Abstract
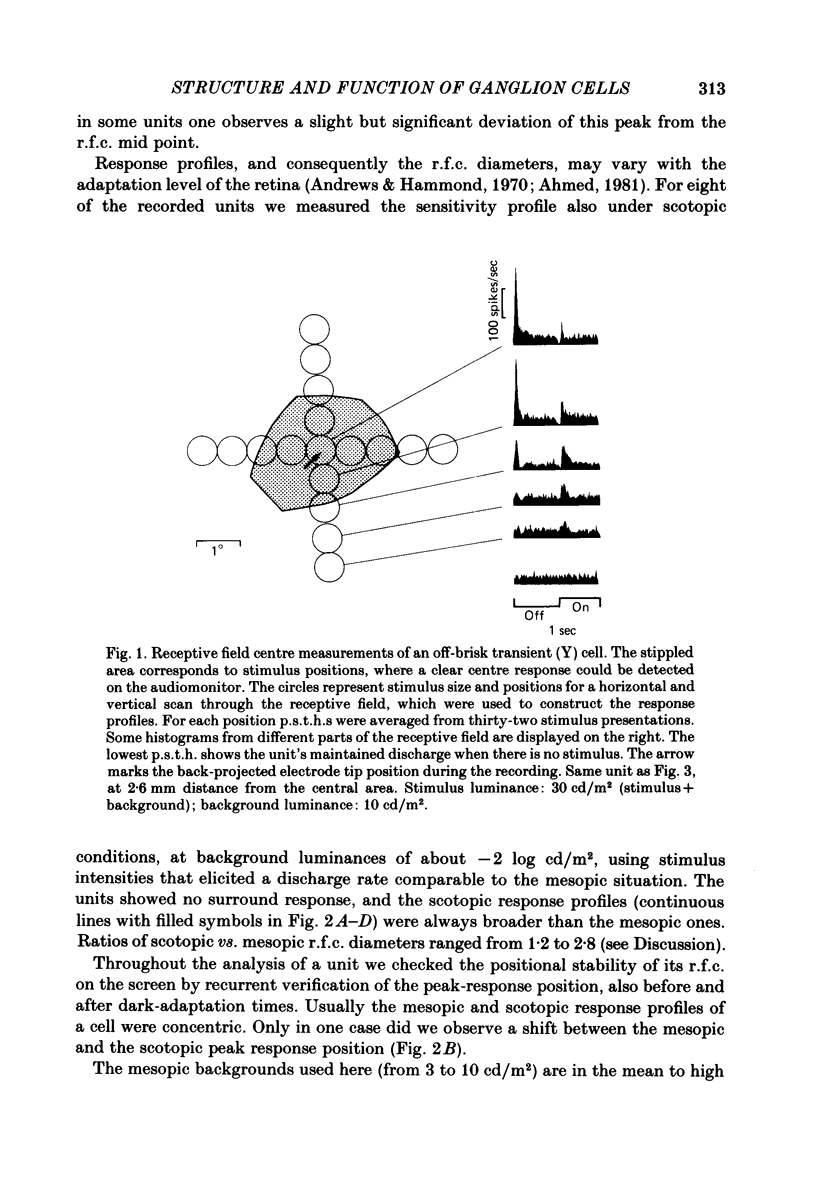
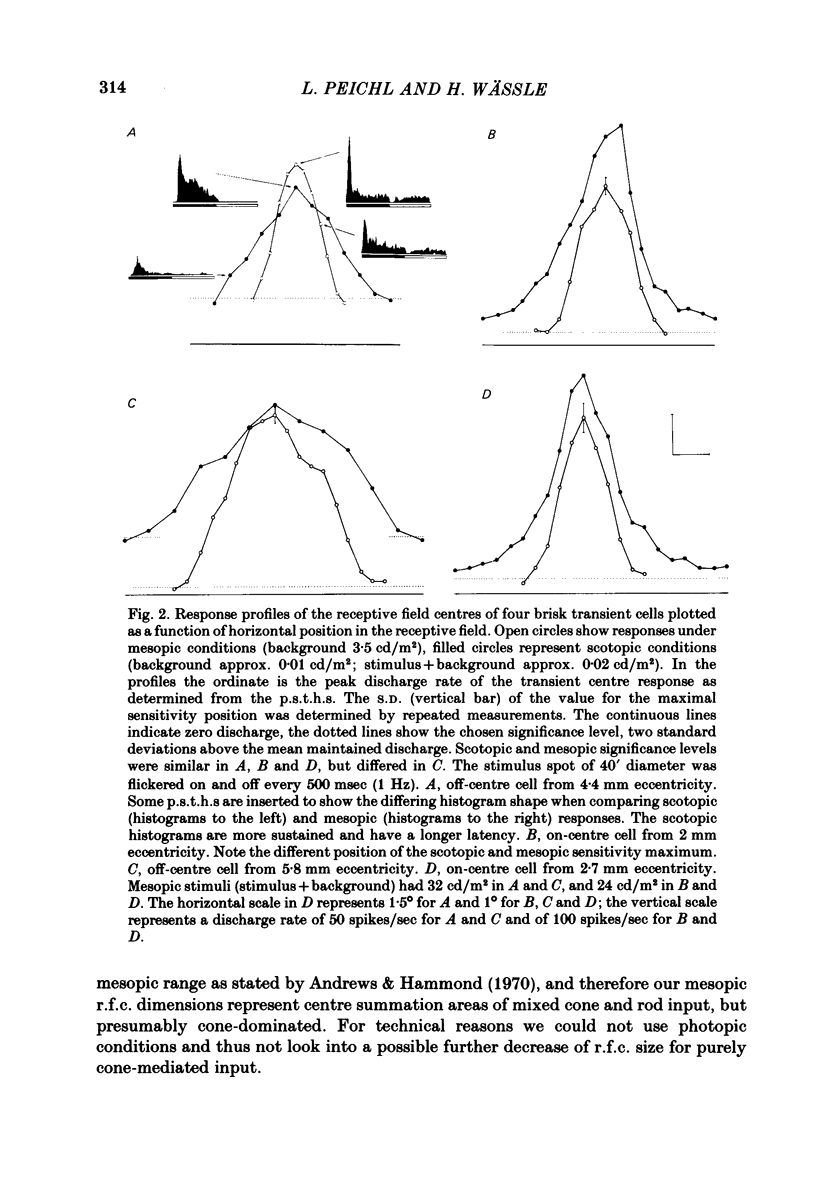
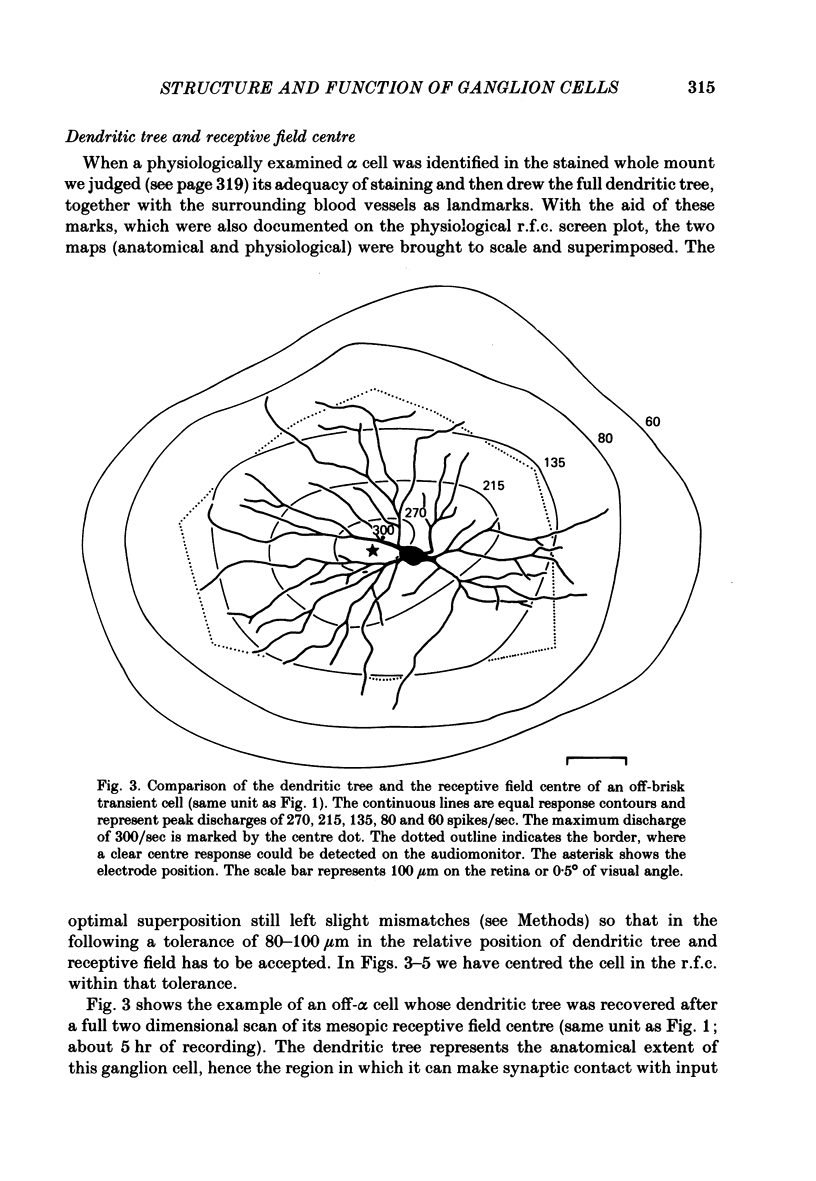
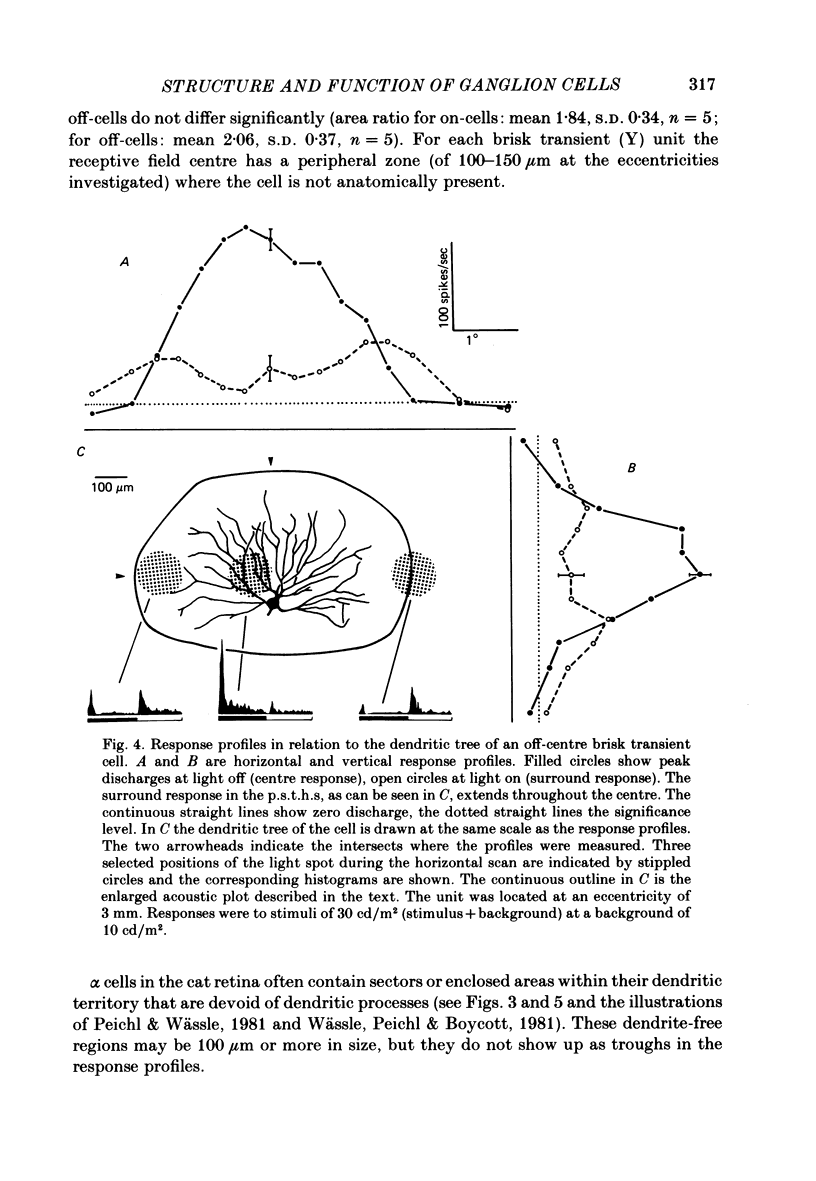
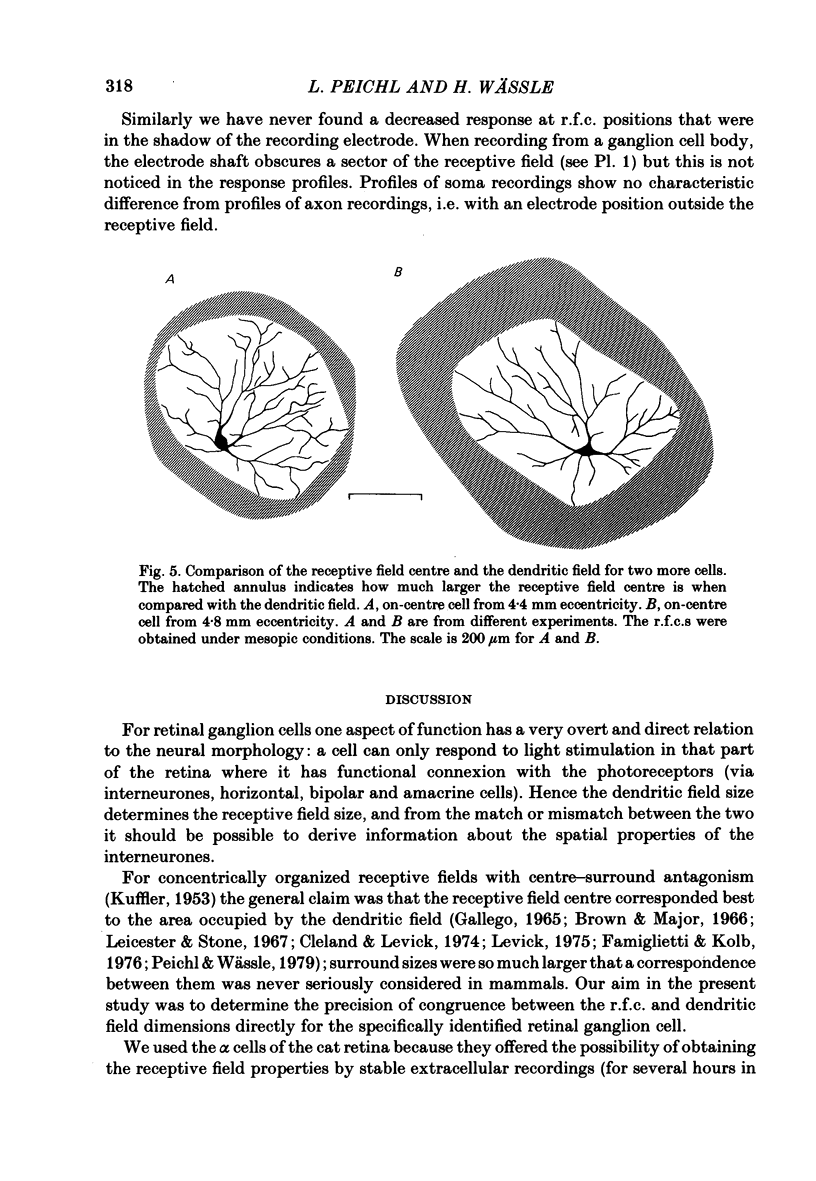
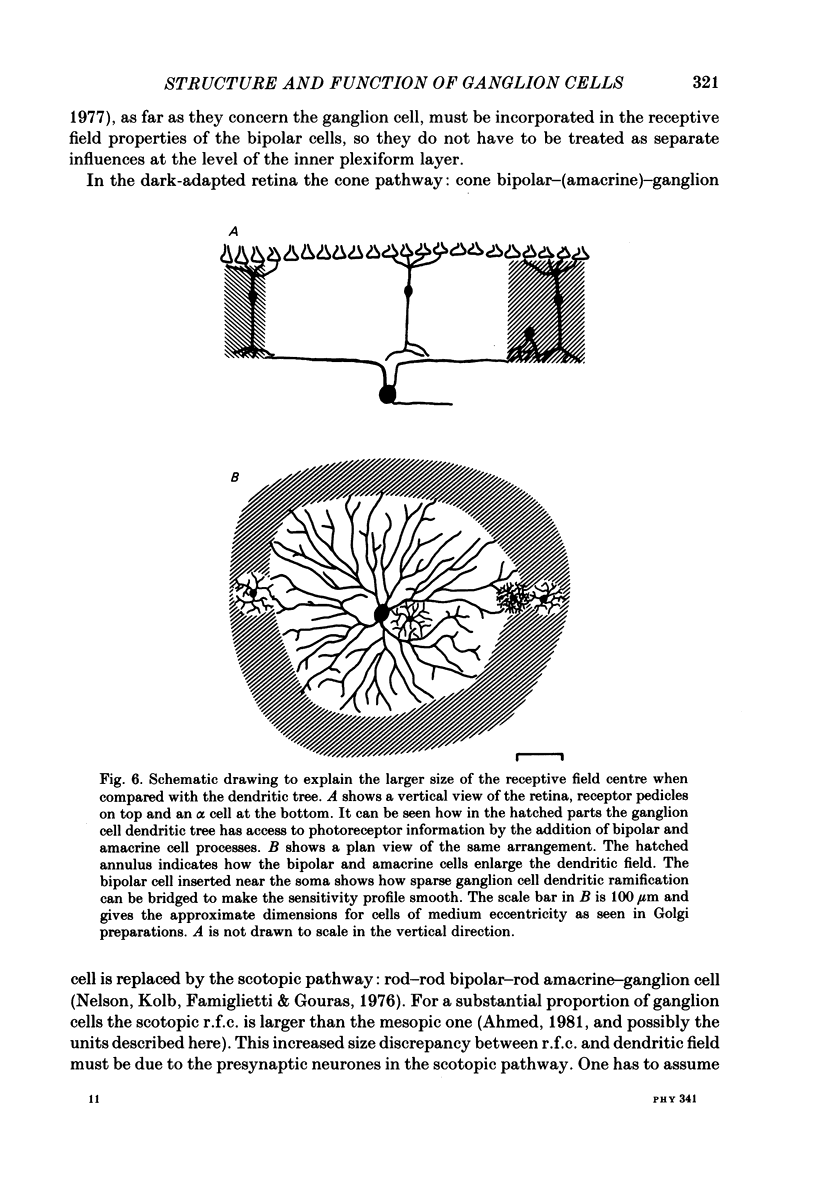
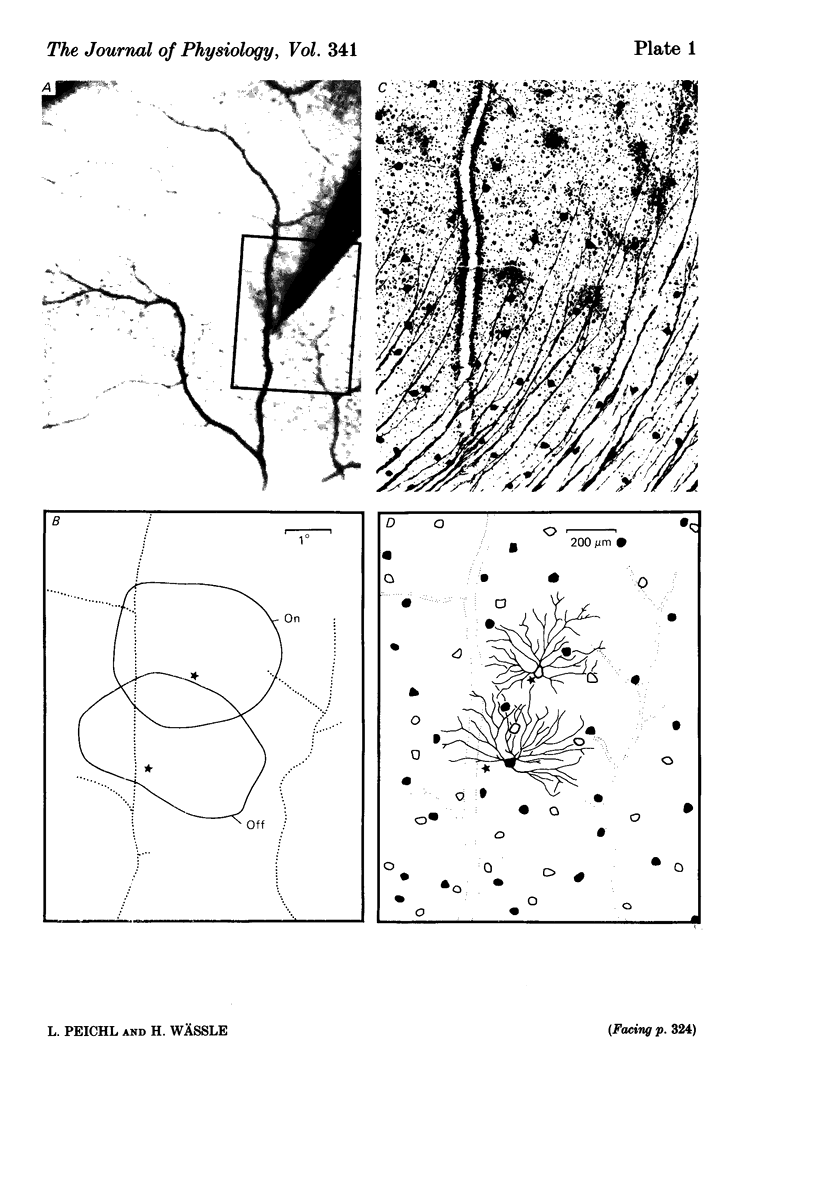
The correlation between the receptive field centre and the dendritic tree of individual brisk transient, or alpha, ganglion cells in the cat retina was investigated by a combination of physiological and anatomical techniques. The sizes of receptive field centres of brisk transient (Y) cells were measured with a flickering spot of light. Contour maps and response (or sensitivity) profiles were measured at mesopic and scotopic backgrounds. Recording positions on the retina and nearby blood vessels were back-projected onto the receptive field plots on the tangent screen. After recording, whole amount preparations of the retinae were stained by a reduced silver method to stain all alpha cells together with their dendritic trees. By comparing the landmarks on the screen plot with those of the whole mount it was possible to identify the recorded cells in the preparation and to study their morphology. The dendritic tree of an alpha cell determines the position, size and shape of its receptive field centre. The mesopic receptive field centres were found to be a factor of 1.4 +/- 0.13 larger than their respective dendritic fields. It is suggested that the dendritic fields of presynaptic neurones (bipolar and amacrine cell processes) add to the ganglion cell dendritic tree to produce the larger centre summating area.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed B. The size and shape of rod and cone centres of cat retinal ganglion cells. Exp Brain Res. 1981;43(3-4):422–428. doi: 10.1007/BF00238386. [DOI] [PubMed] [Google Scholar]
- Andrews D. P., Hammond P. Suprathreshold spectral properties of single optic tract fibres in cat, under mesopic adaptation: cone-rod interaction. J Physiol. 1970 Jul;209(1):83–103. doi: 10.1113/jphysiol.1970.sp009157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B., FITZHUGH R., KUFFLER S. W. Change of organization in the receptive fields of the cat's retina during dark adaptation. J Physiol. 1957 Aug 6;137(3):338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boycott B. B., Kolb H. The connections between bipolar cells and photoreceptors in the retina of the domestic cat. J Comp Neurol. 1973 Mar 1;148(1):91–114. doi: 10.1002/cne.901480106. [DOI] [PubMed] [Google Scholar]
- Boycott B. B., Wässle H. The morphological types of ganglion cells of the domestic cat's retina. J Physiol. 1974 Jul;240(2):397–419. doi: 10.1113/jphysiol.1974.sp010616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Major D. Cat retinal ganglion cell dendritic fields. Exp Neurol. 1966 May;15(1):70–78. doi: 10.1016/0014-4886(66)90035-5. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R. Brisk and sluggish concentrically organized ganglion cells in the cat's retina. J Physiol. 1974 Jul;240(2):421–456. doi: 10.1113/jphysiol.1974.sp010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt O. D., Sakmann B., Scheich H., Korn A. Sensitivity distribution and spatial summation within receptive-field center of retinal on-center ganglion cells and transfer function of the retina. J Neurophysiol. 1970 Sep;33(5):654–671. doi: 10.1152/jn.1970.33.5.654. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Boycott B. B. Organization of the primate retina: electron microscopy. Proc R Soc Lond B Biol Sci. 1966 Nov 15;166(1002):80–111. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- Famiglietti E. V., Jr Functional architecture of cone bipolar cells in mammalian retina. Vision Res. 1981;21(11):1559–1563. doi: 10.1016/0042-6989(81)90032-8. [DOI] [PubMed] [Google Scholar]
- Famiglietti E. V., Jr, Kolb H. Structural basis for ON-and OFF-center responses in retinal ganglion cells. Science. 1976 Oct 8;194(4261):193–195. doi: 10.1126/science.959847. [DOI] [PubMed] [Google Scholar]
- Hammond P. Inadequacy of nitrous oxide/oxygen mixtures for maintaining anaesthesia in cats: satisfactory alternatives. Pain. 1978 Aug;5(2):143–151. doi: 10.1016/0304-3959(78)90036-2. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Koch C., Poggio T., Torre V. Retinal ganglion cells: a functional interpretation of dendritic morphology. Philos Trans R Soc Lond B Biol Sci. 1982 Jul 27;298(1090):227–263. doi: 10.1098/rstb.1982.0084. [DOI] [PubMed] [Google Scholar]
- Kolb H., Nelson R., Mariani A. Amacrine cells, bipolar cells and ganglion cells of the cat retina: a Golgi study. Vision Res. 1981;21(7):1081–1114. doi: 10.1016/0042-6989(81)90013-4. [DOI] [PubMed] [Google Scholar]
- Kolb H. The inner plexiform layer in the retina of the cat: electron microscopic observations. J Neurocytol. 1979 Jun;8(3):295–329. doi: 10.1007/BF01236124. [DOI] [PubMed] [Google Scholar]
- Leicester J., Stone J. Ganglion, amacrine and horizontal cells of the cat's retina. Vision Res. 1967 Sep;7(9):695–705. doi: 10.1016/0042-6989(67)90033-8. [DOI] [PubMed] [Google Scholar]
- Levick W. R. Form and function of cat retinal ganglion cells. Nature. 1975 Apr 24;254(5502):659–662. doi: 10.1038/254659a0. [DOI] [PubMed] [Google Scholar]
- Nelson R. Cat cones have rod input: a comparison of the response properties of cones and horizontal cell bodies in the retina of the cat. J Comp Neurol. 1977 Mar 1;172(1):109–135. doi: 10.1002/cne.901720106. [DOI] [PubMed] [Google Scholar]
- Nelson R., Famiglietti E. V., Jr, Kolb H. Intracellular staining reveals different levels of stratification for on- and off-center ganglion cells in cat retina. J Neurophysiol. 1978 Mar;41(2):472–483. doi: 10.1152/jn.1978.41.2.472. [DOI] [PubMed] [Google Scholar]
- Peichl L., Wässle H. Morphological identification of on- and off-centre brisk transient (Y) cells in the cat retina. Proc R Soc Lond B Biol Sci. 1981 May 22;212(1187):139–153. doi: 10.1098/rspb.1981.0030. [DOI] [PubMed] [Google Scholar]
- Peichl L., Wässle H. Size, scatter and coverage of ganglion cell receptive field centres in the cat retina. J Physiol. 1979 Jun;291:117–141. doi: 10.1113/jphysiol.1979.sp012803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson J. G., Enroth-Cugell C. Light distribution in the cat's retinal image. Vision Res. 1978;18(2):159–173. doi: 10.1016/0042-6989(78)90181-5. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Stone J. Analysis of receptive fields of cat retinal ganglion cells. J Neurophysiol. 1965 Sep;28(5):832–849. doi: 10.1152/jn.1965.28.5.833. [DOI] [PubMed] [Google Scholar]
- Sterling P. Microcircuitry of the cat retina. Annu Rev Neurosci. 1983;6:149–185. doi: 10.1146/annurev.ne.06.030183.001053. [DOI] [PubMed] [Google Scholar]
- Stevens J. K., McGuire B. A., Sterling P. Toward a functional architecture of the retina: serial reconstruction of adjacent ganglion cells. Science. 1980 Jan 18;207(4428):317–319. doi: 10.1126/science.7350663. [DOI] [PubMed] [Google Scholar]
- Wässle H., Peichl L., Boycott B. B. Morphology and topography of on- and off-alpha cells in the cat retina. Proc R Soc Lond B Biol Sci. 1981 May 22;212(1187):157–175. doi: 10.1098/rspb.1981.0032. [DOI] [PubMed] [Google Scholar]