Abstract
The aim of the study was to determine if immunomodulation of host defense with recombinant murine granulocyte colony-stimulating factor (G-CSF) improves the efficacy of trovafloxacin or moxifloxacin in abscesses containing Bacillus fragilis ATCC 23745 and different Escherichia coli strains varying in virulence. Treatment of mice inoculated with 107 CFU B. fragilis and 105 CFU low-virulence E. coli with either trovafloxacin (150 mg/kg/day every 24 hours, days 3 to 7) or moxifloxacin (96 mg/kg/day every 12 hours, days 3 to 7), significantly reduced the number of B. fragilis to 6.9 ± 0.35 and 5.8 ± 0.10 and that of E. coli to 4.9 ± 0.09 and 4.2 ± 0.07 log CFU/abscess for trovafloxacin and moxifloxacin, respectively, compared to controls (B. fragilis 8.7 and E. coli 7.4 log CFU/abscess) on day 8. Also, moxifloxacin was more potent than trovafloxacin. Addition of G-CSF prophylaxis (1 μg once on day −1) or therapy (1 μg/day on days 3 to 7) to fluoroquinolone treatment did not improve the efficacy of fluoroquinolone therapy alone. The effect of moxifloxacin with or without G-CSF prophylaxis on abscesses with a virulent hemolytic E. coli strain was also studied. In moxifloxacin-treated mice, 75% survived infection compared to 10% of controls. Combining moxifloxacin with G-CSF prophylaxis significantly decreased survival (30%) compared to moxifloxacin alone. In addition, G-CSF prophylaxis resulted in a threefold (E. coli) to 100-fold (B. fragilis) increased outgrowth in the abscesses of surviving mice. In conclusion, the addition of G-CSF to a fluoroquinolone is not advisable since, depending on the virulence of the E. coli strains, this might detrimentally influence the outcome of therapy.
Intra-abdominal abscesses are persistent infections which, in the absence of adequate therapy, can be the cause of considerable morbidity and mortality. The conventional treatment of intra-abdominal abscesses involves percutaneous abscess drainage in conjunction with an intensive course of antimicrobial therapy (11). However, when abscesses are multiple and/or small, drainage procedures are not always possible and, under these circumstances, antibiotic treatment alone is the only option available. Despite improvement in antimicrobial therapy, therapeutic failure of mixed bacterial infections remains a major concern and augmentation of nonspecific host defenses with immunomodulating agents may provide an adjunctive approach to manage bacterial abscesses.
Granulocyte colony-stimulating growth factor (G-CSF) is such an agent and G-CSF has been shown to enhance the nonspecific host resistance to mixed bacterial infections (3, 10, 26). However, except for a minor beneficial influence of G-CSF on established Candida abscesses (27), no data are available regarding the influence of G-CSF on established mixed bacterial abscesses.
In our laboratory, we have employed a subcutaneous mouse model to study the treatment of small mixed-infection abscesses (22, 23). The aim of the present study was to investigate whether immunomodulation of host defenses with recombinant murine G-CSF (rmG-CSF) would improve the efficacy of trovafloxacin or moxifloxacin treatment of established abscesses in our mouse model. In addition, since the efficacy of G-CSF against a bacterial infection appears to vary with species and severity of the infection (7, 16), we also investigated the effect of rmG-CSF prophylaxis, with and without moxifloxacin treatment, on abscesses induced by combinations of Bacillus fragilis with different Escherichia coli strains varying in virulence.
(This study was presented in part at the 44th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, D.C., 2004, abstr. B-1696, p. 60.)
MATERIALS AND METHODS
Materials.
Recombinant murine G-CSF was supplied by Amgen (Thousand Oaks, CA). Trovafloxacin (Trovan) solution for infusion, containing 5 mg/ml, was supplied by Pfizer B.V. (Capelle a/d IJssel, The Netherlands) and moxifloxacin (Avelox) solution for infusion, containing 400 mg/250 ml, was supplied by Bayer B.V. (Mijdrecht, The Netherlands). Wilkens Chalgren (WC) broth, Wilkens Chalgren (WC) agar, and eosin methylene blue (EM) agar all came from Unipath Ltd. (Haarlem, The Netherlands).
Bacterial strains.
The strains used were B. fragilis ATCC 23745, a low-virulence E. coli strain (ATCC 25922), a virulent hemolytic E. coli strain (ATCC 35218), and a virulent nonhemolytic clinical blood isolate, E. coli B14349. All strains were first passaged in BALB/c mice by injecting 108 to 109 CFU of overnight cultures (intraperitoneal). After 24 to 48 h, strains were recovered from the liver and spleen, cultured on blood agar plates, and standardized bacterial suspensions were made in brain heart infusion containing 20% glycerol and stored at −80°C until required. Overnight cultures were obtained by inoculating 30-ml volumes of WC broth with 0.1 ml of the standardized frozen bacterial suspensions and incubating aerobically (E. coli) or anaerobically (B. fragilis) at 37°C for 18 h.
Animals.
Female specific-pathogen-free BALB/c mice (Charles River, Maastricht, The Netherlands) weighing 20 to 25 g were used throughout the study. The cecal contents of male specific-pathogen-free Swiss mice (Charles River) were used for the production of autoclaved cecal contents as preciously described (24). All animals received water and food ad libitum. The study was approved by the Institutional Animal Care and Use Committee of the Erasmus MC University Medical Center, Rotterdam, The Netherlands.
MICs.
The MICs of trovafloxacin and moxifloxacin were determined using the standard broth microdilution method and inocula of 105 CFU/ml as previously described (24).
Abscess model.
The subcutaneous abscess model described previously (23) was used. Inocula were prepared by diluting overnight cultures of B. fragilis and an E. coli strain (ATCC 25922, ATCC 35218, or B14349) in WC broth, which were then mixed together with autoclaved cecal contents in a volume ratio of 1:1:2. Final inocula contained 4 mg autoclaved cecal contents (dry weight) and 107 CFU B. fragilis along with 105 CFU E. coli ATCC 25922 or E. coli B14349 or 103 E. coli ATCC 35218 in a total volume of 0.25 ml. Mice were injected subcutaneously on both flanks. Abscesses were allowed to develop for 1 to 14 days.
Treatment regimens of established abscesses.
Abscesses were induced with B. fragilis and the low-virulence E. coli strain. To determine the effect of rmG-CSF with or without fluoroquinolone therapy on established abscesses, groups of four to six mice were injected subcutaneously with 1,000 ng (50 μg/kg) rmG-CSF in 100 μl pyrogen-free saline. Injections were given as a single dose 24 h before inoculation (rmG-CSF prophylaxis) or five daily doses given 3 to 7 days after inoculation (rmG-CSF therapy). Subgroups receiving antibiotic therapy were treated for 5 days (day 3 to day 7 after inoculation) with single daily doses of trovafloxacin at 150 mg/kg subcutaneously or twice-daily doses of moxifloxacin at 48 mg/kg subcutaneously given at 12-hour intervals (total daily dose, 96 mg/kg). The trovafloxacin dosing regimen was previously shown to result in a significant reduction in abscess weight and bacterial counts in this model (23). The moxifloxacin doses were the maximum that could be injected subcutaneously in mice and, for comparison, were given twice daily due to the shorter half-life of this fluoroquinolone in mice compared to trovafloxacin (21, 23). Control mice received injections of pyrogen-free saline. Abscess weights, bacterial counts, and histology were assessed 3, 8, or 14 days after inoculation as outlined in the section on outcome assessments.
Treatment regimen of abscesses induced by B. fragilis and different E. coli strains varying in virulence.
To ascertain the influence of the severity of the infection on the efficacy of rmG-CSF with and without moxifloxacin treatment, mice were inoculated with the same B. fragilis strain combined with either E. coli ATCC 25922 (n = 4) or the more virulent E. coli strains ATCC 35218 (n = 10) and B14349 (n = 10). Mice were given rmG-CSF prophylaxis with or without the moxifloxacin therapy outlined above, which was administered on days 1 to 5 after inoculation. Mouse survival was checked daily, and abscess, spleen, and blood bacterial counts were determined on day 1 (three mice per group) and on day 6 (all surviving mice) of infection.
Outcome assessments.
On different days of infection blood samples were obtained by orbital or heart puncture and the leukocytes were counted in a Coulter Counter (Coulter Electronics, Mijdrecht, The Netherlands). The total numbers of granulocytes, lymphocytes, and monocytes per ml were calculated from the total number of leukocytes per ml and the differential counts of 200 leukocytes in Giemsa-stained blood smears.
Mice were killed by CO2 asphyxiation and the abscesses were dissected, weighed, and homogenized in 1 ml phosphate-buffered saline for 10 seconds (Pro 200, B.V. Centraal Magazijn, Abcoude, The Netherlands). Bacterial counts were performed on the resulting suspensions by making duplicate serial 10-fold dilutions in phosphate-buffered saline and plating 20 μl of each dilution onto EM agar (E. coli) or WC agar containing 100 mg/liter gentamicin (B. fragilis). Plates were incubated at 37°C aerobically for 24 h (EM agar) or anaerobically for 48 h (WC agar).
In experiments comparing combinations of B. fragilis with different E. coli strains varying in virulence, spleen and blood bacterial counts were also determined.
For histology of established abscesses, the abscesses from at least two mice per group (including a section of surrounding skin) were removed on days 3, 8, and 14 after inoculation, fixed in 10% buffered formaldehyde, and embedded in paraffin. Sections of 5 μm were cut on a microtome and mounted on slides. Preparations were deparaffinized in two changes of xylene, hydrated, and stained with Goldner's trichrome or hematoxylin/eosin stains.
Cytokine assays.
Blood was collected on different days by orbital or heart puncture in EDTA tubes, centrifuged 5 min at 7,500 × g, and the plasma was stored at −20°C until measured. Tumor necrosis factor alpha (TNF-α) was determined by a specific radioimmunoassay described previously (12) and had a detection limit of 40 pg/ml. Interleukin-6 (IL-6) and IL-10 concentrations were measured using an enzyme-linked immunosorbent assay kit (Biosource Europe) according to the manufacturer's instructions. The detection limits were 150 and 8 pg/ml, respectively.
Statistical analysis.
Survival between experimental groups and controls was compared by the log-rank test (Graph Pad software Inc., San Diego, CA). Abscess weights and bacterial counts are given as the means ± standard error of the mean. The Mann-Whitney test was used to compare differences between two groups. To analyze the differences between three or more groups, the Kruskal-Wallis one-way analysis of variance was used with the Tukey-Kramer multiple comparison as posttest. A P value (two-sided) of < 0.05 was considered significant. Experiments were carried out two to three times, and the data represent the average results of all experiments performed.
RESULTS
MICs.
The MIC of trovafloxacin against the B. fragilis and the low-virulence E. coli (ATCC 25922) strain was 0.25 and 0.06 μg/ml, respectively (24). The MIC of moxifloxacin against the B. fragilis strain was 0.5 μg/ml and against E. coli strains ATCC 25922, ATCC 35218, and B14349 was 0.125, 0.06 and 0.125 μg/ml, respectively.
Treatment of established abscesses with fluoroquinolones alone.
Consistent with our previous report (23), a 5-day treatment regimen of trovafloxacin (150 mg/kg on days 3 to 7 of infection) significantly reduced bacterial counts and abscess weights compared with saline-treated controls. Moxifloxacin was even more effective than trovafloxacin in reducing the counts of both bacterial strains (P < 0.05; Table 1) although, in contrast, it did not significantly reduce the weight of abscesses 8 days after inoculation (Table 1). Unlike trovafloxacin (23), moxifloxacin demonstrated no visible toxic effects with the doses administered. Cytokine concentrations of TNF-α, IL-10, and IL-6 of all groups were around the detection limit (data not shown).
TABLE 1.
Effect of rmG-CSF, with or without trovafloxacin or moxifloxacin treatment, on established B. fragilis ATCC 23745/low-virulence E. coli ATCC 25922 abscesses 8 days after inoculationa
| rmG-CSF | Abscess parameter | Treatment (days 3 to 7)
|
||
|---|---|---|---|---|
| Saline | Trovafloxacin (150 mg/kg daily) | Moxifloxacin (48 mg/kg twice a day) | ||
| None | Weight (mg ± SEM) | 74 ± 5 | 44 ± 3b | 69 ± 4 |
| E. coli (log10 CFU/abscess ± SEM) | 7.4 ± 0.1 | 4.9 ± 0.1b | 4.2 ± 0.1b,c | |
| B. fragilis | 8.7 ± 0.1 | 6.9 ± 0.4b | 5.8 ± 0.1b,c | |
| Prophylaxis 1,000 ng daily on day 1 | Weight | 60 ± 4 | 45 ± 3b | 70 ± 5 |
| E. coli | 7.4 ± 0.0 | 4.7 ± 0.2b | 3.8 ± 0.1b,c | |
| B. fragilis | 8.7 ± 0.0 | 7.1 ± 0.3b | 6.5 ± 0.2b | |
| Therapy 1,000 ng daily on days 3 to 7 | Weight | 83 ± 8 | 53 ± 6b | 70 ± 15 |
| E. coli | 7.6 ± 0.1 | 5.5 ± 0.1b,d | 4.3 ± 0.2b,c | |
| B. fragilis | 8.9 ± 0.1 | 7.6 ± 0.2b | 5.9 ± 0.2b,c | |
P values are shown as follows:
significantly different from respective saline-treated group (P < 0.05);
significantly different from respective trovafloxacin-treated group (P < 0.05);
significantlly different from group treated with trovafloxacin alone (P < 0.05).
Effect of rmG-CSF on established abscesses.
The number of circulating granulocytes at the time of inoculation was 0.35 × 109± 0.03 × 109/liter in untreated mice and 1.4 × 109± 0.02 × 109/liter (P = 0.057) in mice given 1 μg of rmG-CSF prophylaxis 24 h earlier, indicating that rmG-CSF was biologically active. On day 1 of infection, the granulocyte numbers in control mice had not changed (0.93 ± 0.3 × 109/liter) and remained stable until day 8 (1.78 ± 0.8 × 109/liter, P > 0.05). In mice that had received rmG-CSF prophylaxis, the granulocyte response throughout the infection paralleled that of the control mice, with no significant differences observed.
Both rmG-CSF prophylaxis and rmG-CSF therapy (1 μg daily, days 3 through 7 of infection) did not beneficially influence the bacterial counts in the abscesses (Table 1). Histological examination of untreated abscesses 8 days after inoculation revealed a collagen capsule surrounding an abscess core comprising large numbers of granulocytes and cell debris. A layer of macrophages was evident immediately under the abscess capsule. Abscesses of mice pretreated with rmG-CSF prophylaxis showed a deeper macrophage layer and a greater number of macrophages than in control abscesses. In contrast, in abscesses of mice treated with rmG-CSF therapy, very few macrophages were seen although, compared to controls, there was a greater number of granulocytes both within and surrounding the abscesses and more cell debris was present in the abscess core.
Circulating cytokine concentrations of TNF-α, IL-10, and IL-6 of all groups were around the detection limit (data not shown).
Treatment of established abscesses with fluoroquinolones and rmG-CSF.
The addition of rmG-CSF prophylaxis to the trovafloxacin therapy did not enhance the beneficial effect of trovafloxacin treatment alone (Table 1). To determine whether rmG-CSF prophylaxis would demonstrate a posttreatment synergistic effect, abscesses were also examined 7 days after the termination of trovafloxacin therapy. Although the killing of E. coli was minimally enhanced compared to trovafloxacin therapy alone (3.1 ± 0.4 versus 3.8 ± 0.3 log10 CFU/abscess, P < 0.01), the addition of rmG-CSF prophylaxis to trovafloxacin had no significant synergistic effect on B. fragilis numbers or abscess weights 14 days after inoculation compared to trovafloxacin alone. To assess if rmG-CSF prophylaxis could improve the efficacy of less effective trovafloxacin doses (23), subgroups of mice were also treated with doses of 37.5 mg/kg. Prophylaxis with rmG-CSF failed to enhance the efficacy of these reduced trovafloxacin doses, since E. coli and B. fragilis counts were similar in both treated and control abscesses on day 8 or 14 (data not shown).
RmG-CSF therapy had no synergistic effect on abscess weights or B. fragilis counts but it did have a significant adverse effect on the killing of E. coli by trovafloxacin (5.5 ± 0.1 versus 4.9 ± 0.1 log10 CFU/abscess for trovafloxacin alone, P < 0.05, Table 1).
The addition of rmG-CSF prophylaxis or therapy to moxifloxacin treatment had no synergistic or antagonistic effect on abscess weights and bacterial counts.
All cytokine concentrations of TNF-α, IL-10, and IL-6 of all groups of mice were around the detection limit and no differences between groups were observed (data not shown).
Abscess model with B. fragilis and E. coli strains varying in virulence and survival rates.
On day 1 after inoculation with 107 CFU B. fragilis and 105 CFU E. coli ATCC 25922, the low-virulence E. coli strain, mice displayed normal activity. Abscess weights were 31.5 ± 2.5 mg and B. fragilis and E. coli bacterial counts were 8.4 ± 0.1 and 8.6 ± 0.1 log10 CFU/abscess, respectively (Table 2). Both strains had disseminated to the spleen (3.9 ± 0.4 and 4.2 ± 0.4 log10 CFU/spleen, respectively), but were not isolated from blood samples taken at the same time. On the other hand, 24 h after inoculation with combinations containing the virulent hemolytic E. coli strain ATCC 35218 (103 CFU) or the virulent nonhemolytic E. coli B14349 (105 CFU), the mice appeared ill and were weak and lethargic, and both virulent E. coli strains could be cultured from the blood of all mice examined (Table 2). Abscess and spleen bacterial counts were similar to those of mice inoculated with B. fragilis and E. coli ATCC 25922.
TABLE 2.
Comparison of abscess and mouse parameters 24 h after inoculation with mixtures comprising B. fragilis with E. coli strains varying in virulence
| Determination | Parametera |
E. coli strain (CFU)
|
||
|---|---|---|---|---|
| Low-virulence ATCC 25922 (105) | Virulent hemolytic ATCC 35218 (103) | Virulent nonhemolytic B14349 (105) | ||
| Abscess | Weight | 31.5 ± 3 | 31.8 ± 3 | 19.3 ± 1.3 |
| E. coli | 8.6 ± 0.1 | 8.2 ± 0.1 | 8.9 ± 0.0 | |
| B. fragilis | 8.4 ± 0.1 | 8.2 ± 0.0 | 8.4 ± 0.1 | |
| Spleen | E. coli | 4.2 ± 0.4 | 4.8 ± 0.3 | 4.2 ± 0.4 |
| B. fragilis | 3.9 ± 0.4 | 3.4 ± 0.5 | 3.2 ± 0.6 | |
| Blood | E. coli | <0.5 | 1.0 ± 0.5b | 2.4 ± 0.3b |
| B. fragilis | <0.5 | <0.5 | <0.5 | |
| Cytokines (pg/ml) | TNF | <40 | <40 | <40 |
| IL-10 | 60 ± 12 | 72 ± 11 | 37 ± 8 | |
Weights, mg ± SEM; bacterial counts, log10 CFU/abscess (spleen) ± SEM.
Bacteremia was present in 3/3 mice.
No circulating TNF-α concentrations were detected 24 h after inoculation and no significant differences between IL-10 concentrations were observed (Table 2).
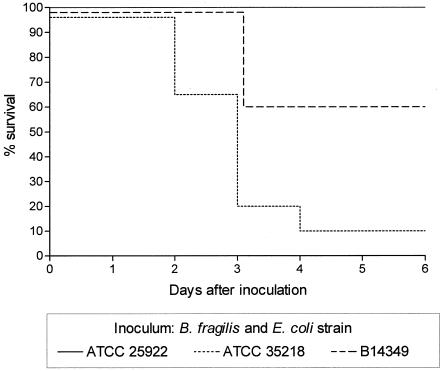
Figure 1 shows the effect of the different B. fragilis/E. coli combinations on mouse survival. Within 3 days of inoculation with the virulent hemolytic E. coli ATCC 35218 or the virulent nonhemolytic E. coli B14349, mouse survival was reduced to 20% and 60%, respectively, compared to 100% for the combination with the low-virulence E. coli ATCC 25922. By day 4 of infection, only 10% of mice inoculated with the virulent hemolytic E. coli strain ATCC 35218 had survived the infection.
FIG. 1.
Survival of mice inoculated subcutaneously with mixtures comprising B. fragilis (107 CFU) and either low-virulence E. coli strain ATCC 25922 (105 CFU) or the more virulent strain ATCC 35218 (103 CFU) or B14349 (105 CFU).
Treatment of abscesses induced by B. fragilis and E. coli strains varying in virulence with rmG-CSF and fluoroquinolones.
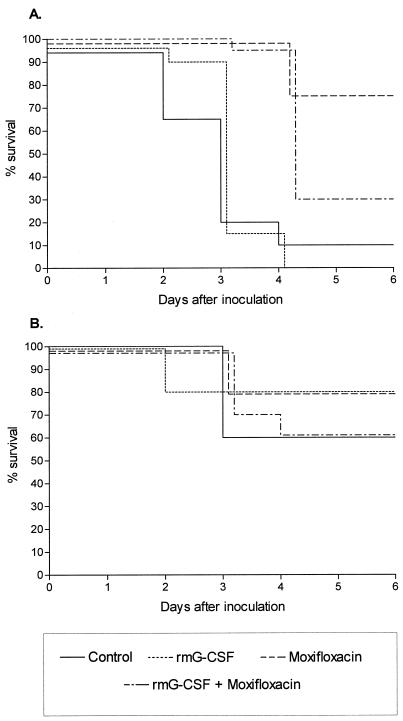
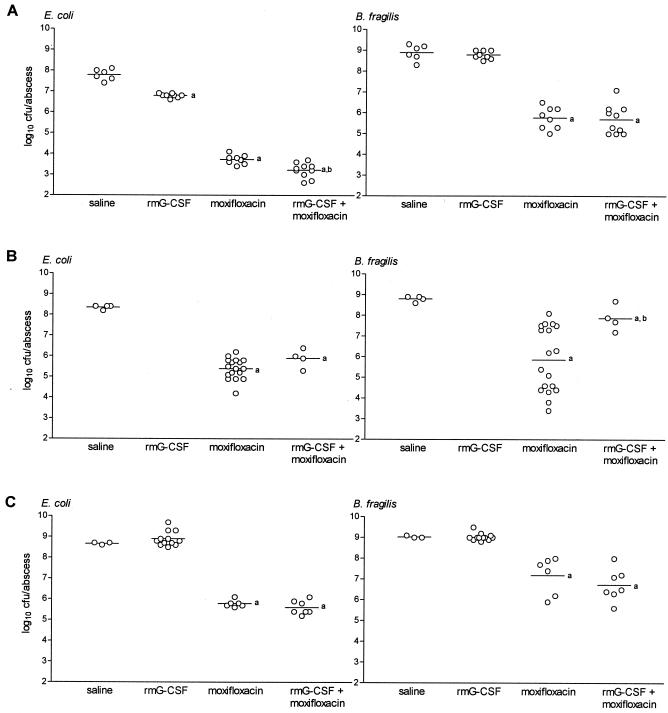
In mice inoculated with B. fragilis and the virulent hemolytic E. coli strain ATCC 35218, treatment with moxifloxacin that started 24 h after inoculation had a significant effect on mouse survival (75% versus 10%, P < 0.0001; Fig. 2A) compared to saline-treated controls. This beneficial effect of moxifloxacin was associated with significantly reduced bacterial counts of both B. fragilis and E. coli in mice that had survived on day 6 of infection (Fig. 3B). rmG-CSF prophylaxis did not influence survival of mice compared to control mice, however, the addition of rmG-CSF prophylaxis to the moxifloxacin treatment significantly reduced mouse survival compared to the group given moxifloxacin alone (30% versus 75%, P < 0.005; Fig. 2A). The detrimental influence of rmG-CSF prophylaxis in mice was accompanied by a threefold (virulent hemolytic E. coli strain) and 100-fold (B. fragilis, P < 0.05) increased outgrowth in the abscesses of mice that had survived on day 6 of infection and were treated with rmG-CSF and moxifloxacin compared to moxifloxacin treatment alone (Fig. 3B).
FIG. 2.
Effect of rmG-CSF prophylaxis (1,000 ng on day −1), with or without moxifloxacin treatment (96 mg/kg/day) on the survival of mice inoculated with mixtures comprising B. fragilis (107 CFU) and the more virulent E. coli strains (A) ATCC 35218 (103 CFU) and (B) B14349 (105 CFU).
FIG. 3.
Effect of rmG-CSF prophylaxis (1,000 ng on day −1), with or without moxifloxacin treatment (96 mg/kg/d days 1 to 5) on abscess bacterial counts 6 days after inoculation with combinations comprising B. fragilis and E. coli strain (A) ATCC 25922, (B) ATCC 35218, or (C) B14349. Horizontal bars indicate the means. a, P < 0.01 compared to saline-treated controls; b, P < 0.05 compared to the group treated with moxifloxacin alone.
In contrast, addition of rmG-CSF prophylaxis to moxifloxacin treatment did not have a detrimental influence on the survival of mice inoculated with B. fragilis and the virulent nonhemolytic E. coli strain B 14349 (Fig. 2B), nor did it detrimentally influence the bacterial counts in abscesses of mice that had survived on day 6 of infection (Fig. 3C).
Six days after inoculation and compared to saline-treated controls, moxifloxacin treatment significantly reduced both B. fragilis and E. coli bacterial counts in abscesses containing the low-virulence E. coli ATCC 25922 (Fig. 3A). Administration of rmG-CSF prophylaxis alone significantly reduced low-virulence E. coli abscess bacterial counts and had a significant synergistic effect on the killing of the low-virulence E. coli strain by moxifloxacin (Fig. 3A).
In the surviving mice of all groups, moxifloxacin significantly reduced the bacterial counts in the spleens by 1.5 to 4.2 log10 CFU/spleen compared to saline-treated controls and addition of rmG-CSF prophylaxis did not alter the effect of moxifloxacin (P < 0.05) (results not shown). The observed differences in survival between the groups could not be explained by differences in circulating concentrations of TNF-α (all below the detection limit) or IL-10 (all around 17 pg/ml).
Macroscopic abscess appearance in surviving mice.
In mice surviving to day 6, there were visible differences in the external and internal appearance of the abscesses that developed from the three inocula combinations. Externally, E. coli ATCC 25922 abscesses formed a “ball-shaped” swelling (measuring approximately 9 by 7 mm) protruding from under the skin on both flanks of the animals. There was no necrosis of the overlying skin and, internally, the pus of the abscess was very easy to remove.
In contrast, the abscesses of mice surviving the virulent hemolytic E. coli ATCC 35218 infection (including those treated with rmG-CSF and/or moxifloxacin) were not protuberant and had spread over a wide area (approximately 10 by 15 mm). Necrosis of the overlying skin was found in 89% (41 of 46) of the abscesses. Internally, the pus appeared as a thickened capsule, making the abscesses more difficult and, in some cases, impossible to remove. In some animals (four mice), the abscesses on both flanks of the mice had merged together forming one large abscess and could not, therefore, be included in bacterial count determinations. A total of 26 abscesses were cultured.
Necrosis of the overlying skin was found in 52% (29 of 56) of abscesses containing the virulent nonhemolytic E. coli B14349. Although these abscesses were similar in size and appearance to those with the low-virulence E. coli ATCC 25922 strain, internally a thickened capsule had formed, making these abscesses also more difficult or impossible to remove. A total of 28 abscesses were cultured.
DISCUSSION
This study demonstrated the efficacy of both trovafloxacin and moxifloxacin in reducing B. fragilis and low-virulence E. coli numbers in established abscesses, with moxifloxacin being the most potent agent. The addition of rmG-CSF prophylaxis or therapy provided no further beneficial effect to fluoroquinolone treatment of established abscesses. In contrast, in our model with the virulent hemolytic E. coli strain, addition of rmG-CSF antagonized the effect of moxifloxacin therapy, as indicated by reduced survival and increased outgrowth of bacteria in the abscesses.
Moxifloxacin was more efficacious than trovafloxacin in reducing established abscess bacterial counts 8 days after inoculation with B. fragilis and low-virulence E. coli ATCC 25922. This occurred despite the fact that the MICs of moxifloxacin against both bacterial strains were higher, lower daily doses of moxifloxacin were administered, and the AUC0-24 h for moxifloxacin in mice is lower (21, 23). Consequently, our results suggest that, in this animal model at least, moxifloxacin is more potent than trovafloxacin. Previous studies have shown that the activities of both trovafloxacin and moxifloxacin are unaffected by dead bacteria, albumin globulin, pus, or anaerobic conditions (17). It is unlikely, therefore, that the activity of trovafloxacin was affected by the adverse conditions present in the abscesses and the superior activity of moxifloxacin may be due to its lower protein binding (21, 23).
Interestingly, trovafloxacin reduced the weights of the established abscesses, whereas moxifloxacin treatment did not. In our previous study, it was suggested that the reduction in B. fragilis numbers and concomitant reduction in inflammation was responsible for the decreased abscess weights (23). However, in the present study, B. fragilis counts in abscesses of moxifloxacin-treated mice were significantly reduced compared to those in abscesses of trovafloxacin-treated mice. Thus, it is more likely that bacterial degradation products, due to the increased or different killing capacity of moxifloxacin compared to trovafloxacin, induced a strong proinflammatory response that resulted in increased abscess weights. Moreover, this indicates that a reduction of bacterial numbers does not necessarily coincide with a diminished inflammatory response. Alternatively, the influence on abscess weights may be the consequence of differences in the immunomodulatory capacity of the two fluoroquinolones.
Both trovafloxacin and moxifloxacin play a dual role in infections, an antimicrobial role and an immunomodulatory role by inhibiting lipopolysaccharide-stimulated secretion of IL-1α, IL-1β, and TNF-α by monocytes (2, 8). However, both fluoroquinolones differ with respect to the additional structures that are placed at the 1 position of the quinolone ring. Moxifloxacin has a cyclopropyl moiety at position 1 of the quinolone ring, whereas trovafloxacin does not. Only quinolones with the cyclopropyl group are able to stimulate white-blood-cell production (4), and moxifloxacin has been shown to significantly ameliorate the white-blood-cell and neutrophil counts of cyclophosphamide-injected mice (20). Thus, it is likely that by increasing the amount of granulocytes, moxifloxacin has an additional beneficial immunomodulating property compared to trovafloxacin. If this was indeed true, then the addition of rmG-CSF to (reduced amounts of) trovafloxacin would raise the level of treatment to moxifloxacin levels and increase abscess weights. However, this was not the case on day 8 of infection. In fact, addition of rmG-CSF to fluoroquinolone therapy did not have any beneficial effect and moxifloxacin treatment alone was superior.
Although rmG-CSF did not influence bacterial counts on day 8 of infection, 7 days after inoculation with B. fragilis/low-virulence E. coli ATCC 25922, rmG-CSF prophylaxis significantly reduced E. coli bacterial counts and, combined with moxifloxacin treatment, improved the efficacy of the fluoroquinolone against the E. coli strain. This indicates that we either assessed bacterial outgrowth at the wrong time point or, at best, the beneficial and synergistic effects of rmG-CSF appeared to be transient. This is corroborated by a previous study that also reported a similar temporary antibacterial effect of G-CSF in an endocarditis infection model (25). Furthermore, although rmG-CSF displayed a posttreatment synergistic effect on the low-virulence E. coli strain 14 days after inoculation, we are cautious in drawing any conclusions from these findings due to the increased range in bacterial counts at this stage of abscess development and, therefore, the limitations of this animal model.
Quinolones readily accumulate in granulocytes and enhance intracellular killing mechanisms (13-15). Therefore, it was assumed that, by increasing the numbers of granulocytes following rmG-CSF treatment, these cells would contribute to achieving higher drug concentrations in the abscesses by serving as secondary transport systems for the fluoroquinolones. In our experiments, however, these putative effects have not led to synergistic effects of rmG-CSF and quinolones. In addition, because TNF-α and IL-1 are important activators of granulocyte function (19, 29), the presence of fluoroquinolones might suppress polymorphonuclear neutrophil function as they have been shown to reduce secretion of these cytokines (2, 8). Also, the absence of synergy may have been due to inhibited neutrophil function within the abscesses (1, 5).
Depending on the severity of the infection, rmG-CSF prophylaxis could detrimentally affect the efficacy of moxifloxacin. In our model with the more virulent hemolytic E. coli ATCC 35218, rmG-CSF prophylaxis reduced mouse survival which was accompanied by a threefold (E. coli) and 100-fold (B. fragilis) increased outgrowth in abscesses compared to mice treated with moxifloxacin alone. This negative effect of rmG-CSF is in line with previous research showing a detrimental influence of G-CSF on survival from E. coli sepsis (16), E. coli pneumonia (7), and on antibiotic treatment of E. coli peritonitis (18). The endotoxin released after moxifloxacin treatment might have induced an increase in circulating TNF-α concentrations. Surprisingly, however, no correlation between circulating TNF-α concentrations and mortality was observed. Possibly the TNF-α concentrations were not increased due to the down-regulatory influence of the fluoroquinolones and G-CSF (6, 10).
Since the abscesses of mice surviving the virulent hemolytic E. coli infection had spread over a wide area, i.e., no optimal compartmentalization occurred, rmG-CSF-stimulated granulocytes may have increased the detrimental effect of the inflammatory process, leading to greater numbers of bacteria in the bloodstream and organs. Furthermore, G-CSF has been shown to increase CD14 expression in response to endotoxin, which results in up-regulation of adherence receptors on granulocytes (28). In addition, hemolysins produced by this E. coli strain may have worsened outcome of infection by inducing reactive oxygen metabolites and proinflammatory mediators (9).
In conclusion, the administration of G-CSF, either prophylactically before the formation of abscesses or during fluoroquinolone treatment of established abscesses, is not considered beneficial. Depending on the severity of infection caused by differences in virulence of the E. coli strains, the administration of G-CSF prophylaxis in conjunction with fluoroquinolones can impair the outcome compared to fluoroquinolone treatment alone.
Acknowledgments
This study was financially supported by Pfizer BV, Cappelle a/d Ijssel, The Netherlands.
REFERENCES
- 1.Abdul-Majid, K. B., P. A. Kenny, and J. J. Finlay-Jones. 1997. The effect of the bacterial product, succinic acid, on neutrophil bactericidal activity. FEMS Immunol. Med. Microbiol. 17:79-86. [DOI] [PubMed] [Google Scholar]
- 2.Araujo, F. G., T. L. Slifer, and J. S. Remington. 2002. Effect of moxifloxacin on secretion of cytokines by human monocytes stimulated with lipopolysaccharide. Clin. Microbiol. Infect. 8:26-30. [DOI] [PubMed] [Google Scholar]
- 3.Barsig, J., D. S. Bundschuh, T. Hartung, A. Bauhofer, A. Sauer, and A. Wendel. 1996. Control of fecal peritoneal infection in mice by colony-stimulating factors. J. Infect. Dis. 174:790-799. [DOI] [PubMed] [Google Scholar]
- 4.Dalhoff, A., and I. Shalit. 2003. Immunomodulatory effects of quinolones. Lancet 3:359-371. [DOI] [PubMed] [Google Scholar]
- 5.Finlay-Jones, J. J., K. V. L. Davies, L. P. Sturm, P. A. Kenny, and P. H. Hart. 1999. Inflammatory processes in a murine model of intra-abdominal abscess formation. J. Leukoc. Biol. 66:583-587. [DOI] [PubMed] [Google Scholar]
- 6.Gorgen, I., T. Hartung, M. Leist, M. Niehorster, G. Tiegs, S. Uhlig, F. Weitzel, and A. Wendel. 1992. Granulocyte colony-stimulating factor treatment protects rodents against lipopolysaccharide-induced toxicity via suppression of systemic tumor necrosis factor-alpha. J. Immunol. 149:918-924. [PubMed] [Google Scholar]
- 7.Karzai, W., B. U. von Specht, C. Parent, J. Haberstroh, K. Wollersen, C. Natanson, S. M. Banks, and P. Q. Eichacker. 1999. G-CSF during Escherichia coli versus Staphylococcus aureus pneumonia in rats has fundamentally different and opposite effects. Am. J. Respir. Crit. Care Med. 159:1377-1382. [DOI] [PubMed] [Google Scholar]
- 8.Khan, A. A., T. L. Slifer, and J. S. Remington. 1998. Effect of trovafloxacin on production of cytokines by human monocytes. Antimicrob. Agents Chemother. 42:1713-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konig, B., W. Konig, J. Scheffer, J. Hacker, and W. Goebel. 1986. Role of Escherichia coli alpha-hemolysin and bacterial adherence in infection: requirement for release of inflammatory mediators from granulocytes and mast cells. Infect. Immun. 54:886-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lundblad, R., J. M. Nesland, and K.-E. Giercksky. 1996. Granulocyte colony-stimulating factor improves survival rate and reduces concentrations of bacteria, endotoxin, tumor necrosis factor, and endothelin-1 in fulminant intra-abdominal sepsis in rats. Crit. Care Med. 24:820-826. [DOI] [PubMed] [Google Scholar]
- 11.McClean, K. L., G. J. Sheehan, and G. K. M. Harding. 1994. Intra-abdominal Infection: a review. Clin. Infect. Dis. 19:100-116. [DOI] [PubMed] [Google Scholar]
- 12.Netea, M. G., P. N. Demacker, B. J. Kullberg, O. C. Boerman, I. Verschueren, A. F. Stalenhoef, and J. W. van der Meer. 1996. Low-density lipoprotein receptor-deficient mice are protected against lethal endotoxemia and severe gram-negative infections. J. Clin. Investig. 97:1366-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nielsen, S. L., N. Obel, M. Storgaard, and P. L. Andersen. 1997. The effect of quinolones on the intracellular killing of Staphylococcus aureus in neutrophil granulocytes. J. Antimicrob. Chemother. 39:617-622. [DOI] [PubMed] [Google Scholar]
- 14.Pascual, A., I. Garcia, S. Ballesta, and E. J. Perea. 1999. Uptake and intracellular activity of moxifloxacin in human phagocytes and tissue-cultured epithelial cells. Antimicrob. Agents Chemother. 43:12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pascual, A., I. Garcia, S. Ballesta, and E. J. Perea. 1997. Uptake and intracellular activity of trovafloxacin in human phagocytes and tissue-cultured epithelial cells. Antimicrob. Agents Chemother. 41:274-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quezado, Z., C. Parent, W. Karzai, M. Depietro, C. Natanson, W. Hammond, R. L. Danner, X. Cui, Y. Fitz, S. M. Banks, E. Gerstenberger, and P. Q. Eichacker. 2001. Acute G-CSF therapy is not protective during lethal E. coli sepsis. Am. J. Physiol. Regulatory Integrative Comp. Physiol. 281:R1177-R1185. [DOI] [PubMed] [Google Scholar]
- 17.Rubinstein, E., L. Diamantstein, G. Yoseph, G. Gruzman, B. Rubinovitch, A. Barzilai, and N. Keller. 2000. The effect of albumin globulin, pus and dead bacteria in aerobic and anaerobic conditions on the antibacterial activity of moxifloxacin, trovafloxacin and ciprofloxacin against Streptococcus pneumoniae, Staphylococcus aureus and E. coli. Clin. Microbiol. Infect. 6:675-686. [DOI] [PubMed] [Google Scholar]
- 18.Saba, R., M. Guler, D. Inan, D. Ogunc, S. Atalay, L. Mamikoglu, and F. Gunseren. 2003. The effect of granulocyte colony-stimulating factor in the treatment of Escherichia coli peritonitis with or without ceftriaxone in a nonneutropenic rat model. Surg. Today 33:504-508. [DOI] [PubMed] [Google Scholar]
- 19.Shalaby, M. R., B. B. Aggarwal, E. Rinderknecht, L. P. Sverdersky, B. S. Finkle, and J. Palladino. 1985. Activation of human polymorrphonuclear neutrophil functions by interferon-gamma and tumor necrosis factors. J. Immunol. 135:2069-2073. [PubMed] [Google Scholar]
- 20.Shalit, I., Y. Kletter, D. Halperin, D. Waldman, E. Vasserman, A. Nagler, and I. Fabian. 2001. Immunomodulatory effects of moxifloxacin in comparison to ciprofloxacin and G-CSF in a murine model of cyclophosphamide-induced leukopenia. Eur. J. Haematol. 66:287-296. [DOI] [PubMed] [Google Scholar]
- 21.Siefert, H. M., A. Domdey-Bette, K. Henninger, F. Hucke, C. Kohlsdorfer, and H. H. Stass. 1999. Pharmacokinetics of the 8-methoxyquinolone, moxifloxacin: a comparison in humans and other mammalian species. J. Antimicrob. Chemother. 43(Suppl. B):69-76. [DOI] [PubMed] [Google Scholar]
- 22.Stearne, L. E. T., S. L. Buijk, J. W. Mouton, and I. C. Gyssens. 2002. Effect of a single percutaneous abscess drainage puncture and imipenem therapy, alone or in combination, in treatment of mixed-infection abscesses in mice. Antimicrob. Agents Chemother. 46:3712-3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stearne, L. E. T., I. C. Gyssens, W. H. Goessens, J. W. Mouton, W. J. Oyen, J. W. van der Meer, and H. A. Verbrugh. 2001. In vivo efficacy of trovafloxacin against Bacteroides fragilis in mixed infections with either Escherichia coli or a vancomycin-resistant strain of Enterococcus faecium in an established-abscess murine model. Antimicrob. Agents Chemother. 45:1394-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stearne, L. E. T., C. Kooi, W. H. Goessens, I. A. J. M. Bakker-Woudenberg, and I. C. Gyssens. 2001. In vitro activity of trovafloxacin against Bacteroides fragilis in mixed culture with either Escherichia coli or a vancomycin resistant strain of Enterococcus faecium using an anaerobic time kill technique. Antimicrob. Agents Chemother. 45:243-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vignes, S., B. Fantin, C. Elbim, F. Walker, M.-A. Gougerot-Pocidalo, and C. Carbon. 1995. Critical influence of timing of administration of granulocyte colony-stimulating factor on antibacterial effect in experimental endocarditis due to Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 39:2702-2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villa, P., L. Shaklee, C. Meazza, D. Agnello, P. Ghezzi, and G. Sendaldi. 1998. Granulocyte colony-stimulating factor and antibiotics in the prophylaxis of a murine model of polymicrobial peritonitis and sepsis. J. Infect. Dis. 178:471-477. [DOI] [PubMed] [Google Scholar]
- 27.Vonk, A. G., M. G. Netea, J. H. van Krieken, P. E. Verweij, J. W. van der Meer, and B. J. Kullberg. 2003. Treatment of intra-abdominal abscesses caused by Candida albicans with antifungal agents and recombinant murine granulocyte colony-stimulating factor. Antimicrob. Agents Chemother. 47:3688-3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright, S. D., R. A. Ramos, A. Hermanowski-Vosatka, P. Rockwell, and P. A. Detmers. 1991. Activation of the adhesive capacity of CR3 on neutrophils by endotoxin: dependence on lipopolysaccharide binding protein and CD14. J. Exp. Med. 173:1281-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yagisawa, M., A. Yuo, S. Kitagawa, Y. Yazaki, and F. Takaku. 1995. Stimulation and priming of human neutrophils by IL-1 alpha and IL-1 beta: complete inhibition by IL-1 receptor antagonist and no interaction with other cytokines. Exp. Hematol. 23:603-608. [PubMed] [Google Scholar]