Abstract
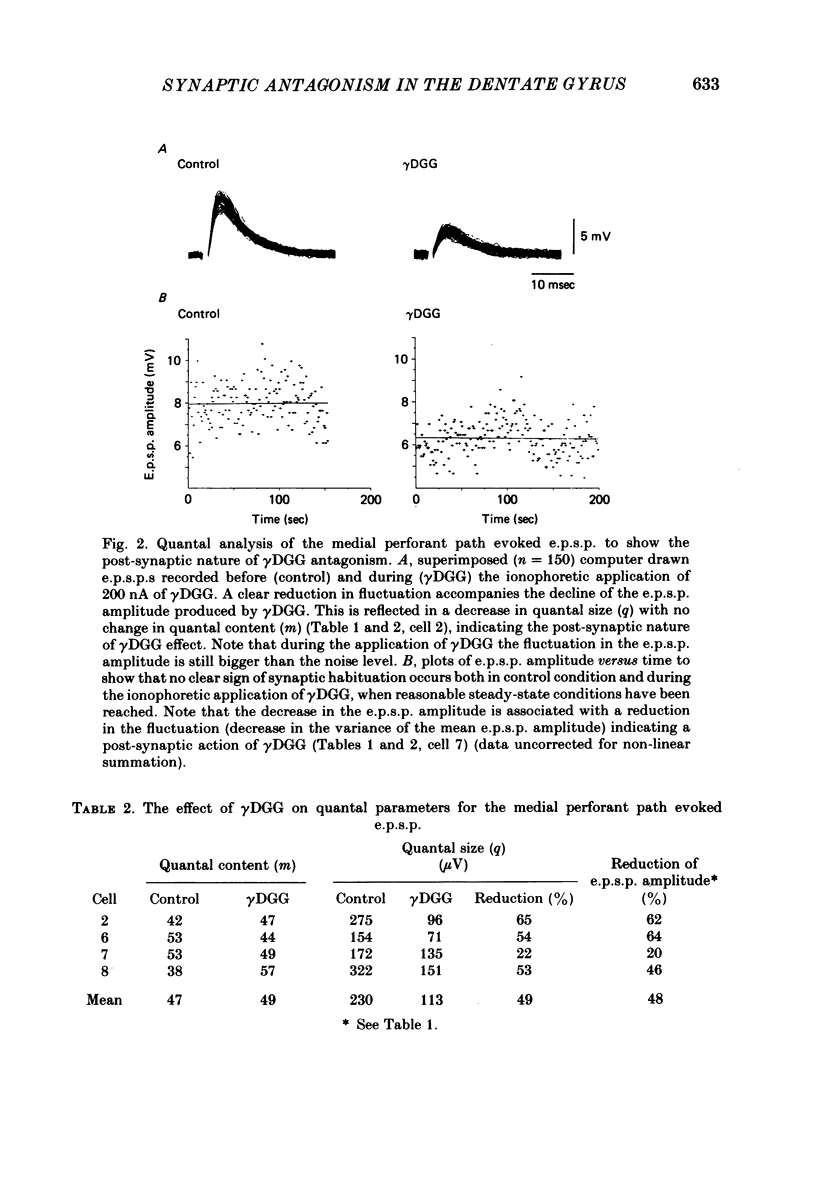
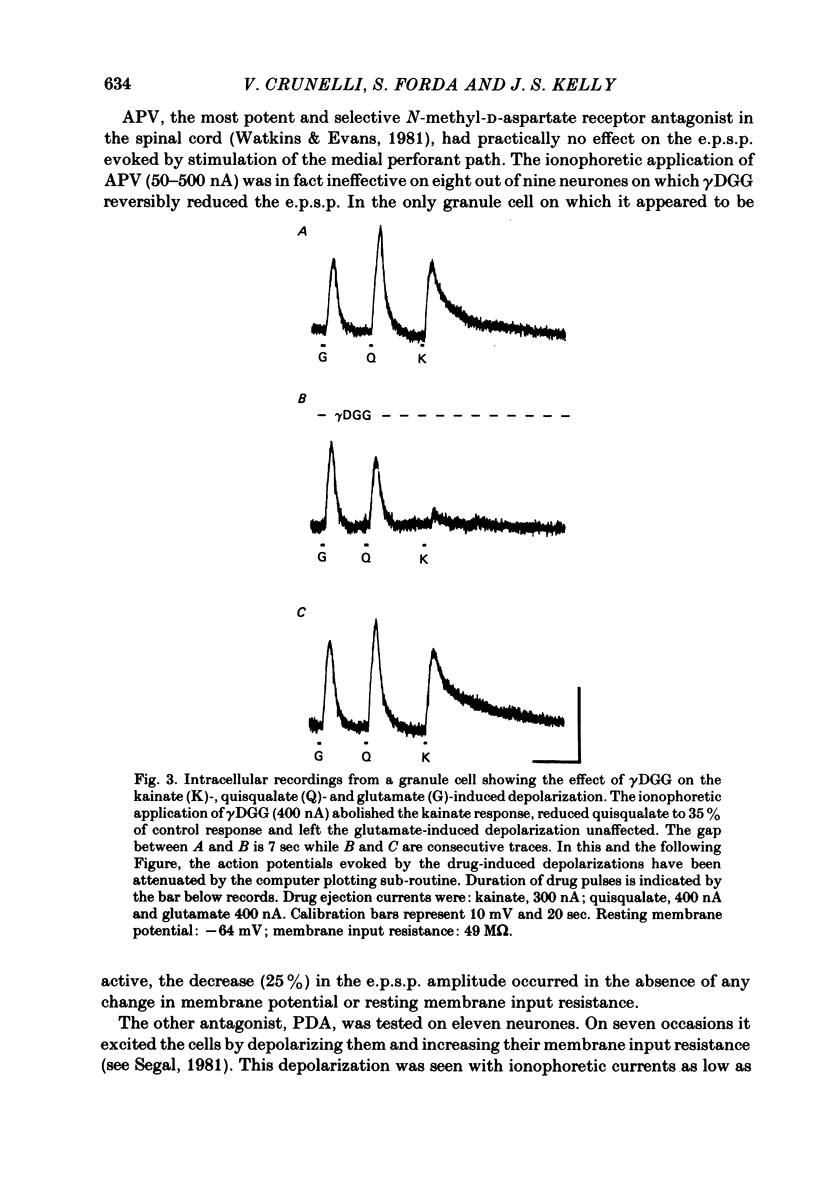
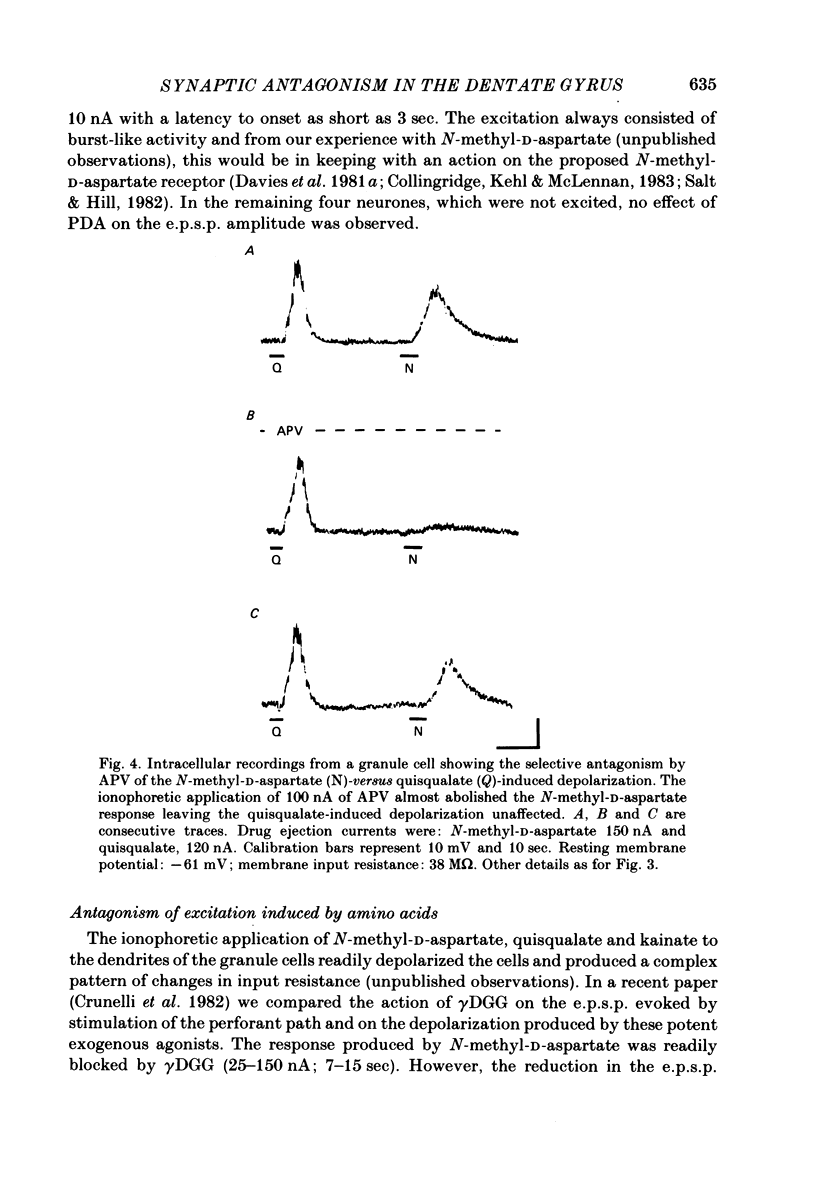
Excitatory post-synaptic potentials (e.p.s.p.s) evoked by stimulation of the medial perforant path and depolarizations induced by excitatory amino acids were recorded from granule cells in the preparation of the hippocampal slice from the rat. The effects of (+/-)-2-amino-5-phosphonovalerate (APV), gamma-D-glutamylglycine (gamma DGG) and cis-2,3-piperidinedicarboxylate (PDA), antagonists of excitatory amino acids on these phenomena were compared. gamma DGG was the most effective antagonist of the e.p.s.p. Its action was reversible and not associated with any change in the passive membrane properties of the granule cells or in the apparent reversal potential of the e.p.s.p. Quantal analysis showed that the reduction in the e.p.s.p. paralleled the decrease in quantal size rather than quantal content, confirming a post-synaptic site of the action of gamma DGG. The potency of gamma DGG against the exogenous agonists was N-methyl-D-aspartate greater than kainate greater than or equal to quisqualate. APV had very little effect on the e.p.s.p. but was a selective antagonist of N-methyl-D-aspartate-induced depolarizations. PDA depolarized granule cells and increased their membrane input resistance. Although gamma DGG was a potent antagonist of both glutamate- and aspartate-induced depolarizations, no clear pattern of specificity could be found. The action of glutamate was unaffected by APV. These results indicate that the receptor for the transmitter at the synapses formed by the fibres of the perforant path with the granule cells is of the quisqualate and/or kainate type. The present data are consistent with the biochemical evidence that glutamate may be the endogenous transmitter at his synapse.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Holmqvist B., Voorhoeve P. E. Entorhinal activation of dentate granule cells. Acta Physiol Scand. 1966 Apr;66(4):448–460. doi: 10.1111/j.1748-1716.1966.tb03223.x. [DOI] [PubMed] [Google Scholar]
- Barnes C. A., McNaughton B. L. Physiological compensation for loss of afferent synapses in rat hippocampal granule cells during senescence. J Physiol. 1980 Dec;309:473–485. doi: 10.1113/jphysiol.1980.sp013521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. H., Fricke R. A., Perkel D. H. Passive electrical constants in three classes of hippocampal neurons. J Neurophysiol. 1981 Oct;46(4):812–827. doi: 10.1152/jn.1981.46.4.812. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. The antagonism of amino acid-induced excitations of rat hippocampal CA1 neurones in vitro. J Physiol. 1983 Jan;334:19–31. doi: 10.1113/jphysiol.1983.sp014477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V., Forda S., Collingridge G. L., Kelly J. S. Intracellular recorded synaptic antagonism in the rat dentate gyrus. Nature. 1982 Dec 2;300(5891):450–452. doi: 10.1038/300450a0. [DOI] [PubMed] [Google Scholar]
- Davies J., Evans R. H., Francis A. A., Jones A. W., Watkins J. C. Antagonism of excitatory amino acid-induced and synaptic excitation of spinal neurones by cis-2,3-piperidine dicarboxylate. J Neurochem. 1981 Mar;36(3):1305–1307. doi: 10.1111/j.1471-4159.1981.tb01736.x. [DOI] [PubMed] [Google Scholar]
- Davies J., Francis A. A., Jones A. W., Watkins J. C. 2-Amino-5-phosphonovalerate (2APV), a potent and selective antagonist of amino acid-induced and synaptic excitation. Neurosci Lett. 1981 Jan 1;21(1):77–81. doi: 10.1016/0304-3940(81)90061-6. [DOI] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. Actions of D and L forms of 2-amino-5-phosphonovalerate and 2-amino-4-phosphonobutyrate in the cat spinal cord. Brain Res. 1982 Mar 11;235(2):378–386. doi: 10.1016/0006-8993(82)91017-4. [DOI] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. Differentiation of kainate and quisqualate receptors in the cat spinal cord by selective antagonism with gamma-D(and L)-glutamylglycine. Brain Res. 1981 Feb 9;206(1):172–177. doi: 10.1016/0006-8993(81)90111-6. [DOI] [PubMed] [Google Scholar]
- Dingledine R., Dodd J., Kelly J. S. The in vitro brain slice as a useful neurophysiological preparation for intracellular recording. J Neurosci Methods. 1980 Aug;2(4):323–362. doi: 10.1016/0165-0270(80)90002-3. [DOI] [PubMed] [Google Scholar]
- Evans R. H., Francis A. A., Jones A. W., Smith D. A., Watkins J. C. The effects of a series of omega-phosphonic alpha-carboxylic amino acids on electrically evoked and excitant amino acid-induced responses in isolated spinal cord preparations. Br J Pharmacol. 1982 Jan;75(1):65–75. doi: 10.1111/j.1476-5381.1982.tb08758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsborg B. L. Ion movements in junctional transmission. Pharmacol Rev. 1967 Sep;19(3):289–316. [PubMed] [Google Scholar]
- Hicks T. P., Hall J. G., McLennan H. Ranking of excitatory amino acids by the antagonists glutamic acid diethylester and D-alpha-aminoadipic acid. Can J Physiol Pharmacol. 1978 Dec;56(6):901–907. doi: 10.1139/y78-143. [DOI] [PubMed] [Google Scholar]
- Hjorth-Simonsen A., Jeune B. Origin and termination of the hippocampal perforant path in the rat studied by silver impregnation. J Comp Neurol. 1972 Feb;144(2):215–232. doi: 10.1002/cne.901440206. [DOI] [PubMed] [Google Scholar]
- Koerner J. F., Cotman C. W. Micromolar L-2-amino-4-phosphonobutyric acid selectively inhibits perforant path synapses from lateral entorhinal cortex. Brain Res. 1981 Jul 6;216(1):192–198. doi: 10.1016/0006-8993(81)91288-9. [DOI] [PubMed] [Google Scholar]
- Kuno M. Quantum aspects of central and ganglionic synaptic transmission in vertebrates. Physiol Rev. 1971 Oct;51(4):647–678. doi: 10.1152/physrev.1971.51.4.647. [DOI] [PubMed] [Google Scholar]
- Lomo T. Patterns of activation in a monosynaptic cortical pathway: the perforant path input to the dentate area of the hippocampal formation. Exp Brain Res. 1971;12(1):18–45. [PubMed] [Google Scholar]
- McLennan H., Liu J. The action of six antagonists of the excitatory amino acids on neurones of the rat spinal cord. Exp Brain Res. 1982;45(1-2):151–156. doi: 10.1007/BF00235774. [DOI] [PubMed] [Google Scholar]
- McLennan H. On the nature of the receptors for various excitatory amino acids in the mammalian central nervous system. Adv Biochem Psychopharmacol. 1981;27:253–262. [PubMed] [Google Scholar]
- McNaughton B. L., Barnes C. A., Andersen P. Synaptic efficacy and EPSP summation in granule cells of rat fascia dentata studied in vitro. J Neurophysiol. 1981 Nov;46(5):952–966. doi: 10.1152/jn.1981.46.5.952. [DOI] [PubMed] [Google Scholar]
- McNaughton B. L., Barnes C. A. Physiological identification and analysis of dentate granule cell responses to stimulation of the medial and lateral perforant pathways in the rat. J Comp Neurol. 1977 Oct 15;175(4):439–454. doi: 10.1002/cne.901750404. [DOI] [PubMed] [Google Scholar]
- McNaughton B. L. Evidence for two physiologically distinct perforant pathways to the fascia dentata. Brain Res. 1980 Oct 13;199(1):1–19. doi: 10.1016/0006-8993(80)90226-7. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Alger B. E. A simple chamber for recording from submerged brain slices. J Neurosci Methods. 1981 Aug;4(2):153–156. doi: 10.1016/0165-0270(81)90049-2. [DOI] [PubMed] [Google Scholar]
- Salt T. E., Hill R. G. Differentiation of excitatory amino acid receptors in the rat caudal trigeminal nucleus: a microiontophoretic study. Neuropharmacology. 1982 May;21(5):385–390. doi: 10.1016/0028-3908(82)90020-x. [DOI] [PubMed] [Google Scholar]
- Segal M. The actions of glutamic acid on neurons in the rat hippocampal slice. Adv Biochem Psychopharmacol. 1981;27:217–225. [PubMed] [Google Scholar]
- Steward O. Topographic organization of the projections from the entorhinal area to the hippocampal formation of the rat. J Comp Neurol. 1976 Jun 1;167(3):285–314. doi: 10.1002/cne.901670303. [DOI] [PubMed] [Google Scholar]
- Storm-Mathisen J., Iversen L. L. Uptake of [3H]Glutamic acid in excitatory nerve endings: light and electronmicroscopic observations in the hippocampal formation of the rat. Neuroscience. 1979;4(9):1237–1253. doi: 10.1016/0306-4522(79)90154-4. [DOI] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- Wheal H. V., Miller J. J. Pharmacological identification of acetylcholine and glutamate excitatory systems in the dentate gyrus of the rat. Brain Res. 1980 Jan 20;182(1):145–155. doi: 10.1016/0006-8993(80)90837-9. [DOI] [PubMed] [Google Scholar]
- White W. F., Nadler J. V., Hamberger A., Cotman C. W., Cummins J. T. Glutamate as transmitter of hippocampal perforant path. Nature. 1977 Nov 24;270(5635):356–357. doi: 10.1038/270356a0. [DOI] [PubMed] [Google Scholar]


