Abstract
Currently available primary screens for the selection of candidate antileishmanial compounds are not ideal. These techniques are time-consuming, laborious, and difficult to scale and require macrophages, which limit their use for high-throughput screening. We have developed Leishmania donovani field isolates that constitutively express the firefly luciferase reporter gene (luc) as a part of an episomal vector. An excellent correlation between parasite number and luciferase activity was observed. luc expression was stable, even in the absence of drug selection, for 4 weeks. The transfectants were infective to macrophages, and intracellular amastigotes exhibited luciferase activity. The suitability of these recombinant field isolates for in vitro screening of antileishmanial drugs was established. The luciferase-expressing sodium stibogluconate-resistant cell lines offer a model for the screening of compounds for resistance. The system is in routine use at the Central Drug Research Institute, Lucknow, India, for high-throughput screening of newly synthesized compounds.
Leishmaniasis, a disease endemic in 88 countries, is a major public health problem worldwide, with approximately 400,000 new cases per year (2). The traditionally exotic disease leishmaniasis is becoming of greater interest for two main reasons: international travel and the importance of Leishmania as an opportunistic pathogen in AIDS patients (www.who.int/emc/diseases/leish/leishdisl.html.). A vertebrate host is infected with flagellated extracellular promastigotes via the bite of a sand fly. The promastigotes are rapidly transformed into nonflagellated amastigotes which actively divide within mononuclear phagocytes of vertebrate host (30). Since vaccines against leishmaniasis are still under development (7), control of the disease relies on chemotherapy. The leishmanicidal drugs used at present are mostly toxic and expensive and require long-term administration (4). Further, a large-scale increase in the rates of clinical resistance to the drug of first choice, pentavalent antimonials, has been reported (17, 26). Therefore, there is an urgent need for the development of new, effective, safe, and nontoxic drugs for treatment of the disease.
The current outlook for the chemotherapy of leishmaniasis is more promising than it has been for several years. With the advances in combinatorial chemistry, a number of libraries of different chemical entities can be synthesized in less time. Screening of these libraries is an effective and rapid way to find new, active drugs. However, efficient screening of antileishmanial compounds is severely impeded by the lack of a simple, reliable, and rapid drug evaluation and screening system that can be used for high-throughput screening (HTS). The cytotoxic effects of compounds on amastigotes in cell cultures or in animal models are mostly evaluated by microscopy with Geimsa-stained, fixed, infected macrophages or impression smears (9). Although these tests represent standard procedures, they are difficult to scale, labor-intensive, expensive, and lengthy and are restricted to use with a very few number of compounds. Rapid cell viability assays like the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction assay (3), the Almar blue assay (28), and the incorporation of radiolabeled precursors have been devised for use with Leishmania promastigotes (6). However, none of these assays could be widely adopted because of various drawbacks (13), and the tedious promastigote counting has remained the screening assay of choice (1).
The reporter gene technology is being widely used to monitor cell growth and proliferation under in vitro culture systems and also to monitor the cellular events associated with gene expression and signal transduction. The use of reporter genes in a number of intracellular microorganisms, e.g., Mycobacterium tuberculosis (12), Trypanosoma cruzi (8), and Toxoplasma gondii (27), has facilitated antimicrobial drug testing and discovery. The same strategy should be of interest for Leishmania too. Green fluorescence protein has been transfected into Leishmania promastigotes (23) and used for the screening of natural products for their antileishmanial activities by flow cytometry (31). The assay could not be developed for HTS due to the limitation of the low level of fluorescence to be detected by fluorimeter. The firefly luciferase (22) represents one of the most efficient biological reporter molecules which allows rapid testing of cellular viability and thus is most suitable for biological screening. The luciferase gene had been expressed in Leishmania infantum, Leishmania major, and the Leishmania donovani Sudan strain for assessment of the amastigote stage within infected macrophages (34); but no reports on its use for screening are available. Further, all of these species or strains have been in prolonged continuous in vitro cultures. A recent keynote article in TDR News urged the need for the development of reporter gene assays for malaria and leishmaniasis (36). They emphasized the need for the development of drug-resistant and -sensitive transgenic strains. Consequently, we focused on the development of transgenic cell lines of L. donovani field isolates (both sodium stibogluconate [SAG]-resistant and -sensitive ones) that constitutively express the firefly luciferase reporter gene (luc) as a part of an episomal vector. We also established the suitability of these transgenic cell lines as target cells for in vitro screening of antileishmanial compounds. The method is rapid, very sensitive, and highly reproducible and does not require any very expensive specialized instrument or training.
MATERIALS AND METHODS
Cell cultures. (i) Clinical isolates.
The patients were selected from the Kala-azar Medical Centre of the Institute of Medical Sciences, Banaras Hindu University, Varanasi, India, and also from its affiliated hospital at Muzzafarpur, Bihar, India. The criterion for a diagnosis of visceral leishmaniasis (VL) was the presence of Leishman-Donovan bodies in splenic aspirates which were graded according to standard criteria (11). After establishment of a diagnosis of VL, the patients were administered a course of SAG intravenously (20 mg/kg of body weight once daily for 30 days). The response to treatment was evaluated by repeating the splenic aspiration on day 30 of treatment. A positive response to treatment was based on the absence of fever, clinical improvement with a reduction in spleen size, and the absence of the parasite in splenic aspirates. Patients who showed the presence of parasites were considered unresponsive. These patients were subsequently treated with amphotericin B. Two unresponsive isolates, namely, Ld03 (R1) and Ld39 (R2), and two responsive isolates, Ld01 (S1) and Ld87 (S2), were taken for use in transfection studies after their resistance phenotypes were confirmed under laboratory conditions.
(ii) Reference strain.
L. donovani Dd8 promastigotes (World Health Organization designation MHOM/IN/80/Dd8; originally obtained as a gift from the late P. C. C. Granham and routinely maintained at the Central Drug Research Institute in golden hamsters) were used as the reference sensitive organisms.
Culture conditions.
The splenic aspirates of patients were inoculated into NNN medium (blood agar base with an overlay of RPMI 1640 medium), grown at 25°C ± 1°C, and subcultured every sixth day. The positive cultures were then adapted to medium 199 (Sigma Chemical Co., St. Louis, Mo.) supplemented with 10% fetal calf serum (FCS; GIBCO BRL) and 1% penicillin (50 U/ml) and streptomycin (50 μg/ml) solution (Sigma) (15).
Drugs.
The standard antileishmanial drugs amphotericin B, pentamidine, potassium antimony tartrate trihydrate (SbIII), and miltefosine were procured from Sigma. Sodium stibogluconate (SbV; 30% weight by volume) was the product of Gluconate India, India.
DNA constructs and gene transfections.
The complete open reading frame of the firefly luciferase gene was amplified from pGEM-Luc plasmid DNA (Promega) with forward primer 5′-GGCCACTAGTATGGAAGACGCCAAAAAC-3′ and reverse primer 5′-CCGGACTAGTTTACAATTTGGACTTTCCGCC-3′, which included the SpeI restriction site (underlined). The resulting amplicon (1.7 kb) was ligated into the pCRII-TOPO vector (Invitrogen) to create plasmid pCRT-luc and was selected for ampicillin (100 μg/ml) resistance in the presence of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside and isopropyl-β-d-thiogalactopyranoside.
Two clones (pCRT-luc4 and pCRT-luc8) were sequenced completely in both directions by the dideoxy method to confirm the sequence of the amplicon and to detect the presence of any deletions, additions, or point mutations in the sequence that may have appeared during the amplification of the gene. The sequence of pCRT-luc8 was clean, while pCRT-luc4 had one point mutation at position 1287. Therefore, pCRT-luc8 was pursued for use in the expression of the luc open reading frame (ORF) in the Leishmania parasite.
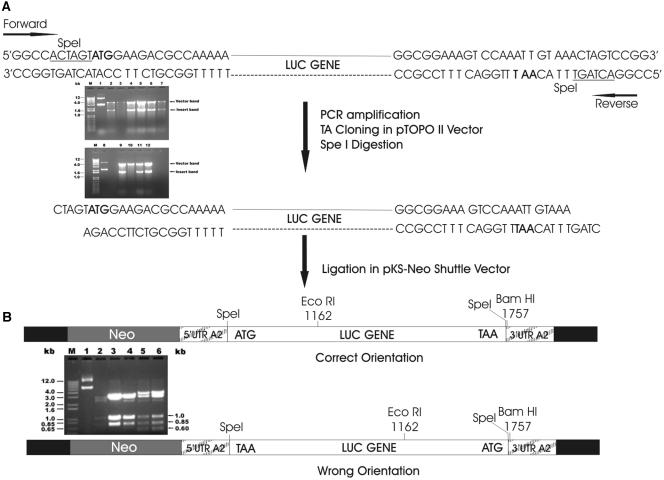
The luciferase gene cloned in the pCRII-TOPO vector was digested with the SpeI restriction enzyme, and the purified insert was cloned at the SpeI site of the linearized, dephosphorylated pKS-Neo leishmania shuttle vector (37) containing the neomycin phosphotransferase gene to obtain the pKS-Neo-luc construct.
The orientation of the luc insert in the expression vector was determined by double digestion with EcoRI (which cuts once within the luc ORF) and BamHI (which cuts once within the plasmid shuttle vector). One pKS-Neo-luc clone with the luc ORF insert in the correct orientation, i.e., clone 4pKS-Neo-luc8, was selected, expanded in Escherichia coli, and used for transfection.
Promastigotes of L. donovani field isolates (both sensitive and resistant strains) grown to mid-log phase (until the parasite count reached 2 × 107 to 3 × 107/ml) were transfected by electroporation with a Gene Pulser (Bio-Rad) by using the conditions described previously (14). The transfected organisms were allowed to grow in medium M199 plus 10% FCS at 25 ± 1°C for 24 h and were then selected with minimum doses of G418 (20 μg/ml). Drug (G418)-resistant parasites were usually selected after 11 to 20 days. After the establishment of transgenic parasites, clones were derived by limiting dilutions, which were evaluated for luciferase expression after the second subculture. Transfectants were routinely maintained in the presence of 20 μg/ml of G418, as an increase in the drug pressure did not result in an increase in the number of relative light units.
Luciferase assay.
To estimate the luciferase activities of the transgenic Leishmania cells lines developed, promastigotes from stationary-phase culture were harvested by centrifugation washed and suspended in phosphate-buffered saline at a final concentration of 2 × 106 cells/ml. A 50-μl cell suspension was mixed with 50 μl Steady Glo reagent, according to the manufacturer's instruction (Promega), in a black 96-well plate (Nunc). After 2 min, the plate was read either by a Polar Star Galaxy or by a Micro Beta Trilux instrument. The light output was measured for 1 s/well with a 0.2-s delay (Polar Star Galaxy instrument) or for 1 min/well after a 1-s delay (Micro Beta Trilux instrument).
Promastigote drug susceptibility assay.
Exponentially growing transgenic promastigotes were seeded in 96-well flat-bottom tissue culture plates (Cell Star; Greiner) to a final concentration of 0.5 × 106/ml and allowed to grow for 72 h in medium alone or in the presence of various concentrations of standard drugs (in triplicate for each concentration) at 26°C. After incubation, 50 μl of the parasite suspension was mixed with 50 μl Steady Glo reagent (Promega), incubated for 2 min, and read in the Polar Star Galaxy instrument. The inhibition of parasite growth was determined by comparison of the luciferase activity of drug-treated parasites with that of untreated control parasites.
Macrophage infection and intracellular amastigote drug susceptibility assay.
The infectivity, growth, and expression of the luciferase gene in the amastigote stage within macrophages (J774) were evaluated according to a method described previously (16). Briefly, 0.5 × 105 J774 macrophages were plated in a 16-well chamber slide (Nunc) and 96-well tissue culture plates and allowed to adhere for 24 h in RPMI 1640 medium plus 10% FCS at 37°C in a 5% CO2-95% air mixture. Adherent macrophages (in duplicate) were infected with stationary-phase recombinant promastigotes at a ratio of 1:5 for 24 h at 37°C in a 5% CO2-95% air mixture. After incubation, noninternalized parasites were removed by washing, and infected macrophage cultures were incubated further at 37°C in a 5% CO2-95% air mixture for 48 h in the absence or in the presence of standard drugs at several dilutions. After incubation, the chamber slides were fixed in methanol, stained with Geimsa, and observed under a microscope to determine the mean number of amastigotes per macrophage. While in the tissue culture plate, the luciferase activity of the amastigotes was determined.
RESULTS
L. donovani isolate(s) expressing the firefly luciferase.
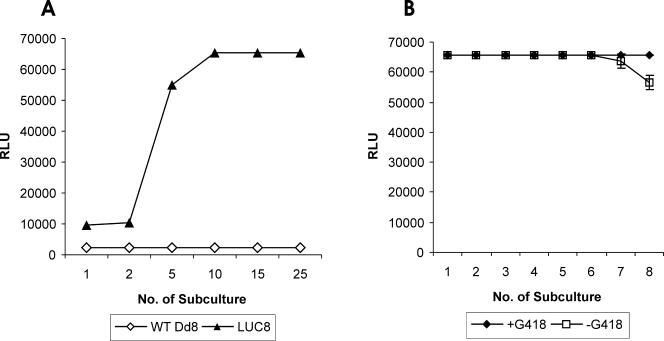
Figure 1 depicts the strategy and schematic presentation of cloning of the luciferase (luc) gene in Leishmania shuttle expression vector. The luc gene was amplified by PCR and cloned into the pCRII-TOPO vector to obtain the pCRT-luc4 and pCRT-luc8 constructs (Fig. 1A, lanes 5 and 9). Complete sequencing of the luc ORF in both clones revealed that pCRT-luc8 did not have any mutations, additions, or deletions, while pCRT-luc4 had one point mutation at position 1287. Therefore, only one luc ORF insert of pCRT-luc8 was subcloned into the pKS-Neo expression vector, resulting in two clones, 4pKS-Neo-luc2 and 4pKS-Neo-luc8, with the luc ORF in the correct orientation (Fig. 1B, lane 3 and 4). The luc expression construct 4pKS-Neo-luc8 was electroporated, and the luciferase activity was found to be severalfold higher in the luc expression transfectant than in the pKS-Neo control (Fig. 2A). Initially, the level of luciferase expression was less, but after three subcultures in the presence of 20 μg/ml G418, the luciferase activity reached a maximum level and the level of activity was maintained. Interestingly, even after the removal of drug pressure, the luciferase activity of the parasites did not decline for up to six subcultures (every 5th day) for a period of 30 days (Fig. 2B). After that, there was a decrease in the luciferase activity.
FIG. 1.
Schematic presentation of steps for construction of the luciferase expression vector for transfection in L. donovani promastigotes. Partial maps with the relevant restriction sites are shown. (A) pCRII-TOPO-luc construct digested with SpeI and (B) pKS-Neo-luc clones digested with BamHI and EcoRI for determination of orientation of the luc ORF within the construct.
FIG. 2.
Expression of luciferase activity in recombinant promastigotes of Dd8. (A) Transfection with Leishmania shuttle vector pKS-Neo and luc expression constructs, i.e., 4pKS-Neo-luc8; (B) stability of expression of luc gene in the absence of G418. Results are expressed as mean of triplicate experiments ± standard deviations. RLU, relative light units; WT, wild type.
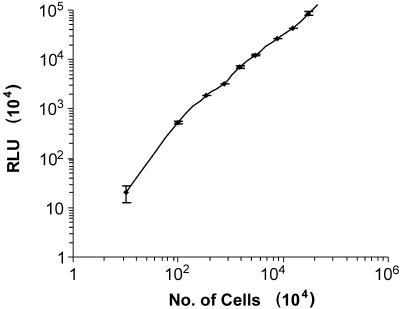
To test whether luciferase activity correlated well with the number of parasites, serial 10-fold dilutions were made and luciferase activity was measured. An excellent linear correlation was observed between the number of transgenic promastigotes and the luciferase activity (Fig. 3). The sensitivity of the assay was very high, as it was possible to detect as few as 10 promastigotes.
FIG. 3.
Correlation between the luciferase activity and the number of Leishmania promastigotes (Dd8) transfected with the 4pKS-Neo-luc8 construct. Results are expressed as mean of triplicate experiments ± standard deviations. Tenfold serial dilutions were applied, and the parasites were counted with a hemocytometer, while the number of relative light units (RLU) was determined as described in Materials and Methods.
Infectivity of the transfectants and luciferase activity of amastigotes within macrophages.
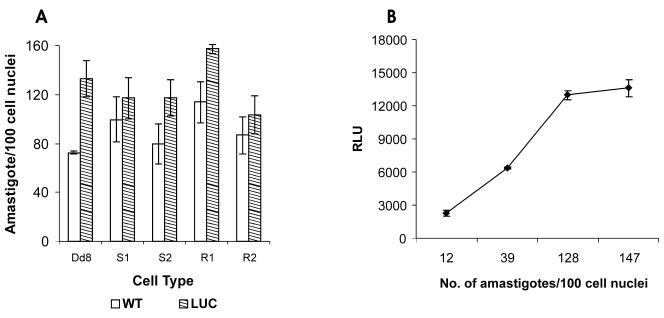
The transfectants were also tested for their ability to infect the macrophages. The stationary-phase recombinant promastigotes were used to infect J774 macrophages. The intracellular Leishmania infection was visualized microscopically after Giemsa staining. Except for reference sensitive strain Dd8, none of the field isolates (both sensitive and resistant) exhibited any significant change in infectivity after transfection (Fig. 4A). Further, a good correlation was observed between number of amastigotes/100 macrophages and the number of relative light units (Fig. 4B).
FIG. 4.
Relationship between number of intracellular amastigotes and luciferase activity. L. donovani promastigotes transfected with 4pKS-Neo-luc8 were used to infect cells of the J774 macrophage cell line, as indicated in Materials and Methods. (A) Cells were counted by using Geimsa staining and microscopic analysis; (B) the number of relative light units (RLU) was determined as described in Materials and Methods. The results are expressed as the means of triplicate experiments ± standard deviations.
Efficacies of standard drugs.
In order to establish the suitability of these transgenic cell lines for rapid in vitro screening of antileishmanial compounds in the HTS mode, the study was performed with four antileishmanial reference drugs. These included SbIII (the active form of antimony); pentamidine; amphotericin B; and miltefosine, a drug which has been licensed in India and which is undergoing phase IV clinical trials (Table 1). The resistant isolates exhibited almost three- to fourfold higher 50% inhibitory concentration (IC50) values for SbIII than the sensitive ones. On the other hand, these isolates were more susceptible to amphotericin B. Miltefosine had more or less the same effect on all the isolates.
TABLE 1.
IC50s of standard antileishmanials for transgenic promastigotes of field isolates determined by LUC assay
| Strain or isolate | IC50 (μg/ml)a
|
|||
|---|---|---|---|---|
| Petamidine | Amphotericin B | SbIII | Miltefosine | |
| Dd8 | 0.6425 | 0.024 | 31.1893 | 3.3534 |
| S1 | 0.3704 | 0.017 | 23.6797 | 3.5525 |
| S2 | 0.4673 | 0.0124 | 21.5431 | 3.296 |
| R1 | 0.5407 | 0.0096 | 82.9582 | 2.658 |
| R2 | 0.3796 | 0.0075 | 84.4716 | 2.942 |
Representative data from two separate experiments.
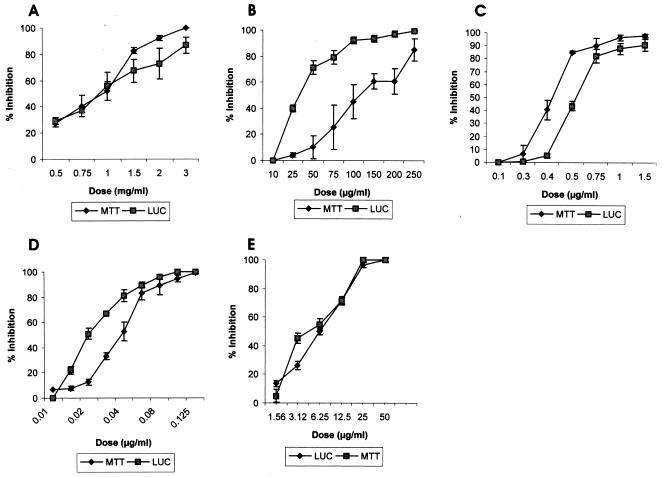
The IC50 values obtained by LUC assay were also compared with those obtained by the more conventional MTT method by using transgenic promastigotes of laboratory sensitive strain Dd8 (Table 2; Fig. 5). As expected, sodium stibogluconate was found to be toxic only at very high concentrations (mg/ml), while SbIII was active in microgram quantities (Fig. 5A and B, respectively). Pentamidine, amphotericin B, and miltefosine exhibited dose-dependent efficacies (Fig. 5C to E, respectively). Except for SbIII, the IC50 values obtained for these drugs by the luciferase assay were more or less consistent with the values obtained by the MTT assay (Table 2). Further, the results for all four antileishmanial agents were significantly different between the promastigote and the intracellular amastigote assays. The reference antileishmanial agent sodium stibogluconate was much less active against promastigotes than against intracellular amastigotes, while the other three agents, namely, pentamidine, amphotericin B, and miltefosine, were more active against the promastigotes.
TABLE 2.
Comparative susceptibilities of luc transfectant promastigote and intracellular amastigote forms of L. donovani (Dd8 strain) to drugs currently used to treat leishmaniasis
| Antileishmanial agent | IC50 (μg/ml)a
|
||
|---|---|---|---|
| Promastigote
|
Intracellular amastigote LUC assay | ||
| MTT assay | LUC assay | ||
| SbV | 878.86 | 946.52 | 117.3289 |
| SbIII | 134.11 | 31.19 | ND |
| Pentamidine | 0.433 | 0.643 | 15.2577 |
| Amphotericin B | 0.036 | 0.024 | 0.017 |
| Miltefosine | 3.823 | 3.353 | 33.0393 |
Representative data from two separate experiments. ND, not determined.
FIG. 5.
Use of luciferase-expressing parasites (Dd8) to monitor drug toxicity: comparison of the MTT and LUC assays. The results are expressed as the means of triplicate experiments ± standard deviations. (A) SbIII; (B) SbV; (C) pentamidine; (D) amphotericin B; (E) miltefosine.
We also compared the levels of resistance of the clinical isolates to SbIII by using these transfectants (Table 3). Two clinical noresponsive isolates, R1 (Ld39) and R2 (Ld03), exhibited threefold greater resistance to SbIII than strain Ld01 (S1), the clinical sensitive strain, and Dd8, the reference sensitive strain. This established the utility of these luc-expressing resistant isolates for the detection of resistance in in vitro assays well as their suitability for the screening of compounds against resistant cell lines.
TABLE 3.
In vitro resistance profile of L. donovani field isolates to SbIII determined by microscopy and LUC assay
| Strain | Resistance indexa
|
|
|---|---|---|
| LUC assay | Microscopy | |
| Dd8 | Nil | Nil |
| S1 | 0.759 | 0.912 |
| S2 | 0.690 | 0.886 |
| R1 | 2.708 | 3.324 |
| R2 | 2.659 | 3.011 |
Values are the means of two separate experiments. The resistance index is the IC50 of field isolate/IC50 of reference strain Dd8.
DISCUSSION
The use of reporter genes in a number of intracellular pathogenic microorganisms has facilitated antimicrobial drug discovery and testing (8, 12, 24, 27). Recently, the green fluorescence protein has been introduced into Leishmania spp. and used for the in vitro screening of herbal products. However, this reporter assay cannot be used at the high-throughput level because of the use of flow cytometry (31). The firefly LUC assay (22) overcomes these shortcomings and offers a highly reliable method for the screening of compounds. The luciferase gene had been transfected transiently (19) and stably (29, 34, 35) in Leishmania promastigotes and axenic amastigotes. We have focused on virulent clinical isolates (SAG sensitive and resistant) while developing rapid screening methods for use in the HTS mode for the screening of large numbers of antileishmanial compounds synthesized at the Central Drug Research Institute. Our aim is to establish HTS assays by using these luciferase-expressing recombinant cell lines of L. donovani for in vitro and semi-in vivo screening.
In order to achieve this goal, the compete ORF of the luciferase gene was amplified and cloned into the pKS-Neo Leishmania shuttle expression vector (Fig. 1) This expression vector has the 5′ untranslated region and the 3′ untranslated region of the A2 gene, which is an amastigote-specific gene (37); therefore, transformation of the promastigote stage to the amastigote stage will not affect the expression of the luciferase gene. The L. donovani cell lines transfected with 4pKS-Neo-luc8 (luc expression construct) exhibited luciferase activity at very high levels compared to the levels expressed by the pKS-Neo control (Fig. 2A). Maximum expression was achieved after three subcultures in G418 (20 μg/ml)-containing medium, and the luciferase expression was then maintained. Interestingly, there was no decrease in the expression of luciferase activity of the recombinant parasites even in the absence of G418 for up to six subcultures (every 5th day), and the activity remained the same for up to 30 days (Fig. 2B). This suggests that the copy number of the episomal vector per parasite does not decline during this period in the absence of G418. The correlation between luciferase activity and parasite number was excellent (Fig. 3). The minimum number of parasites that could be detected by the assay was 10. This level of sensitivity is up to the level required for automation of the method for the screening of libraries of compounds and for the setting of cutoff values.
The infectivity of the recombinant promastigotes of field isolates was not affected upon transfection with the luc expression construct and drug selection. However, transfectants of the reference Dd8 strain were found to be more infective than the wild type (Fig. 4A). No suitable explanation can be offered for this response. Again, a good correlation was observed between the number of amastigotes per 100 macrophage cell nuclei and luciferase activity (Fig. 4B).
The toxicities of both the first-line drugs (antimonials) and the second-line drugs (pentamidine and amphotericin B) as well as those of the newly introduced compound miltefosine were determined by using luc-expressing promastigotes of individual isolates. The inhibition of luminescence correlated well with the dose-dependent efficacies of the drugs (Table 1). Resistant isolates Ld39 and Ld03 exhibited significantly higher IC50 values of SbIII than the sensitive ones. Interestingly these isolates are much more sensitive to amphotericin B. Miltefosine and pentamidine are more or less equally toxic to all isolates.
We also compared the LUC assay with that of more conventional MTT assay for the evaluation of drug toxicity in HTS with Dd8 luc transfectants. The literature shows significant differences between the IC50s of the four antileishmanial agents tested in this study for promastigotes and those for intracellular amastigotes. The same pattern was observed in our experiments (Table 2). Pentavalent antimonial (sodium stibogluconate) inhibited promastigote growth at a very high concentration, i.e., mg/ml concentrations (Fig. 5A), while SbIII was highly toxic at a concentration 500 times less (Fig. 5B). Previous reports suggest that SbIII is the active form of SbV that is toxic to the promastigotes of different Leishmania species (32). The relatively nontoxic drug SbV is considered a prodrug that is converted to highly toxic SbIII either in macrophages (21) or near the site of action (33). In agreement with these findings, we showed, using the Dd8 transfectants, that intracellular amastigotes are much more susceptible to SbV (SAG), with the IC50 being almost ninefold less than that for the promastigotes (Table 2). Pentamidine and miltefosine exhibited higher IC50s for intracellular amastigotes than for promastigotes, while amphotericin B showed almost same the toxicity to both forms of the parasite (Table 2). In India, pentamidine is used as the second-line drug (25), while amphotericin B has evolved as a drug effective for the treatment of antimony- and pentamidine-resistant cases (20). Except SbIII, the IC50 values of the other three drugs determined by using the transfectants were almost comparable to the values obtained by the MTT assay and reported in literature (Table 2) (5). The LUC assay resulted in fourfold lower IC50 of SbIII compared to those obtained by the MTT assay. However, this value is equivalent to the IC50 obtained by the microscopic assay (Table 3). This clearly demonstrates the higher sensitivity of the LUC assay than the MTT assay. Miltefosine is the first oral antileishmanial drug recently licensed in India and is undergoing phase IV trials (18). Further, using the luciferase assay, we could determine the IC50 value for SAG, which otherwise could be determined only by either parasite counting or the hazardous [3H]thymidine incorporation method (1).
The introduction of the luc marker into field strains nonresponsive to the SAG treatment generated an in vitro model (Table 3) for the screening of compounds active against SAG-resistant parasites. The resistance index for isolates determined by the LUC assay was in substantial agreement with the values obtained by Giemsa staining and microscopic analysis. These cell lines can be used for evaluation of the activities of compounds against strains refractory to SAG treatment.
Thus, we established a simple, rapid, reliable, quantitative screening method for antileishmanial compounds using luciferase-expressing clinical isolates of Leishmania donovani. The assay is much faster than other reporter gene assays. The measurement of luciferase activity is straightforward and basically requires nothing but the Steady Glo reagent and a luminometer. The luc-expressing recombinant L. donovani cell lines are now in routine use at the Central Drug Research Institute for the evaluation of large numbers of compounds for their antileishmanial activities (10). The method has been automated in a 96-well plate format. The infectivity of a transgenic cell line prompted us to monitor infection within macrophages and develop a semi-in vivo amastigote-macrophage method to study the effects of compounds on intracellular amastigotes in the HTS mode. Further, luc-expressing resistant L. donovani cell lines provide a useful tool for the screening of compounds with activities against this resistant organism model. These cell lines can also be used to study the mechanism of drug resistance and to screen for clones in functional complementation studies.
Acknowledgments
This work was supported by an in-house grant from the Central Drug Research Institute. We gratefully acknowledge CSIR for financial support to Ashutosh and Ramesh.
We thank A. Kausar for computational help.
Footnotes
This paper is Central Drug Research Institute communication number 6796.
REFERENCES
- 1.Al-Abdely, H. M., J. R. Graybill, R. Bocanegra, L. Najvar, E. Montalbo, S. Regen, and P. C. Melby. 1998. Efficacies of KY 62 against Leishmania amazonensis and Leishmania donovani in experimental murine cutaneous leishmaniasis and visceral leishmaniasis. Antimicrob. Agent Chemother. 42:2542-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashford, R., P. Desjeux, and P. deRaadt. 1992. Estimation of population at risk of infection and number of cases of leishmaniasis. Parasitol. Today 8:104-105. [DOI] [PubMed] [Google Scholar]
- 3.Avlonitis, N., E. Lekka, A. Detsi, M. Koufaki, T. Calogerpoulou, E. Scoulica, E. Siapi, I. Kyrikou, T. Mavromoustakos, A. Tsotinis, S. G. Grdadolnik, and A. Makriyannis. 2003. Antileishmanial ring substituted ether phospholipids. J. Med. Chem. 46:755-767. [DOI] [PubMed] [Google Scholar]
- 4.Berman, J. 1998. Chemotherapy of leishmaniasis: recent advances in the treatment of visceral diseases. Curr. Opin. Infect. Dis. 11:707-710. [PubMed] [Google Scholar]
- 5.Berman, J. D., and J. V. Gallaee. 1985. Semiautomated assessment of in vitro activity of potential antileishmanial drugs. Antimicrob. Agents Chemother. 28:713-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bodley, A. L., M. W. Mcgarry, and T. A. Shapiro. 1995. Drug cytotoxicity assay for African trypanosomes and Leishmania species. J. Infect. Dis. 172:1157-1159. [DOI] [PubMed] [Google Scholar]
- 7.Brandonisio, D., and R. Spinelli. 2002. Immune response to parasitic infections—an introduction. Curr. Drug Target Immunol. Endocr. Metab. Disorder 2:193-199. [DOI] [PubMed] [Google Scholar]
- 8.Buckner, F. S., C. L. Verlinde, A. C. LaFlamme, and W. L. VonVoorhis. 1996. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasite expressing beta-galactosidase. Antimicrob. Agents Chemother. 40:2592-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrio, J., C. Riera, M. Gallego, and M. Portus. 2001. In vitro activity of pentavalent antimony derivatives on promastigotes and intracellular amastigotes of Leishmania infantum strains from humans and dogs in Spain. Acta Trop. 79:179-183. [DOI] [PubMed] [Google Scholar]
- 10.Chandra, N., Ramesh, Ashutosh, N. Goyal, S. N. Suryawanshi, and S. Gupta. 2005. Antileishmanial agents. Part IV. Synthesis and antileishmanial activity of novel terpenyl pyrimididines. Eur. J. Med. Chem. 40:552-556. [DOI] [PubMed]
- 11.Chulay, J. D., and A. D. M. Bryceson. 1983. Quantitation of amastigotes of Leishmania donovani in smears of spleenic aspirates from patients with visceral leishmaniasis. Am. J. Trop. Med. Hyg. 32:475-479. [DOI] [PubMed] [Google Scholar]
- 12.Collins, L. A., M. N. Torrero, and S. G. Fravizblau. 1998. Green fluorescence protein reporter microplate assay for high-throughput screening of compounds against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 42:344-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Croft, S. L. 1986. In vitro screens in experimental chemotherapy of leishmaniasis and trypanosomiasis. Parasitol. Today 2:64-69. [DOI] [PubMed] [Google Scholar]
- 14.Debrabant, A., E. Ghedin, and D. M. Dwyer. 2000. Dissection of functional domains of the Leishmania surface membrane 3′-nucleotidase/nuclease, a unique member of class I nuclease family. J. Biol. Chem. 275:16366-16372. [DOI] [PubMed] [Google Scholar]
- 15.Debrabant, A., M. Gottilieb, and D. M. Dwyer. 1995. Isolation and characterization of the gene encoding the surface membrane 3′ nucleotidase/nuclease of Leishmania donovani. Mol. Biochem. Parasitol. 71:51-63. [DOI] [PubMed] [Google Scholar]
- 16.Dumas, C., M. Ouellette, J. Tovar, et al. 1997. Distruption of trypanothione reductase gene of Leishmania decreases its ability to survive oxidative stress in macrophages. EMBO J. 16:2590-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faraut-Gambbarelli, F., R. Pioroux, M. Deniau, B. Giusiano, G. Marty, B. Faugere, and H. Dumon. 1997. In vitro resistance of Leihmania infantum to meglumine antimoniate: a study of 37 strains collected from patients with visceral leishmaniasis. Antimicrob. Agents Chemother. 41:827-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganguly, N. K. 2002. Oral miltefosine may revolutionize treatment of visceral leishmaniasis. TDR News 68:2. [Google Scholar]
- 19.Gay, L. S., M. E. Wilson, and J. E. Donelson. 1996. The promoter for ribosomal RNA genes of Leishmania chagasi. Mol. Biochem. Parasitol. 77:1993-2000. [DOI] [PubMed] [Google Scholar]
- 20.Giri, O. P. 1994. Treatment of visceral leishmaniasis unresponsive to pentamidine with amphotericin B. J. Assoc. Physician India 42:688-689. [PubMed] [Google Scholar]
- 21.Goodwin, L. G. 1995. Pentostam (sodium stibogluconate); a 50 year personal reminiscence. Trans. R. Soc. Trop. Med. 89:339-341. [DOI] [PubMed] [Google Scholar]
- 22.Gould, S. J., and S. Subramani. 1988. Firefly luciferase as a tool in molecular and cell biology. Anal. Biochem. 175:5-13. [DOI] [PubMed] [Google Scholar]
- 23.Ha, D. S., J. K. Schwarz, S. J. Turca, and S. M. Beverly. 1996. Use of green fluorescence protein as a marker in the transfected Leishmania. Mol. Biochem. Parasitol. 77:57-64. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs, W. R., R. G. Barletta, Jr., R. Udani, J. Chan, G. Kalkut, T. Kieser, G. T. Sarkis, G. F. Hatfull, and B. R. Bloom. 1993. Rapid assessment of drug susceptibility of Mycobacterium tuberculosis by means of luciferase reporter phages. Science 260:819-822. [DOI] [PubMed] [Google Scholar]
- 25.Jha, T. K. 1983. Evaluation of diamidine compounds (pentamidine isethionate) in the treatment of resistant cases of kala-azar occurring in north Bihar, India. Trans. R. Soc. Trop. Med. Hyg. 77:167-170. [DOI] [PubMed] [Google Scholar]
- 26.Lira, R., S. Sunder, A. Makharia, R. Kenney, A. Gam, E. Saraiva, and D. Sack. 1999. Evidence that incidence of treatment failure in Indian kala-azar is due to the emergence of antimony resistant strains of Leishmania donovani. J. Infect. Dis. 180:564-567. [DOI] [PubMed] [Google Scholar]
- 27.McFadden, D. C., F. Seeber, and J. C. Boothroyd. 1997. Use of Toxoplasma gondii expressing beta-galactosidase for colorimeteric assessment of drug activity in vitro. Antimicrob. Agents Chemother. 41:1849-1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mikus, J., and D. Steverding. 2000. A simple method to screen drug cytotoxicity against Leishmania using dye Almar blue. Parasitol. Int. 48:265-269. [DOI] [PubMed] [Google Scholar]
- 29.Mislitz, A., J. C. Mottram, P. Overath, and T. Aebischer. 2000. Targeted integration into a rRNA locus results in uniform and high level expression of transgenes in Leishmania amastigotes. Mol. Biochem. Parasitol. 107:251-261. [DOI] [PubMed] [Google Scholar]
- 30.Molyneux, D., and R. Killick-Kendrick. 1987. Morphology, ultra-structure and life cycles, p. 121-176. In W. Peters and R. Killick-Kendrik (ed.), The leishmaniasis in biology and medicine, vol. 1. Academic Press, Inc., London, England. [Google Scholar]
- 31.Plock, A., W. Sokolowska-Kohler, and W. Presber. 2001. Application of flow-cytometry and microscopical method to characterize effect of herbal drugs on Leishmania spp. Exp. Parasitol. 97:141-153. [DOI] [PubMed] [Google Scholar]
- 32.Robert, W. L., J. D. Berman, and P. M. Rainey. 1995. In vitro antileishmanial properties of tri- and pentavalent antimonial preparations. Antimicrob. Agents Chemother. 39:1234-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robert, W. L., and P. M. Rainey. 1993. Antimony quantification in Leishmania by electrothermal atomic absorption spectroscopy. Anal. Biochem. 217:1-6. [DOI] [PubMed] [Google Scholar]
- 34.Roy, G., C. Dumas, D. Sereno, Y. Wu, A. K. Singh, M. J. Tremblay, M. Ouellette, M. Olivier, and B. Papadopoulou. 2000. Episomal and stable expression of luciferase reporter gene for quantifying Leishmania spp. infection in macrophages and animal models. Mol. Biochem. Parasitol. 110:195-206. [DOI] [PubMed] [Google Scholar]
- 35.Sereno, D., G. Roy, J. L. Lemesre, B. Papadopoulou, and M. Oullette. 2001. DNA transformation of Leishmania infantum axenic amastigotes and their use in drug screening. Antimicrob. Agents Chemother. 45:1168-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.TDR News. 2005. Applying genomics to drug discovery research. 74:2.
- 37.Zhang, W. W., H. Charest, E. Ghedin, and G. Matlashewski. 1996. Identification and overexpression of A2 amastigote specific protein in Leishmania donovai. Mol. Biochem. Parasitol. 78:79-90. [DOI] [PubMed] [Google Scholar]