection in post-transfusion hepatitis. An analysis with first- and second-generation assays. N Engl J Med. 1991 Nov 7;325(19):1325–1329. doi: 10.1056/NEJM199111073251901. [DOI] [PubMed] [Google Scholar]
- Anderson R. M. Mathematical and statistical studies of the epidemiology of HIV. AIDS. 1989 Jun;3(6):333–346. doi: 10.1097/00002030-198906000-00001. [DOI] [PubMed] [Google Scholar]
- Bell J., Batey R. G., Farrell G. C., Crewe E. B., Cunningham A. L., Byth K. Hepatitis C virus in intravenous drug users. Med J Aust. 1990 Sep 3;153(5):274–276. doi: 10.5694/j.1326-5377.1990.tb136900.x. [DOI] [PubMed] [Google Scholar]
- Choo Q. L., Kuo G., Weiner A. J., Overby L. R., Bradley D. W., Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989 Apr 21;244(4902):359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- Copeland K. T., Checkoway H., McMichael A. J., Holbrook R. H. Bias due to misclassification in the estimation of relative risk. Am J Epidemiol. 1977 May;105(5):488–495. doi: 10.1093/oxfordjournals.aje.a112408. [DOI] [PubMed] [Google Scholar]
- Crofts N., Hopper J. L., Bowden D. S., Breschkin A. M., Milner R., Locarnini S. A. Hepatitis C virus infection among a cohort of Victorian injecting drug users. Med J Aust. 1993 Aug 16;159(4):237–241. doi: 10.5694/j.1326-5377.1993.tb137822.x. [DOI] [PubMed] [Google Scholar]
- Donahue J. G., Muñoz A., Ness P. M., Brown D. E., Jr, Yawn D. H., McAllister H. A., Jr, Reitz B. A., Nelson K. E. The declining risk of post-transfusion hepatitis C virus infection. N Engl J Med. 1992 Aug 6;327(6):369–373. doi: 10.1056/NEJM199208063270601. [DOI] [PubMed] [Google Scholar]
- Kaldor J. M., Archer G. T., Buring M. L., Ismay S. L., Kenrick K. G., Lien A. S., Purusothaman K., Tulloch R., Bolton W. V., Wylie B. R. Risk factors for hepatitis C virus infection in blood donors: a case-control study. Med J Aust. 1992 Aug 17;157(4):227–230. doi: 10.5694/j.1326-5377.1992.tb137123.x. [DOI] [PubMed] [Google Scholar]
- Kaldor J., Elford J., Wodak A., Crofts J. N., Kidd S. HIV prevalence among IDUs in Australia: a methodological review. Drug Alcohol Rev. 1993;12(2):175–184. doi: 10.1080/09595239300185611. [DOI] [PubMed] [Google Scholar]
- Kaldor J., Williamson P., Guinan J. J., Imrie A., Gold J. Falling incidence of HIV infection in a cohort of clinic attenders. Aust J Public Health. 1993 Dec;17(4):334–338. doi: 10.1111/j.1753-6405.1993.tb00165.x. [DOI] [PubMed] [Google Scholar]
- Kuo G., Choo Q. L., Alter H. J., Gitnick G. L., Redeker A. G., Purcell R. H., Miyamura T., Dienstag J. L., Alter M. J., Stevens C. E. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989 Apr 21;244(4902):362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- Melbye M., Biggar R. J., Wantzin P., Krogsgaard K., Ebbesen P., Becker N. G. Sexual transmission of hepatitis C virus: cohort study (1981-9) among European homosexual men. BMJ. 1990 Jul 28;301(6745):210–212. doi: 10.1136/bmj.301.6745.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hoek J. A., van Haastrecht H. J., Goudsmit J., de Wolf F., Coutinho R. A. Prevalence, incidence, and risk factors of hepatitis C virus infection among drug users in Amsterdam. J Infect Dis. 1990 Oct;162(4):823–826. doi: 10.1093/infdis/162.4.823. [DOI] [PubMed] [Google Scholar]


