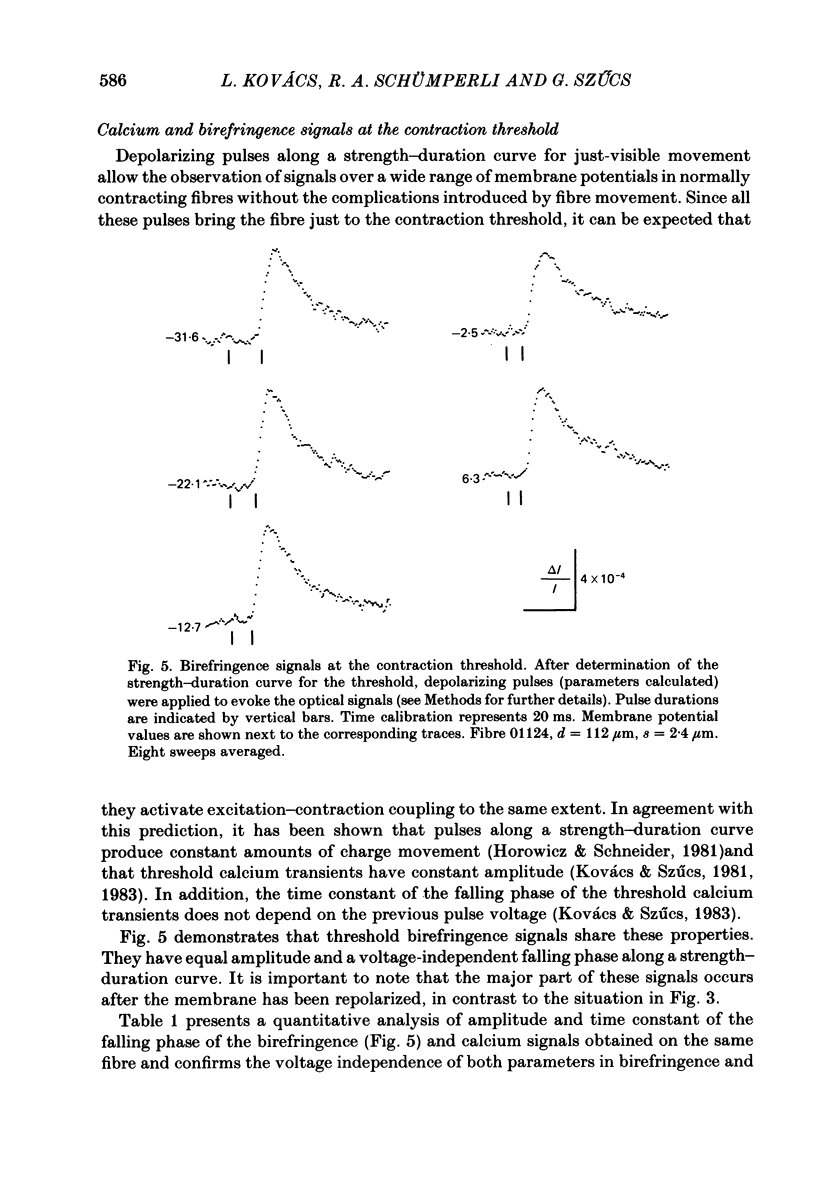
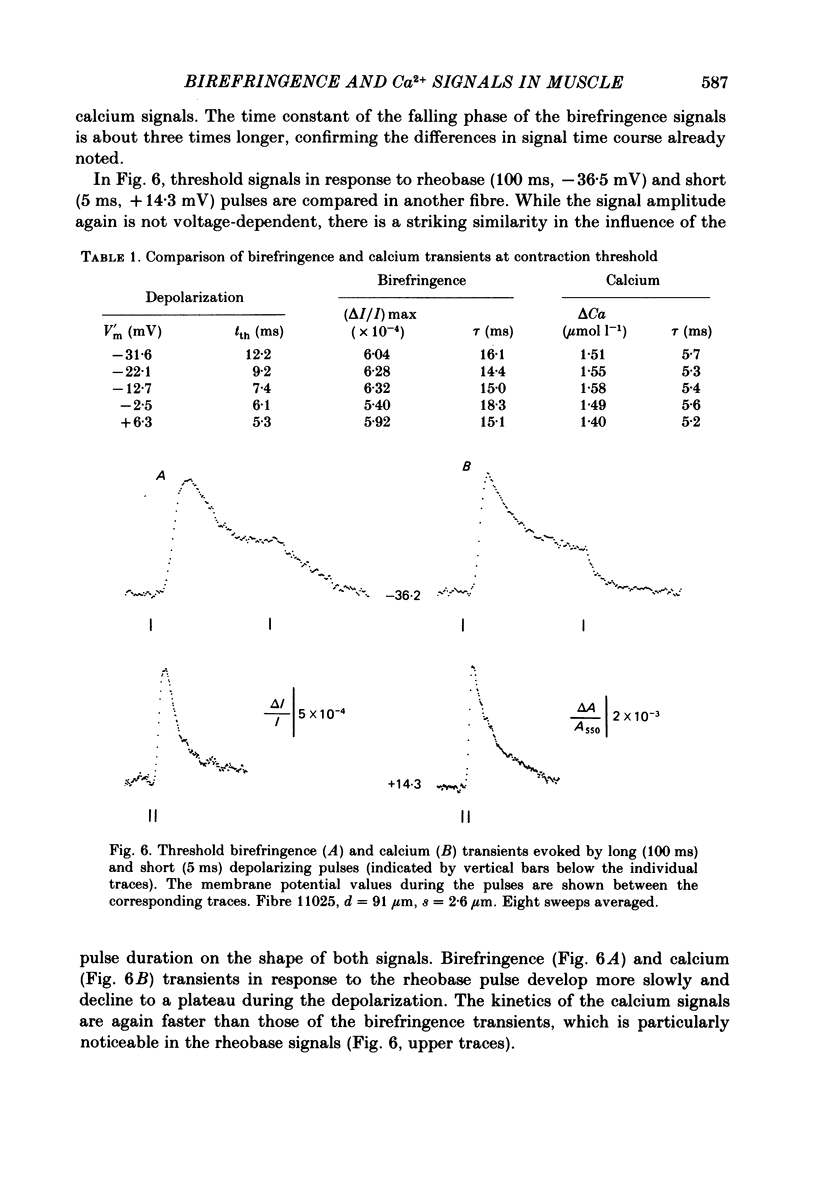
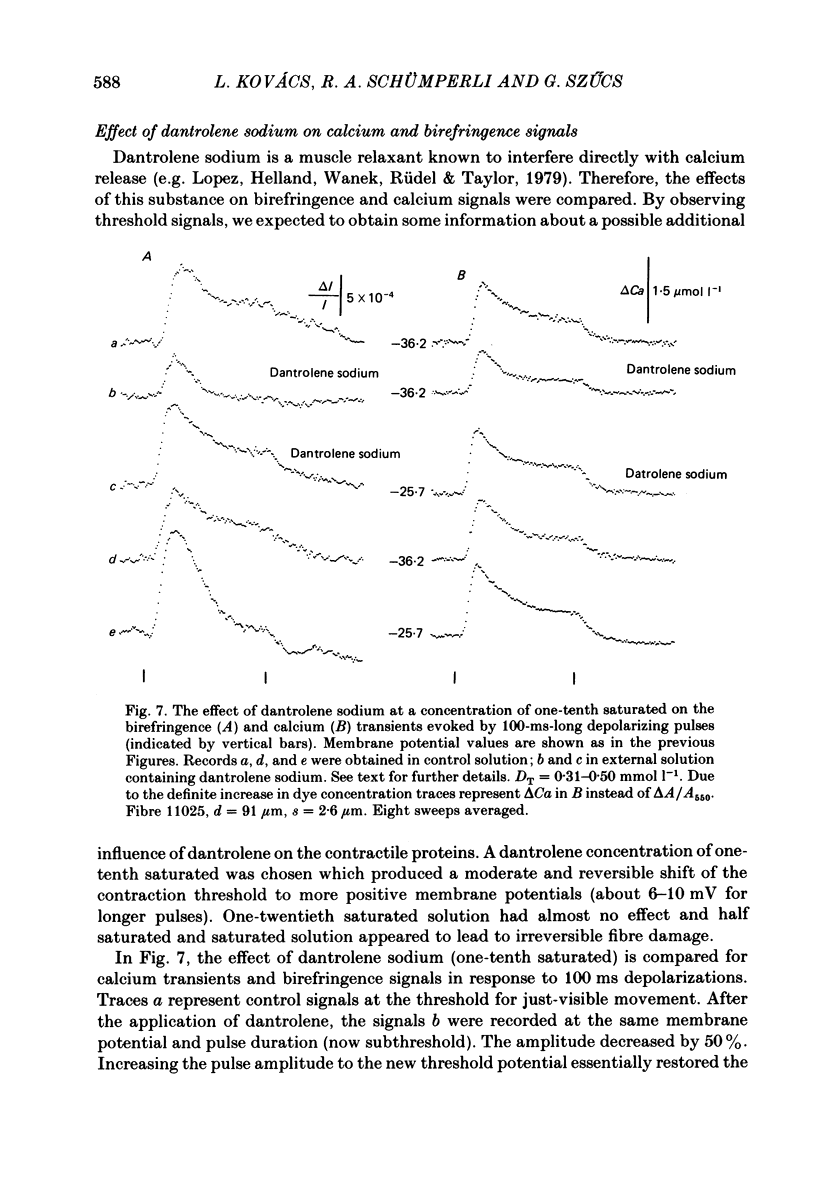
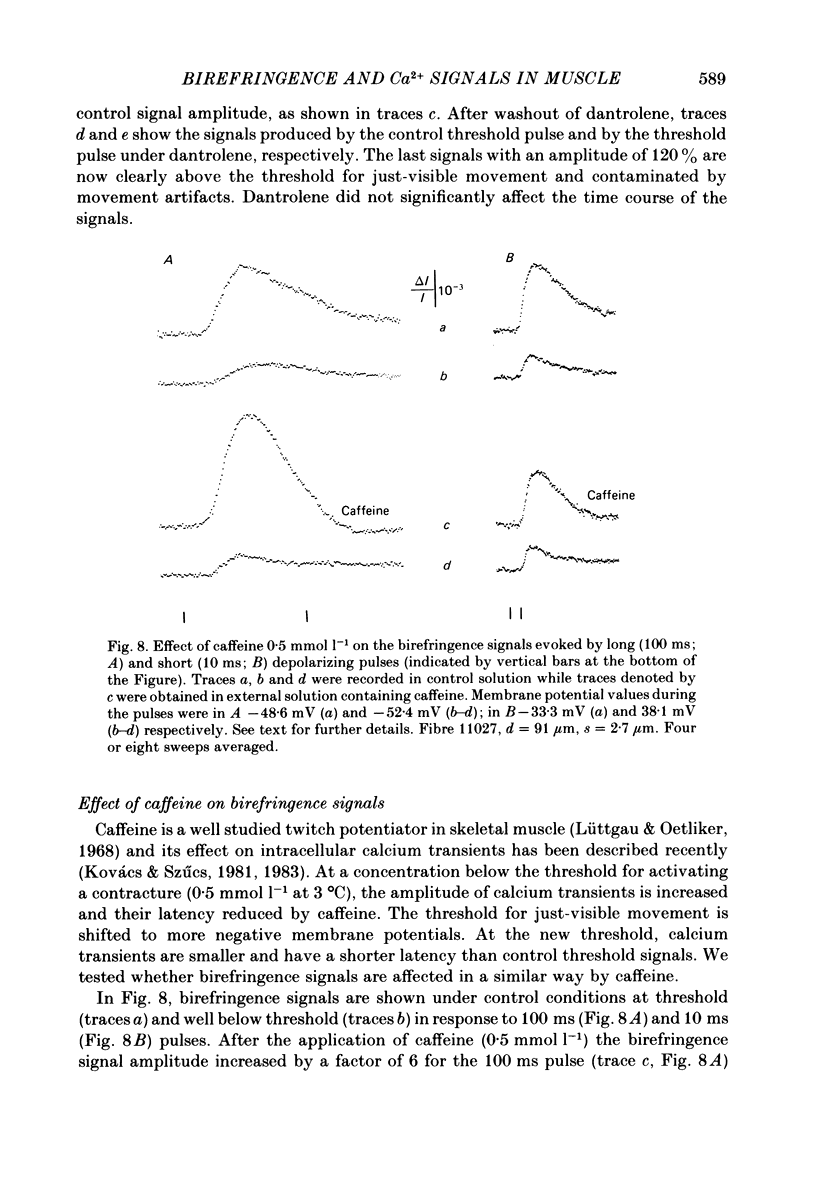
Abstract
The characteristic features of birefringence and calcium transients were compared in voltage-clamped cut skeletal muscle fibres: Birefringence signals were measured by introducing crossed polarizers above and below the fibres (+/- 45 degrees to the fibre axis) and using light of 790 nm. Calcium transients were monitored by the metallochromic indicator dye, Antipyrylazo III recording the changes in fibre absorbance at 720 nm. The dye entered the myoplasmic space by diffusion through the cut end. The early large birefringence signals, related to excitation-contraction coupling had a time course similar to that of calcium transients. The two signals had superimposable onset but the change in optical retardation peaked later and declined more slowly than the calcium signal. Using depolarizing pulses with increasing amplitudes both transients showed the same voltage dependence in the rate of rise, the time-to-peak and the peak amplitude. Birefringence signals recorded at different voltages along the strength-duration curve for contraction threshold had the same amplitudes and similar time constants for the falling phase comparable to the properties of the calcium transients. After applying dantrolene sodium both signals were reduced to the same extent. A shift in the contraction threshold was found towards more positive membrane potential values. The birefringence and calcium transients recorded at the new contraction threshold during the dantrolene treatment showed nearly the same size and time course as threshold transients obtained before the treatment. A subthreshold concentration of caffeine increased the peak amplitude of birefringence signals at a given voltage and decreased the latency of the signals. Birefringence transients at the new contraction threshold under caffeine were smaller than controls. Both effects are very similar to the changes in calcium transients due to caffeine treatment as previously reported. Consequently the voltage-dependent properties of birefringence and calcium transients and their responses to caffeine and dantrolene treatment are nearly the same. These results support the view that the changes in optical retardation of the fibres reflect calcium bound to some sarcoplasmic binding site rather than a potential change of the sarcoplasmic reticulum.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Gilly W. F. Fast and slow steps in the activation of sodium channels. J Gen Physiol. 1979 Dec;74(6):691–711. doi: 10.1085/jgp.74.6.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Optical measurements of intracellular pH and magnesium in frog skeletal muscle fibres. J Physiol. 1982 Oct;331:105–137. doi: 10.1113/jphysiol.1982.sp014367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Use of metallochromic dyes to measure changes in myoplasmic calcium during activity in frog skeletal muscle fibres. J Physiol. 1982 Oct;331:139–177. doi: 10.1113/jphysiol.1982.sp014368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Oetliker H. A large birefringence signal preceding contraction in single twitch fibres of the frog. J Physiol. 1977 Jan;264(1):141–162. doi: 10.1113/jphysiol.1977.sp011661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Oetliker H. Birefringence experiments on isolated skeletal muscle fibres suggest a possible signal from the sarcoplasmic reticulum. Nature. 1975 Jan 10;253(5487):97–101. doi: 10.1038/253097a0. [DOI] [PubMed] [Google Scholar]
- Baylor S. M., Oetliker H. The optical properties of birefringence signals from single muscle fibres. J Physiol. 1977 Jan;264(1):163–198. doi: 10.1113/jphysiol.1977.sp011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Horowicz P. Fluorescence intensity changes associated with contractile activation in frog muscle stained with Nile Blue A. J Physiol. 1975 Apr;246(3):709–735. doi: 10.1113/jphysiol.1975.sp010912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainaut K., Desmedt J. E. Effect of dantrolene sodium on calcium movements in single muscle fibres. Nature. 1974 Dec 20;252(5485):728–730. doi: 10.1038/252728a0. [DOI] [PubMed] [Google Scholar]
- Horowicz P., Schneider M. F. Membrane charge moved at contraction thresholds in skeletal muscle fibres. J Physiol. 1981 May;314:595–633. doi: 10.1113/jphysiol.1981.sp013726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács L., Ríos E., Schneider M. F. Calcium transients and intramembrane charge movement in skeletal muscle fibres. Nature. 1979 May 31;279(5712):391–396. doi: 10.1038/279391a0. [DOI] [PubMed] [Google Scholar]
- Kovács L., Schneider M. F. Contractile activation by voltage clamp depolarization of cut skeletal muscle fibres. J Physiol. 1978 Apr;277:483–506. doi: 10.1113/jphysiol.1978.sp012286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács L., Schneider M. F. Increased optical transparency associated with excitation--contraction coupling in voltage-clamped cut skeletal muscle fibres. Nature. 1977 Feb 10;265(5594):556–560. doi: 10.1038/265556a0. [DOI] [PubMed] [Google Scholar]
- Kovács L., Szücs G. Effect of caffeine on intramembrane charge movement and calcium transients in cut skeletal muscle fibres of the frog. J Physiol. 1983 Aug;341:559–578. doi: 10.1113/jphysiol.1983.sp014824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttgau H. C., Oetliker H. The action of caffeine on the activation of the contractile mechanism in straited muscle fibres. J Physiol. 1968 Jan;194(1):51–74. doi: 10.1113/jphysiol.1968.sp008394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Parker I., Schalow G. Measurement of calcium transients in frog muscle by the use of arsenazo III. Proc R Soc Lond B Biol Sci. 1977 Aug 22;198(1131):201–210. doi: 10.1098/rspb.1977.0094. [DOI] [PubMed] [Google Scholar]
- Oetliker H. An appraisal of the evidence for a sarcoplasmic reticulum membrane potential and its relation to calcium release in skeletal muscle. J Muscle Res Cell Motil. 1982 Sep;3(3):247–272. doi: 10.1007/BF00713037. [DOI] [PubMed] [Google Scholar]
- Oetliker H., Baylor S. M., Chandler W. K. Simultaneous changes in fluorescence and optical retardation in single muscle fibres during activity. Nature. 1975 Oct 23;257(5528):693–696. doi: 10.1038/257693a0. [DOI] [PubMed] [Google Scholar]
- Ríos E., Schneider M. F. Stoichiometry of the reactions of calcium with the metallochromic indicator dyes antipyrylazo III and arsenazo III. Biophys J. 1981 Dec;36(3):607–621. doi: 10.1016/S0006-3495(81)84755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa A., Brinley F. J., Jr, Dubyak G. Antipyrylazo III, a "middle range" Ca2+ metallochromic indicator. Biochemistry. 1978 Apr 18;17(8):1378–1386. doi: 10.1021/bi00601a004. [DOI] [PubMed] [Google Scholar]
- Stromer M., Hasselbach W. Fusion of isolated sarcoplasmic reticulum membranes. Z Naturforsch C. 1976 Nov-Dec;31(11-12):703–707. [PubMed] [Google Scholar]
- Suarez-Kurtz G., Parker I. Birefringence signals and calcium transients in skeletal muscle. Nature. 1977 Dec 22;270(5639):746–748. doi: 10.1038/270746a0. [DOI] [PubMed] [Google Scholar]
- Takauji M., Takahashi N., Nagai T. Effect of dantrolene sodium on excitation-contraction coupling in frog skeletal muscle. Jpn J Physiol. 1975;25(6):747–758. doi: 10.2170/jjphysiol.25.747. [DOI] [PubMed] [Google Scholar]
- Takauji M., Takahashi N., Suzuki T., Nagai T. Inhibitory action of dantrolene sodium on the activation of excitation-contraction coupling in frog skeletal muscle. Jpn J Physiol. 1977;27(6):731–741. doi: 10.2170/jjphysiol.27.731. [DOI] [PubMed] [Google Scholar]
- Vergara J., Bezanilla F., Salzberg B. M. Nile blue fluorescence signals from cut single muscle fibers under voltage or current clamp conditions. J Gen Physiol. 1978 Dec;72(6):775–800. doi: 10.1085/jgp.72.6.775. [DOI] [PMC free article] [PubMed] [Google Scholar]


