Abstract
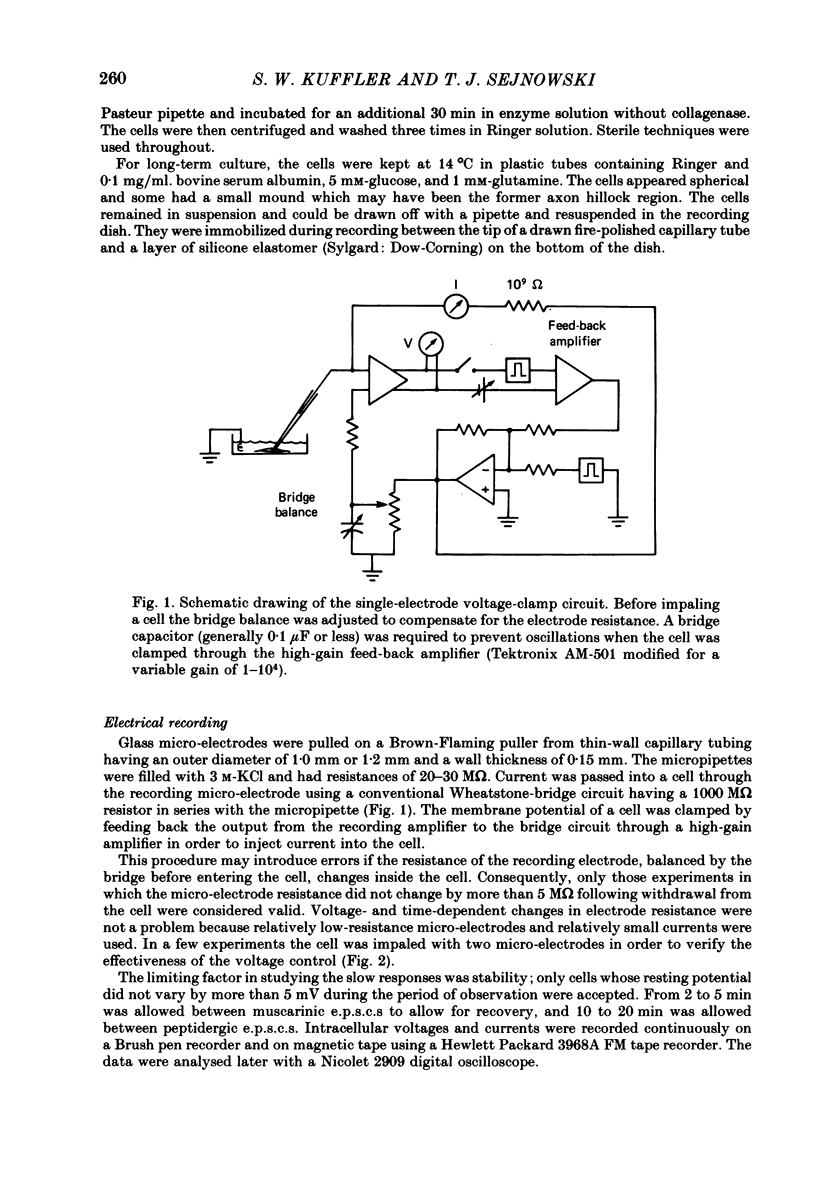
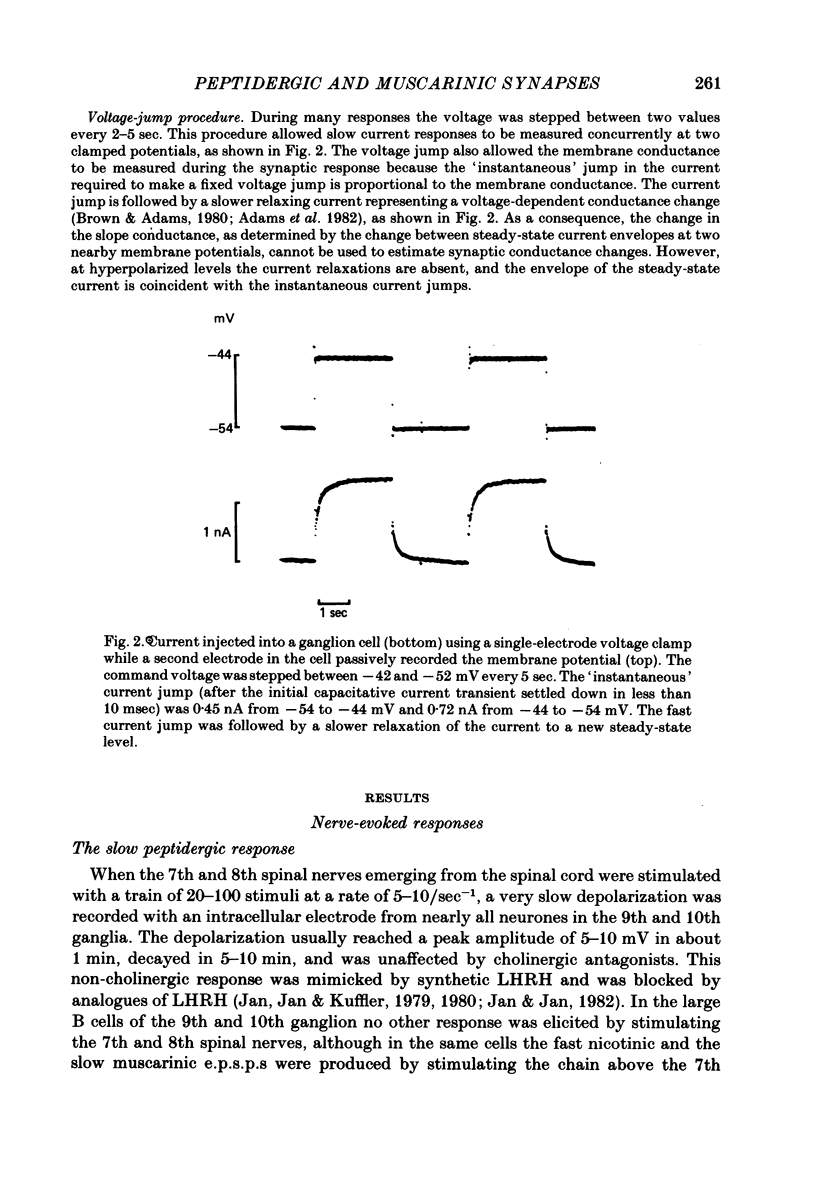
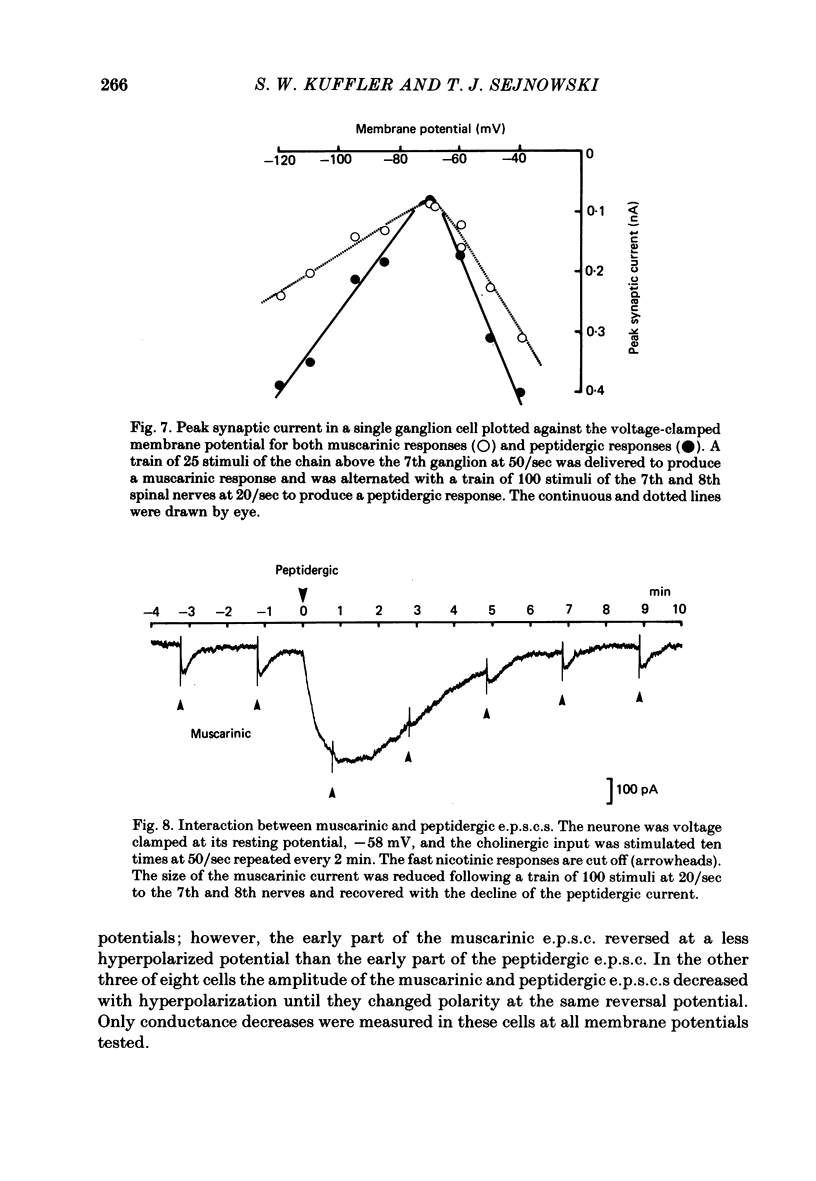
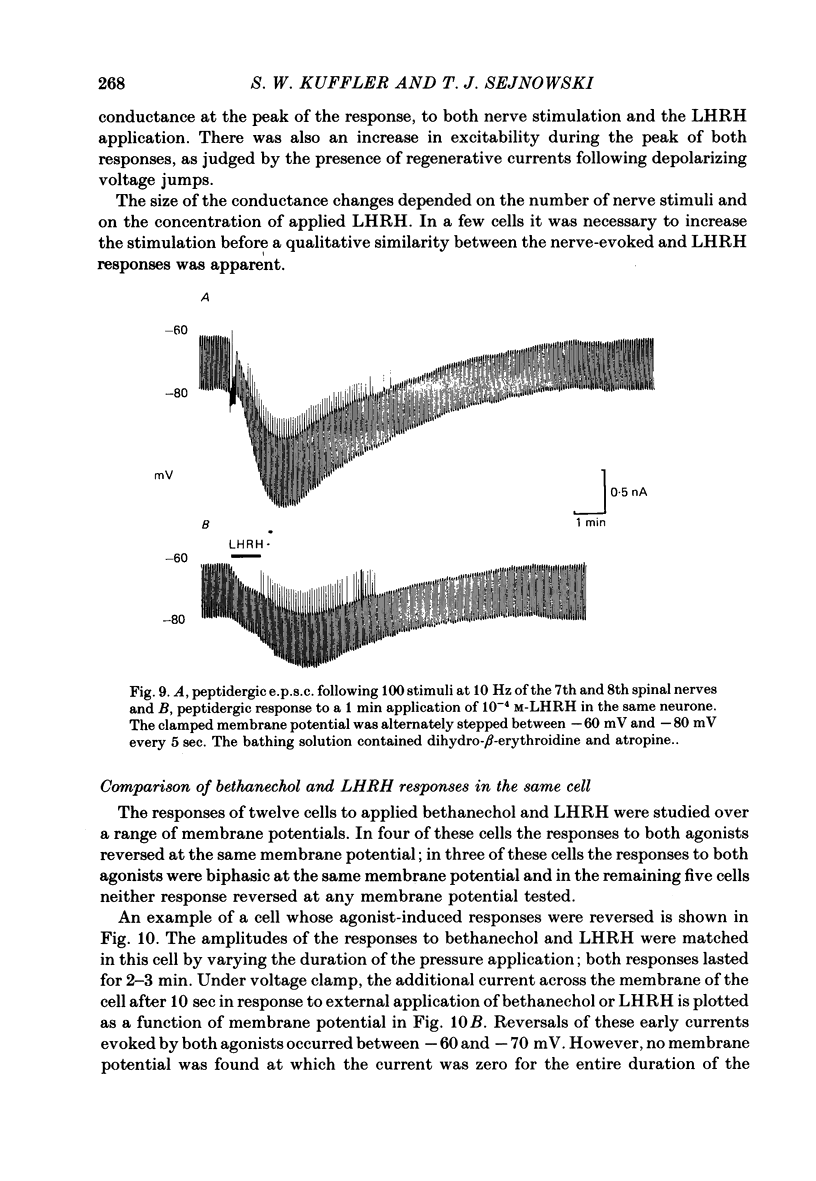
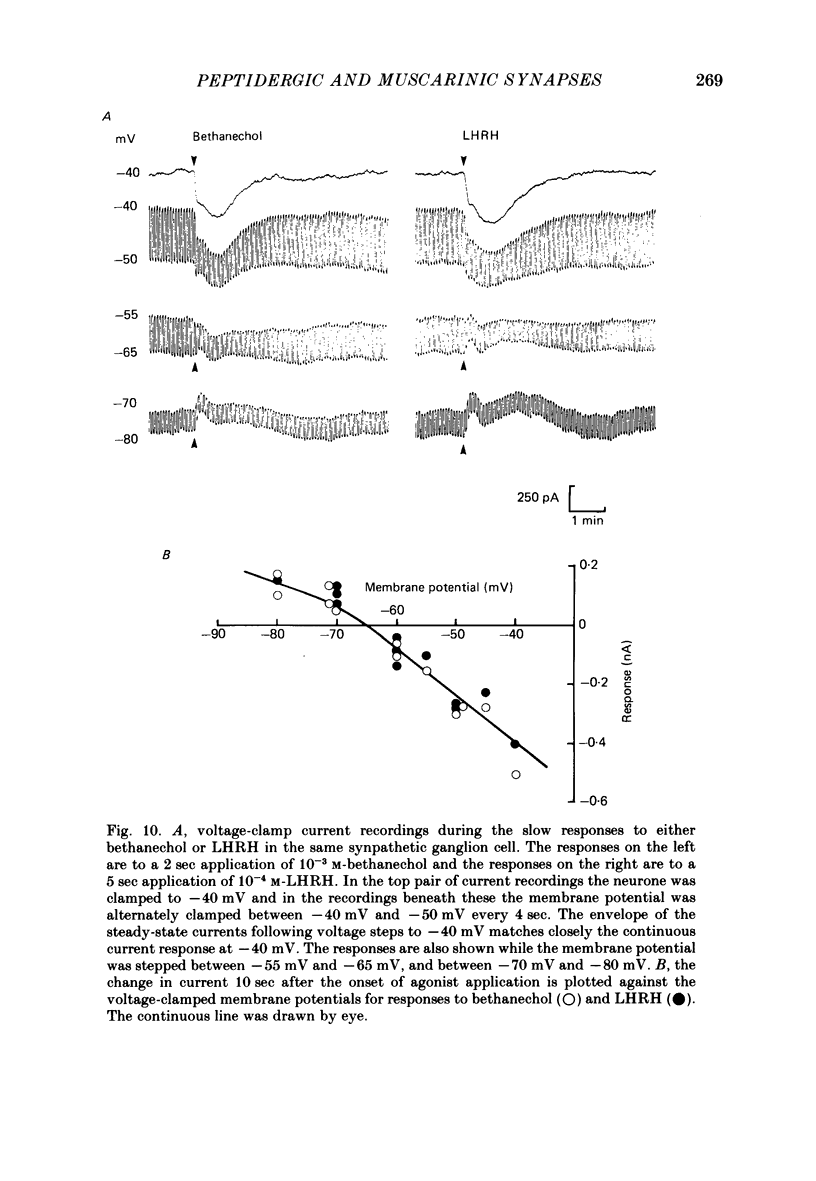
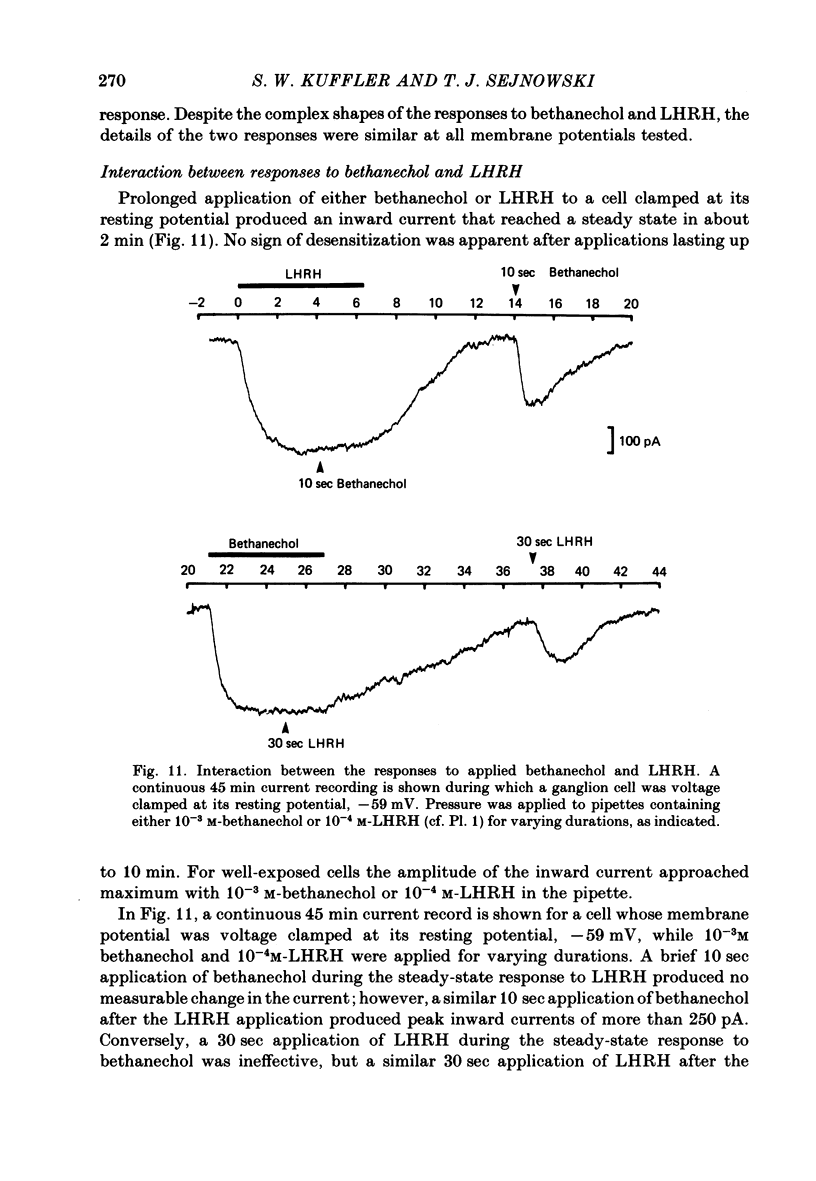
A single-electrode voltage clamp was used to study the slow muscarinic and late slow peptidergic excitatory post-synaptic currents (e.p.s.c.s) in B cells of the paravertebral sympathetic ganglia of the bull-frog. Conductance decreases were measured during peptidergic e.p.s.c.s in nearly all cells at clamped potentials near the resting level. In about half of the cells the size of the peptidergic e.p.s.c.s increased with hyperpolarization and in some of these cells conductance increases were found at hyperpolarized levels. In the remaining cells conductance decreases occurred at all levels of membrane potential tested, and in a few of these the polarity of the e.p.s.c.s reversed at hyperpolarized potentials. A similar diversity was observed among muscarinic e.p.s.c.s. At least two simple ionic mechanisms are required to explain the heterogeneous voltage dependencies observed: a conductance decrease primarily to K+ that dominates at depolarized potentials and a conductance increase to other ions that is more prominent at hyperpolarized potentials. The proportion of these two mechanisms appears to differ among B cells. The two slow e.p.s.c.s recorded in the same neurone had the same voltage dependence and were accompanied by the same conductance changes in each of eight cells despite differences between cells. The muscarinic e.p.s.c. was reduced during the peptidergic e.p.s.c. in each of twenty-five neurones tested over a range of membrane potentials. Externally-applied luteinizing hormone releasing hormone (LHRH) produced currents with the same voltage dependence and conductance changes as the nerve-evoked peptidergic e.p.s.c. in each of fifteen cells tested. Bethanechol, a muscarinic agonist, and LHRH produced currents with the same voltage dependence and conductance changes in each of the twelve cells studied. In several cells a saturating response to a prolonged application of LHRH completely occluded the response to bethanechol, and vice versa. Slow currents were recorded from dissociated cell bodies in response to bethanechol and LHRH; these responses exhibited the same diversity of voltage dependence and conductance changes as was observed in intact ganglia. Activation of muscarinic and peptidergic receptors may control shared ionic mechanisms in single ganglion cells.
Full text
PDF


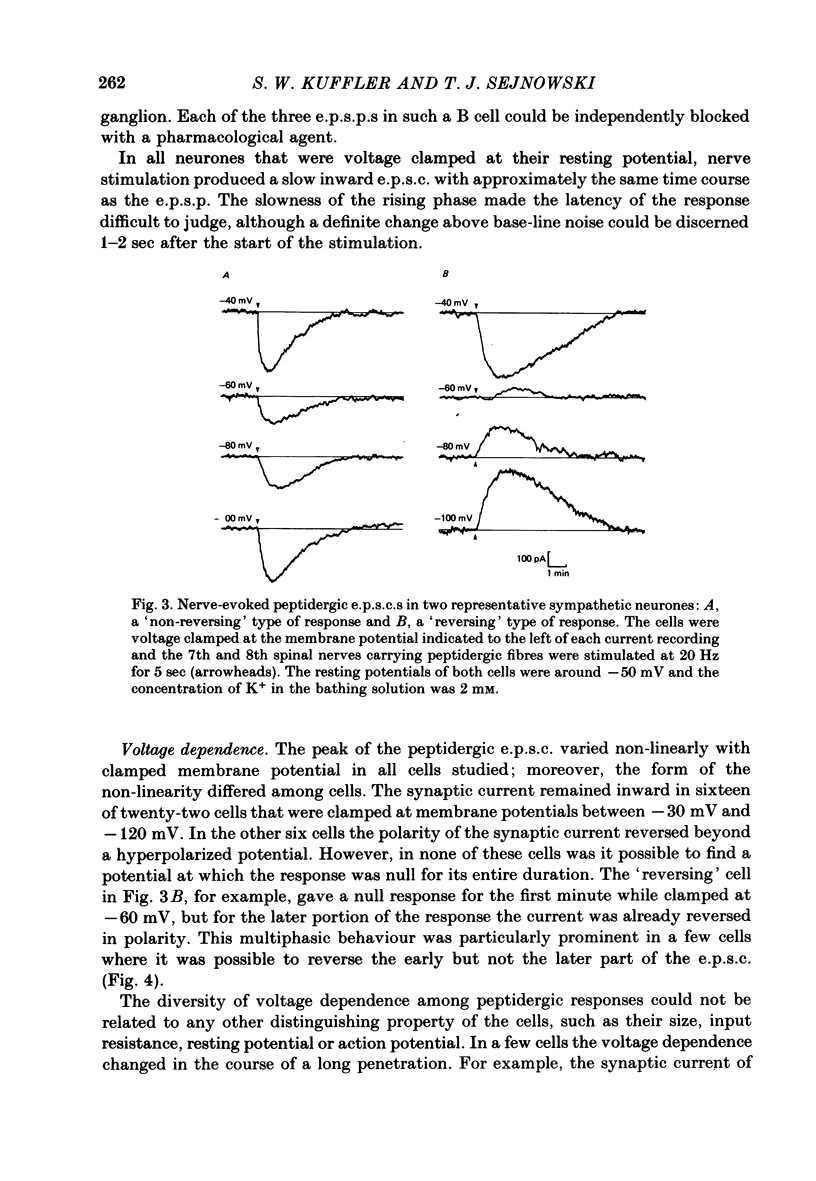
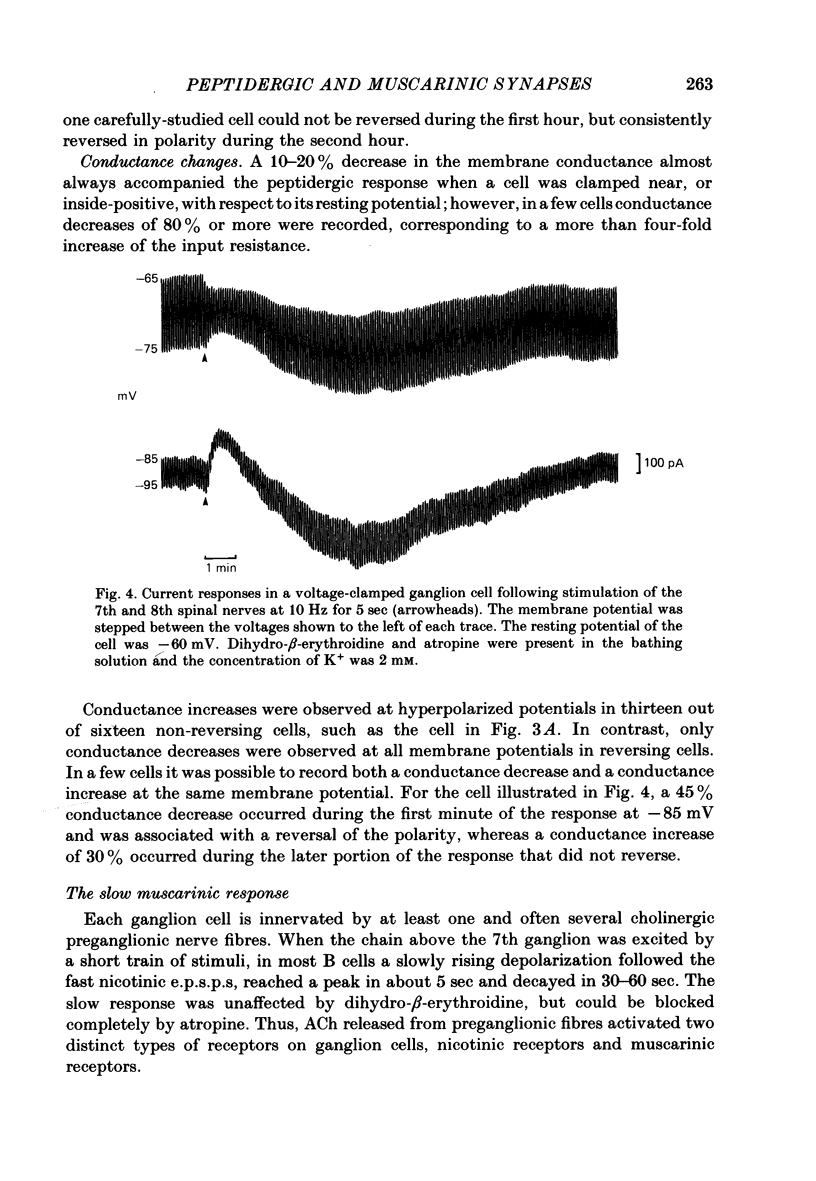
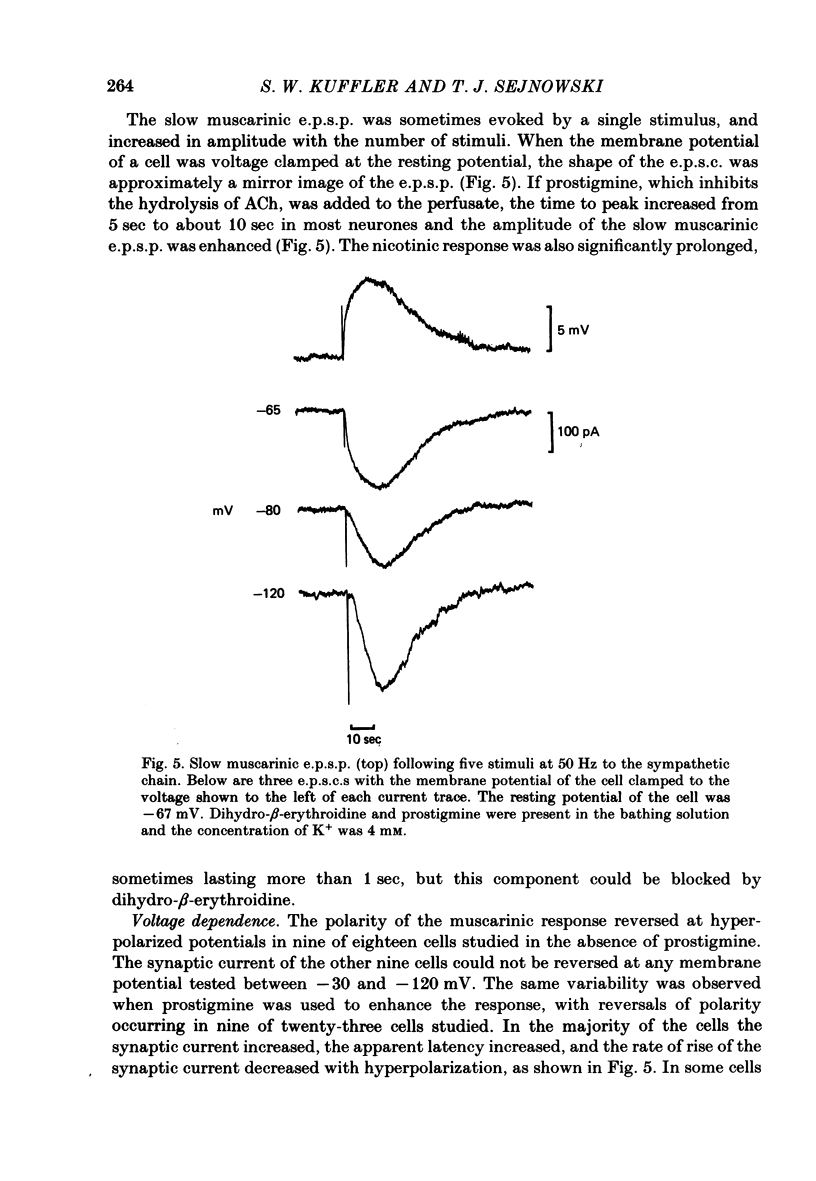
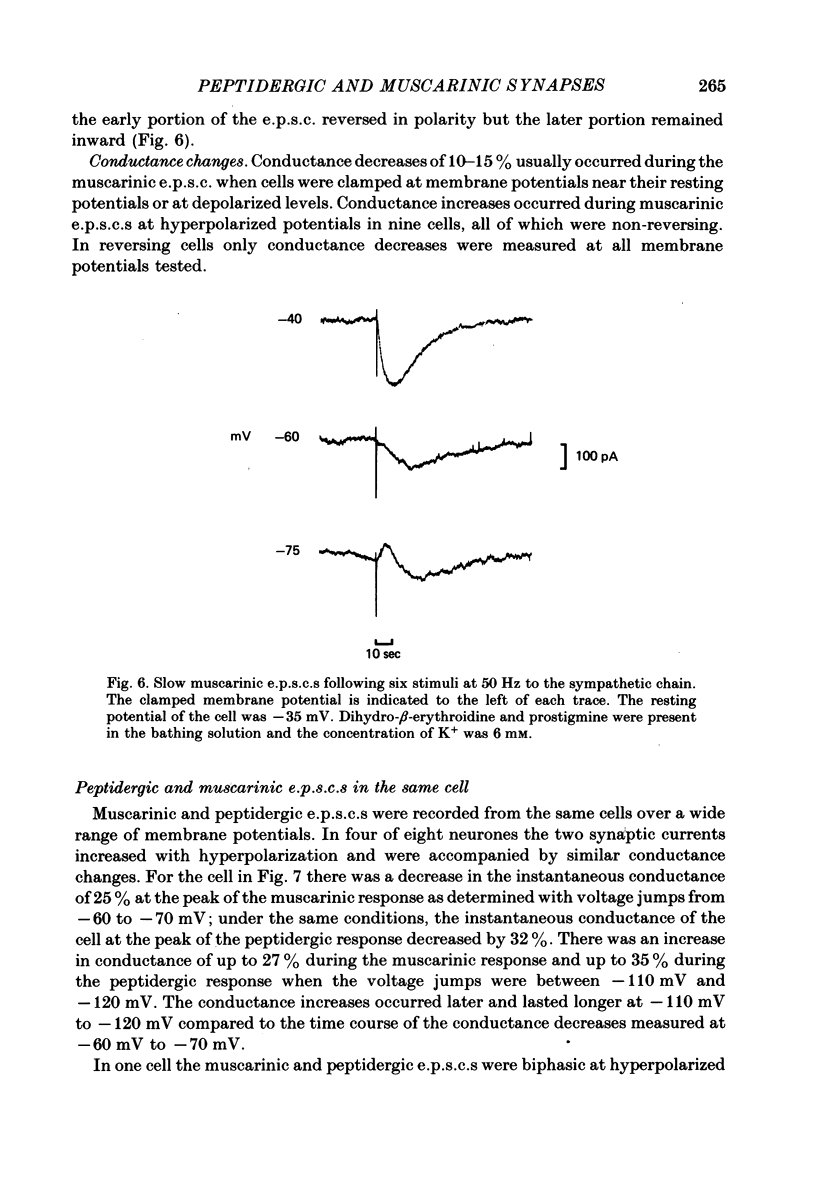








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A., Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J Physiol. 1982 Sep;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Brown D. A. Luteinizing hormone-releasing factor and muscarinic agonists act on the same voltage-sensitive K+-current in bullfrog sympathetic neurones. Br J Pharmacol. 1980 Mar;68(3):353–355. doi: 10.1111/j.1476-5381.1980.tb14547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams W. B., Parnas I., Levitan I. B. Mechanism of long-lasting synaptic inhibition in Aplysia neuron R15. J Neurophysiol. 1980 Dec;44(6):1148–1160. doi: 10.1152/jn.1980.44.6.1148. [DOI] [PubMed] [Google Scholar]
- BLACKMAN J. G., GINSBORG B. L., RAY C. Synaptic transmission in the sympathetic ganglion of the frog. J Physiol. 1963 Jul;167:355–373. doi: 10.1113/jphysiol.1963.sp007155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., McBurney R. N. GABA and glycine may share the same conductance channel on cultured mammalian neurones. Nature. 1979 Jan 18;277(5693):234–236. doi: 10.1038/277234a0. [DOI] [PubMed] [Google Scholar]
- Bennett M. V., Model P. G., Highstein S. M. Stimulation-induced depletion of vesicles, fatigue of transmission and recovery processes at a vertebrate central synapse. Cold Spring Harb Symp Quant Biol. 1976;40:25–35. doi: 10.1101/sqb.1976.040.01.005. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Adams P. R. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980 Feb 14;283(5748):673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Brown J. E., Muller K. J., Murray G. Reversal potential for an electrophysiological event generated by conductance changes: mathematical analysis. Science. 1971 Oct 15;174(4006):318–318. doi: 10.1126/science.174.4006.318. [DOI] [PubMed] [Google Scholar]
- Dodd J., Horn J. P. Muscarinic inhibition of sympathetic C neurones in the bullfrog. J Physiol. 1983 Jan;334:271–291. doi: 10.1113/jphysiol.1983.sp014494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun N. J., Jiang Z. G. Non-cholinergic excitatory transmission in inferior mesenteric ganglia of the guinea-pig: possible mediation by substance P. J Physiol. 1982 Apr;325:145–159. doi: 10.1113/jphysiol.1982.sp014141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun N. J., Karczmar A. G. Actions of substance P on sympathetic neurons. Neuropharmacology. 1979 Feb;18(2):215–218. doi: 10.1016/0028-3908(79)90064-9. [DOI] [PubMed] [Google Scholar]
- Dun N. J., Minota S. Effects of substance P on neurones of the inferior mesenteric ganglia of the guinea-pig. J Physiol. 1981 Dec;321:259–271. doi: 10.1113/jphysiol.1981.sp013982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES R. M., LIBET B. Origin and blockade of the synaptic responses of curarized sympathetic ganglia. J Physiol. 1961 Aug;157:484–503. doi: 10.1113/jphysiol.1961.sp006738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES R. Responses of isolated curarized sympathetic ganglia. J Physiol. 1952 Jun;117(2):196–217. doi: 10.1113/jphysiol.1952.sp004740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallacher D. V., Petersen O. H. Substance P increases membrane conductance in parotid acinar cells. Nature. 1980 Jan 24;283(5745):393–395. doi: 10.1038/283393a0. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Stickgold R., Yoshikami D. Synaptic excitation and inhibition resulting from direct action of acetylcholine on two types of chemoreceptors on individual amphibian parasympathetic neurones. J Physiol. 1977 Oct;271(3):817–846. doi: 10.1113/jphysiol.1977.sp012027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C. Mechanisms of slow postsynaptic potentials. Nature. 1981 Jun 18;291(5816):539–544. doi: 10.1038/291539a0. [DOI] [PubMed] [Google Scholar]
- Horn J. P., Dodd J. Monosynaptic muscarinic activation of K+ conductance underlies the slow inhibitory postsynaptic potential in sympathetic ganglia. Nature. 1981 Aug 13;292(5824):625–627. doi: 10.1038/292625a0. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N., Brownfield M. S. Peptidergic transmitters in synaptic boutons of sympathetic ganglia. Nature. 1980 Nov 27;288(5789):380–382. doi: 10.1038/288380a0. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Peptidergic transmission in sympathetic ganglia of the frog. J Physiol. 1982 Jun;327:219–246. doi: 10.1113/jphysiol.1982.sp014228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y., Kuffler S. W. A peptide as a possible transmitter in sympathetic ganglia of the frog. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1501–1505. doi: 10.1073/pnas.76.3.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y., Kuffler S. W. Further evidence for peptidergic transmission in sympathetic ganglia. Proc Natl Acad Sci U S A. 1980 Aug;77(8):5008–5012. doi: 10.1073/pnas.77.8.5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y., Nishi S. Voltage-clamp analysis of peptidergic slow depolarizations in bullfrog sympathetic ganglion cells. J Physiol. 1982 Dec;333:305–313. doi: 10.1113/jphysiol.1982.sp014455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. S., Marty A. Certain slow synaptic responses: their properties and possible underlying mechanisms. Annu Rev Biophys Bioeng. 1980;9:437–465. doi: 10.1146/annurev.bb.09.060180.002253. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Libet B. Actions of noradrenaline and acetylcholine on sympathetic ganglion cells. J Physiol. 1970 Jun;208(2):353–372. doi: 10.1113/jphysiol.1970.sp009125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Libet B. Generation of slow postsynaptic potentials without increases in ionic conductance. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1304–1311. doi: 10.1073/pnas.60.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Libet B. Is inactivation of potassium conductance involved in slow postsynaptic excitation of sympathetic ganglion cells? Effects of nicotine. Life Sci. 1974 May 16;14(10):1871–1883. doi: 10.1016/0024-3205(74)90404-4. [DOI] [PubMed] [Google Scholar]
- Kuba K., Koketsu K. Ionic mechanism of the slow excitatory postsynaptic potential in bullfrog sympathetic ganglion cells. Brain Res. 1974 Dec 6;81(2):338–342. doi: 10.1016/0006-8993(74)90949-4. [DOI] [PubMed] [Google Scholar]
- Kuba K., Koketsu K. Synaptic events in sympathetic ganglia. Prog Neurobiol. 1978;11(2):77–169. doi: 10.1016/0301-0082(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Kuba K., Nishi S. Characteristics of fast excitatory postsynaptic current in bullfrog sympathetic ganglion cells. Effects of membrane potential, temperature and Ca ions. Pflugers Arch. 1979 Jan 31;378(3):205–212. doi: 10.1007/BF00592737. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W. Slow synaptic responses in autonomic ganglia and the pursuit of a peptidergic transmitter. J Exp Biol. 1980 Dec;89:257–286. doi: 10.1242/jeb.89.1.257. [DOI] [PubMed] [Google Scholar]
- Kuno M. Quantum aspects of central and ganglionic synaptic transmission in vertebrates. Physiol Rev. 1971 Oct;51(4):647–678. doi: 10.1152/physrev.1971.51.4.647. [DOI] [PubMed] [Google Scholar]
- LAPORTE Y., LORENTE de NO R. Potential changes evoked in a curarized sympathetic ganglion by presynaptic volleys of impulses. J Cell Physiol Suppl. 1950 Jul;35(Suppl 2):61–106. doi: 10.1002/jcp.1030350505. [DOI] [PubMed] [Google Scholar]
- Libet B., Chichibu S., Tosaka T. Slow synaptic responses and excitability in sympathetic ganglia of the bullfrog. J Neurophysiol. 1968 May;31(3):383–395. doi: 10.1152/jn.1968.31.3.383. [DOI] [PubMed] [Google Scholar]
- Llinás R., Nicholson C. Reversal properties of climbing fiber potential in cat Purkinje cells: an example of a distributed synapse. J Neurophysiol. 1976 Mar;39(2):311–323. doi: 10.1152/jn.1976.39.2.311. [DOI] [PubMed] [Google Scholar]
- MacDermott A. B., Connor E. A., Dionne V. E., Parsons R. L. Voltage clamp study of fast excitatory synaptic currents in bullfrog sympathetic ganglion cells. J Gen Physiol. 1980 Jan;75(1):39–60. doi: 10.1085/jgp.75.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L. M. Synaptic localization of alpha-bungarotoxin binding which blocks nicotinic transmission at frog sympathetic neurons. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1948–1952. doi: 10.1073/pnas.78.3.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan U. J., Kuffler S. W. Visual identification of synaptic boutons on living ganglion cells and of varicosities in postganglionic axons in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):485–508. doi: 10.1098/rspb.1971.0044. [DOI] [PubMed] [Google Scholar]
- Minota S., Dun N. J., Karczmar A. G. Substance P-induced depolarization in sympathetic neurons: not simple K-inactivation. Brain Res. 1981 Jul 6;216(1):224–228. doi: 10.1016/0006-8993(81)91294-4. [DOI] [PubMed] [Google Scholar]
- NISHI S., KOKETSU K. Electrical properties and activities of single sympathetic neurons in frogs. J Cell Comp Physiol. 1960 Feb;55:15–30. doi: 10.1002/jcp.1030550104. [DOI] [PubMed] [Google Scholar]
- Neild T. O. Slowly-developing depolarization of neurones in the guinea-pig inferior mesenteric ganglion following repetitive stimulation of the preganglionic nerves. Brain Res. 1978 Jan 27;140(2):231–239. doi: 10.1016/0006-8993(78)90457-2. [DOI] [PubMed] [Google Scholar]
- Nishi S., Koketsu K. Early and late after discharges of amphibian sympathetic ganglion cells. J Neurophysiol. 1968 Jan;31(1):109–121. doi: 10.1152/jn.1968.31.1.109. [DOI] [PubMed] [Google Scholar]
- Nishi S., Soeda H., Koketsu K. Studies on sympathetic B and C neurons and patterns of pregnaglionic innervation. J Cell Physiol. 1965 Aug;66(1):19–32. doi: 10.1002/jcp.1030660103. [DOI] [PubMed] [Google Scholar]
- PICK J. Sympathectomy in amphibians; anatomical considerations. J Comp Neurol. 1957 Apr;107(2):169–207. doi: 10.1002/cne.901070202. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Muscarinic, alpha-adrenergic and peptide receptors regulate the same calcium influx sites in the parotid gland. J Physiol. 1977 Jun;268(1):139–149. doi: 10.1113/jphysiol.1977.sp011851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier J. E., Vale W. W. [D-pGlu1,D-Phe2,D-Trp3,6]-LRF. A potent luteinizing hormone releasing factor antagonist in vitro and inhibitor of ovulation in the rat. Life Sci. 1978 Aug 28;23(8):869–876. doi: 10.1016/0024-3205(78)90522-2. [DOI] [PubMed] [Google Scholar]
- Schulman J. A., Weight F. F. Synaptic transmission: long-lasting potentiation by a postsynaptic mechanism. Science. 1976 Dec 24;194(4272):1437–1439. doi: 10.1126/science.188131. [DOI] [PubMed] [Google Scholar]
- Sejnowski T. J. Peptidergic synaptic transmission in sympathetic ganglia. Fed Proc. 1982 Nov;41(13):2923–2928. [PubMed] [Google Scholar]
- Torre V. The contribution of the electrogenic sodium-potassium pump to the electrical activity of toad rods. J Physiol. 1982 Dec;333:315–341. doi: 10.1113/jphysiol.1982.sp014456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weight F. F., Votava J. Slow synaptic excitation in sympathetic ganglion cells: evidence for synaptic inactivation of potassium conductance. Science. 1970 Nov 13;170(3959):755–758. doi: 10.1126/science.170.3959.755. [DOI] [PubMed] [Google Scholar]
- Weight F. F., Weitsen H. A. Identification of small intensely fluorescent (SIF) cells as chromaffin cells in bullfrog sympathetic ganglia. Brain Res. 1977 Jun 10;128(2):213–226. doi: 10.1016/0006-8993(77)90989-1. [DOI] [PubMed] [Google Scholar]
- Weitsen H. A., Weight F. F. Synaptic innervation of sympathetic ganglion cells in the bullfrog. Brain Res. 1977 Jun 10;128(2):197–211. doi: 10.1016/0006-8993(77)90988-x. [DOI] [PubMed] [Google Scholar]