Abstract
Coumarin-resistant mutants of Staphylococcus aureus were isolated by three-step selection with novobiocin at different concentrations. Sequencing analysis of the gyrB and parE genes of the first-, second-, and third-step mutants revealed that successive point mutations first occurred specifically in the gyrB gene, followed by a point mutation in the parE gene and then an additional point mutation in the gyrB gene. These findings demonstrate that DNA gyrase is the primary target and that topoisomerase IV is the secondary target for novobiocin and that the accumulation of point mutations in both the gyrB and the parE genes is associated with high-level resistance to novobiocin in S. aureus. Moreover, our results show that the amino acid substitutions (Asp-89 to Gly and Ser-128 to Leu) found in GyrB are associated with resistance to novobiocin but not to coumermycin A1, suggesting that the interactions of novobiocin and coumermycin A1 with GyrB differ at the molecular level.
DNA gyrase and topoisomerase IV (topo IV) are essential bacterial type II topoisomerases that play important roles in DNA replication, chromosome segregation and DNA compaction (14, 15). DNA gyrase, an A2B2 heterotetramer, introduces negative supercoils in DNA by stabilizing double-stranded DNA break and using ATP hydrolysis to pass another portion of DNA through this break (25). Topo IV, on the other hand, is a C2E2 tetramer (6) involved in decatenation of daughter chromosomes following DNA replication (31). As GyrA and GyrB of DNA gyrase share extensive sequence homology with ParC and ParE, respectively, of topo IV, both topoisomerases are targets of quinolones and coumarin antibacterial agents, such as novobiocin and coumermycin A1. The coumarins inhibit the ATPase activity of GyrB by competing with ATP for binding to the GyrB of DNA gyrase (16). On the other hand, the quinolones, which were first shown to inhibit DNA gyrase (23) and which were subsequently demonstrated to inhibit topo IV (21), form a ternary complex with topoisomerase in the presence of DNA, resulting in lethal double-stranded DNA breaks (11).
The mechanism of resistance to quinolones, especially alteration of target enzymes, has long been studied. Point mutations involved in quinolone resistance have been shown to occur in defined regions of GyrA and GyrB, termed quinolone resistance-determining regions (29, 30), and those in the parC and the parE genes of topo IV have been reported to take place in similar regions (4, 10). For instance, in Escherichia coli, a strain must have a mutation rendering DNA gyrase resistant in order to acquire a subsequent mutation in topo IV genes, since the primary target of quinolones in E. coli is DNA gyrase (10). Interestingly, it has been demonstrated that in some gram-positive organisms, the hierarchy is reversed and topo IV is the primary target (5, 20). Other studies have shown that the primary target (DNA gyrase or topo IV) depends on the type of quinolone used (1, 24). Although the primary target is different among microorganisms, accumulation of alterations in DNA gyrase and topo IV confers high-level resistance to the quinolones (5, 10). On the other hand, the coumarins have long been known to inhibit DNA gyrase (8) and have also been shown to inhibit, at higher concentrations than those required for DNA gyrase inhibition, the activity of purified topo IV (21). Moreover, it has been reported that only gyrB mutations confer resistance to the coumarins and that all mutations reported occur at the periphery of the GyrB ATP-binding cleft (7, 17, 18, 22). Although recent studies have indicated that an amino acid substitution in the topo IV ParE subunit of E. coli confers resistance to novobiocin in vitro (9), no report has shown novobiocin- or coumermycin A1-resistant mutants with point mutations in the parE gene. Thus, detailed information on the mechanism of coumarin resistance is still lacking, and the availability of this information might help with the development of potent DNA gyrase inhibitors that are effective against quinolone-resistant strains with mutations in DNA gyrase genes.
In order to understand better the mechanism of acquisition of high-level coumarin resistance in Staphylococcus aureus, spontaneous novobiocin-resistant mutants of S. aureus were isolated by three-step selection and were characterized in this study.
MATERIALS AND METHODS
Materials and bacterial strains.
Novobiocin and coumermycin A1 were purchased from Sigma Chemical Co. (St. Louis, Mo.). Sparfloxacin and norfloxacin were synthesized at our Chemistry Research Laboratories. Other reagents were purchased from Nacalai Tesque (Kyoto, Japan), unless otherwise indicated. S. aureus RN4220 was kindly provided by R. P. Novick. E. coli KL16 was obtained from B. Bachmann.
Preparation of genomic DNA.
S. aureus genomic DNA was prepared as described by Hudson and Curtiss (12), except that 100 μg/ml mutanolysin was replaced by 50 μg/ml lysostaphin.
Drug susceptibility test.
MICs were determined by the twofold agar dilution method recommended by CLSI (formerly NCCLS) (19).
Isolation of spontaneous coumarin-resistant mutants of S. aureus RN4220.
For isolation of the first-step mutants, a portion of the overnight culture of S. aureus RN4220 (approximately 1010 CFU) was spread onto tryptic soy agar containing novobiocin at one to four times the MIC and incubated at 35°C for 48 h. For the second-step and third-step mutants, the first-step and second-step mutants were incubated on agar containing novobiocin (at one to two times the MIC) at 35°C for 48 h and 72 h, respectively.
PCR amplification and DNA sequence analysis.
Genes corresponding to the amino-terminal 43-kDa regions (ATP-binding domain) of GyrB and ParE subunits from S. aureus RN4220 and novobiocin-resistant S. aureus mutants were amplified by PCR with the following primers: the gyrB forward primer (5′-GGGTGACTGCATTGTCAGATGTA-3′; the 5′ nucleotide is at position 184 [the amino acid is at position 2] in the sequence published by Ito et al. [13]), the gyrB reverse primer (5′-GGACGTGTTACTTCACGCGCTTTTTT-3′; the 5′ nucleotide is at position 1392 [the amino acid is at position 404]), the parE forward primer (5′-GGAATAAACAAAATAATTATTCAGATGAT-3′; the 5′ nucleotide is at position 388 [the amino acid is at position 2] in the sequence published by Yamagishi et al. [27]), and the parE reverse primer (5′-GGACGAGCATCTTCACGAGCTTTAC-3′; the 5′ nucleotide is at position 1575 [the amino acid is at position 397]). PCR was performed with a GeneAmp PCR System 9600 with TAKARA Ex Taq polymerase (Takara Shuzo, Kyoto, Japan). The reactions were repeated for 30 cycles (15 s at 94°C for denaturation, 30 s at 60°C for annealing, and 1 min at 72°C for polymerization). PCR-amplified fragments were sequenced by the cycle sequencing method with an ABI Prism Big Dye terminator, version 3.1, cycle sequencing kit and then applied on a Genetic Analyzer 3100 (Applied Biosystems, Foster, Calif.).
Enzyme assays.
S. aureus DNA gyrase supercoiling assays and topo IV decatenation assays were carried out by the method of Blanche et al. (3).
RESULTS AND DISCUSSION
First-step mutants.
About 5,400 first-step mutants were isolated from S. aureus RN4220 at a frequency of about 10−7 by selection with novobiocin (at one to four times the MIC). The MICs of novobiocin and coumermycin A1 for 579 randomly selected isolates of the first-step mutants were determined, and the isolates tested were classified into seven groups according to their resistance patterns (Fig. 1; Table 1). Since the susceptibilities of the first-step mutants tested to other types of antibiotics, including levofloxacin, ampicillin, tetracycline, gentamicin, and erythromycin, did not change (data not shown), mutations occurred only in genes of the target enzyme of novobiocin, such as the gyrB gene. Hence, we sequenced the regions of the gyrB genes corresponding to the ATP-binding cleft (28) amplified from 1 to 10 strains of the mutants in each group. The regions of the parE genes were also sequenced to identify mutations in the parE gene, if there were any. The mutants in each group had mutations only in gyrB. Mutants with the same mutations always had the same resistance profiles. For reasons of clarity, only one strain with each type of mutation is shown in Table 1. First-step mutants in groups 1 to 5 had 8- to 32-fold decreased susceptibilities to novobiocin and single mutations in their gyrB genes: group 1, Ser-128 (TCA) to Leu (TTA); group 2, Thr-173 (ACT) to Ala (GCT); group 3, Ile-102 (ATT) to Ser (AGT); group 4, Arg-144 (AGA) to Ser (AGT) or Gly-85 (GGT) to Ser (AGT); and group 5, Arg-144 (AGA) to Ile (ATA). All first-step novobiocin-resistant mutants except those in group 1 were also less susceptible to different levels of coumermycin A1 (4- to 128-fold increased MICs compared with that for the parent strain). Strains of groups 6 and 7, which were resistant to higher levels of novobiocin and coumermycin A1, had two amino acid substitutions in GyrB: group 6, Ile-56 (ATC) to Ser (AGC) and Arg-144 (AGA) to Ser (AGT); group 7, Ile-102 (ATT) to Ser (AGT) and Arg-144 (AGA) to Ser (AGT). The amino acid changes Gly-85 to Ser, Ile-102 to Ser, Ser-128 to Leu and Arg-144 to Ile have been reported previously (7, 22); and the amino acid substitutions Arg-144 to Ser and Thr-173 to Ala are analogous to mutated residues found in previously characterized resistance-associated gyrB genes from E. coli and Bartonella bacilliformis, respectively (2, 17). It has also been demonstrated that two amino acid substitutions in GyrB confer higher levels of resistance to coumarins (7, 22). Thus, our results for the first-step mutants are in agreement with those of previous reports. In group 6, we found an amino acid substitution of Ile-56 to Ser in GyrB. Ile-56 in S. aureus is equivalent to Ile-48 in E. coli and is located in the ATP-binding cleft. These findings strongly suggest that the amino acid substitution of Ile-56 to Ser is involved in coumarin resistance. In group 7, strain N276 had double amino acid substitutions, Ile-102 to Ser and Arg-144 to Ser, in GyrB. The MICs of coumarins for strains carrying the individual mutations Ile-102 to Ser (N295) and the Arg-144 to Ser (N175) were 8 and 16 μg/ml of novobiocin and 0.032 and 0.5 μg/ml of coumermycin A1, respectively. The MICs of novobiocin and coumermycin A1 for N276 were 32 and 16 μg/ml, respectively, and compared to the parent strain, N276 had 64- and 2,048-fold decreased susceptibilities to novobiocin and coumermycin A1, respectively. We cannot explain the difference in the susceptibility changes. However, the results might indicate that the double amino acid substitutions Ile-102 to Ser and Arg-144 to Ser make the ATP-binding cleft narrowed and the bulkier molecule, coumermycin A1, enters the cleft harder than the smaller molecule, novobiocin.
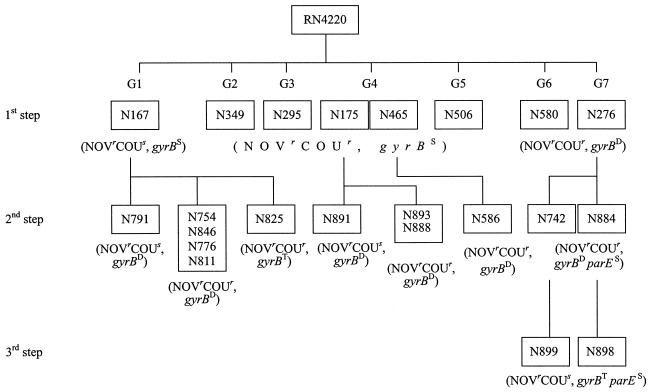
FIG. 1.
Relationships between S. aureus RN4220 and its resistant mutants isolated by stepwise exposure to novobiocin and between phenotype and genotype in first-, second-, and third-step mutants. G1, group 1; G2, group 2; G3, group 3; G4, group 4; G5, group 5; G6, group 6; G7, group 7. NOVs and COUs, mutant susceptibility to NOV and COU, respectively, did not change compared to that of the parent strain; NOVr and COUr, mutant susceptibility to NOV and COU, respectively, decreased compared to that of the parent strain; NOV, novobiocin; COU, coumermycin A1; superscript S, D, or T on gyrB and parE, the gene had a single, a double, or a triple point mutation, respectively.
TABLE 1.
Coumarin resistance mutations in gyrB gene in first-step mutants
| Group | No. of strains | Representative isolate | MIC (μg/ml)a
|
Mutation in:
|
||||
|---|---|---|---|---|---|---|---|---|
| NOV | COU | SPX | NOR | gyrB | parE | |||
| RN4220b | 0.5 | 0.008 | 0.125 | 2 | ||||
| 1 | 220 | N167 | 4 | 0.008 | 0.125 | 2 | S128 (TCA)→L (TTA) | None |
| 2 | 7 | N349 | 4 | 0.125 | 0.125 | 2 | T173 (ACT)→A (GCT) | None |
| 3 | 1 | N295 | 8 | 0.032 | 0.125 | 2 | I102 (ATT)→S (AGT) | None |
| 4 | 331 | N175 | 16 | 0.5 | 0.125 | 2 | R144 (AGA)→S (AGT) | None |
| N465 | 16 | 0.5 | 0.125 | 2 | G85 (GGT)→S (AGT) | None | ||
| 5 | 18 | N506 | 16 | 1 | 0.125 | 2 | R144 (AGA)→I (ATA) | None |
| 6 | 1 | N580 | 32 | 1 | 0.125 | 2 | I56 (ATC)→S (AGC)c | None |
| R144 (AGA)→S (AGT) | ||||||||
| 7 | 1 | N276 | 32 | 16 | 0.125 | 2 | I102 (ATT)→S (AGT) | None |
| R144 (AGA)→S (AGT) | ||||||||
NOV, novobiocin; COU, coumermycin A1; SPX, sparfloxacin; NOR, norfloxacin.
Parent strain.
Mutation not previously reported.
Second-step mutants.
In order to elucidate the mechanism of acquisition of higher levels of resistance to coumarins, second-step mutants were isolated from the first-step mutants (N167, N175, N276, and N465) by selection with novobiocin (at one to two times the MIC) and characterized. Strains N167, N175, and N465 (groups 1 and 4) were the most frequently isolated; and strain N276 had two amino acid substitutions in GyrB. From first-step mutants N167, N175, N276, and N465, 116, 15, 26, and 2 second-step mutants were isolated, respectively. The MICs of novobiocin and coumermycin A1 for all second-step mutants were determined, and the second-step mutants were classified according to their resistance patterns: N167 derivatives, six resistance patterns; N175 derivatives, three resistance patterns; N276 derivatives, two resistance patterns; N465 derivatives, one resistance pattern (Fig. 1). The regions of the ATP-binding cleft of both the gyrB and the parE genes of one to three strains of the second-step mutants in each group were analyzed. Since the mutants with the same resistance pattern had the same mutation(s) in the gyrB and/or the parE gene, the results for representative second-step strains with each type of mutation are shown in Table 2. Compared with the parent strains, all second-step mutants were 2 to 16 times more resistant to novobiocin. In addition, all second-step mutants except N791, N891, and N586 were 4 to 256 times more resistant to coumermycin A1. Without exception, additional one or two point mutations were found in the gyrB and the parE genes of the second-step mutants. The mutations in parE gene were only seen in mutants that had two gyrB mutations. The amino acid substitution in GyrB (Asp-89 [GAT] to Gly [GGT]) found in N791, N891, and N586 was associated with higher levels of resistance to novobiocin but not to coumermycin A1, suggesting that the Asp-89 to Gly substitution and the Ser-128 to Leu substitution found in first-step mutants are not involved in coumermycin A1 resistance. These results demonstrate the molecular-level difference in the modes of action between novobiocin and coumermycin A1; i.e., Asp-89 and Ser-128 in GyrB could interact with novobiocin but not with coumermycin A1. In E. coli, the coumarin ring in both novobiocin and coumermycin A1 forms hydrogen bonds with Gly-77 and Arg-136 in GyrB (equivalent to Gly-85 and Arg-144, respectively, in S. aureus) (16). Therefore, in S. aureus, it is suggested that mutations in these residues direct removal of the hydrogen-bonded interactions associated with resistance to novobiocin and coumermycin A1. Alternatively, since the 3′-isopentenyl-4′-hydroxybenzoate moiety and the 3′-carbamoyl group of novobiocin have specific interactions with residues Asp-89 and Ser-128, respectively, substitutions of these residues can be associated with resistance to novobiocin but not to coumermycin A1. Interestingly, the mutants isolated from N276, N742, and N884, which had two amino acid substitutions in the GyrB, had additional amino acid changes (Gly-78 [GGT] to Ser [AGT] and Arg-136 [CGA] to Gly [GGA], respectively) in ParE. Alignment of GyrB and ParE revealed that Gly-78 and Arg-136 in ParE were equivalent to Gly-85 and Arg-144 in GyrB, respectively, and were in the region of ParE that corresponds to the ATP-binding domain of GyrB (Fig. 2). Moreover, the amino acid changes in GyrB, i.e., Gly-85 to Ser and Arg-144 to Ile, were associated with resistance to the coumarins. These findings strongly suggest that the amino acid residues Gly-78 and Arg-136 in ParE interact with the coumarins and are involved in their resistance and that the mechanism of inhibition of topo IV by the coumarins is the same as that of inhibition of DNA gyrase, namely, blocking of ATP binding.
TABLE 2.
Additional mutations in gyrB and parE genes found in second- and third-step mutants
| Strain (mutation) | MIC (μg/ml)a
|
Additional mutation in:
|
||||
|---|---|---|---|---|---|---|
| NOV | COU | SPX | NOR | gyrB | parE | |
| Second-step mutants | ||||||
| N167 (S128L in GyrB) | 4 | 0.008 | 0.125 | 2 | ||
| N167-derived mutants | ||||||
| N791 (9)c | 32 | 0.008 | 0.125 | 2 | D89 (GAT)→G (GGT)b | None |
| N754 (51) | 16 | 0.25 | 0.125 | 2 | T173 (ACT)→A (GCT) | None |
| N846 (1) | 32 | 0.063 | 0.125 | 2 | I102 (ATT)→S (AGT) | None |
| N776 (51) | 32 | 0.5 | 0.125 | 2 | G85 (GGT)→S (AGT) | None |
| N811 (3) | 32 | 2 | 0.125 | 1 | R144 (AGA)→I (ATA) | None |
| N825 (1) | 64 | 0.5 | 0.125 | 1 | D89 (GAT)→G (GGT)b | None |
| T173 (ACT)→A (GCT) | ||||||
| N175 (R144S in GyrB) | 16 | 0.5 | 0.125 | 2 | ||
| N175-derived mutant | ||||||
| N891 (9) | 64 | 0.5 | 0.125 | 1 | D89 (GAT)→G (GGT)b | None |
| N893 (3) | 32 | 2 | 0.125 | 2 | I102 (ATT)→T (ACT) | None |
| N888 (3) | 32 | 16 | 0.125 | 2 | I102 (ATT)→S (AGT) | None |
| N465 (G85S in GyrB) | 16 | 0.5 | 0.125 | 2 | ||
| N465-derived mutant | ||||||
| N586 (2) | 64 | 1 | 0.125 | 1 | D89 (GAT)→G (GGT)b | None |
| N276 (I102S and R144S in GyrB) | 32 | 16 | 0.125 | 2 | ||
| N276-derived mutant | ||||||
| N742 (1) | 128 | 64 | 0.125 | 2 | None | G78 (GGT)→S (AGT)b |
| N884 (25) | 128 | 64 | 0.125 | 2 | None | R136 (CGA)→G (GGA)b |
| Third-step mutants | ||||||
| N742 (I102S and R144S in GyrB, G78S in ParE) | 128 | 64 | 0.125 | 2 | ||
| N742-derived mutant | ||||||
| N899 (1) | 512 | 64 | 0.125 | 2 | D89 (GAT)→G (GGT)b | None |
| N884 (I102S and R144S in GyrB, R136G in ParE) | 128 | 64 | 0.125 | 2 | ||
| N884-derived mutant | ||||||
| N898 (1) | 256 | 64 | 0.125 | 2 | D89 (GAT)→G (GGT)b | None |
NOV, novobiocin; COU, coumermycin A1; SPX, sparfloxacin; NOR, norfloxacin.
Mutations not previously reported.
The number of isolates that had the same resistance pattern is shown in parentheses.
FIG. 2.
Alignment of GyrB and ParE N-terminal amino acid sequences. Triangles, residues from E. coli GyrB region involved in ATP binding (26); asterisks and dots under the amino acid sequences, identical and similar residues in all four proteins, respectively; dashes, gaps introduced to maximize similarities; boxes, amino acid sequences that underwent changes in the novobiocin-resistant mutants; Ec, E. coli; Sa, S. aureus.
Third-step mutants.
Next, to examine additional amino acid changes in GyrB or ParE, we isolated much higher-level coumarin-resistant mutants from the second-step mutants that already had amino acid changes in ParE (N742 and N884). Only one third-step mutant was isolated from each second-step mutant. The third-step mutants, N899 and N898, derived from N742 and N884, respectively, were two to four times more resistant to novobiocin but not to coumermycin A1 than the parent strains. In these mutants, analysis of the gyrB and the parE genes demonstrated that an additional amino acid change (Asp-89 [GAT] to Gly [GGT]) occurred in GyrB (Table 2).
Characterization of the first-, second-, and third-step mutants revealed that the mechanism of acquisition of high-level novobiocin resistance in S. aureus is as follows: (i) first, successive point mutations specifically occurred in gyrB; (ii) next, a point mutation occurred in parE; (iii) finally, a point mutation occurred in gyrB again (Fig. 1). Therefore, the accumulation of mutations in both the gyrB and the parE genes is associated with high-level resistance to novobiocin.
The concentrations of the compounds that inhibited 50% of the enzymatic activity of novobiocin (0.2 μg/ml) and coumermycin A1 (0.05 μg/ml) for S. aureus DNA gyrase were 125 to 32 times lower than those for S. aureus topo IV (25 μg/ml for novobiocin and 1.56 μg/ml for coumermycin A1), suggesting that the coumarins interact primarily with DNA gyrase. Furthermore, Hardy and Cozzarelli (9) reported that in E. coli, a mutation in the parE gene homologous to a commonly mutated residue in gyrB alleles confers novobiocin resistance. Although these in vitro studies suggest that topo IV is a secondary target for coumarins, our results directly indicate that, in vivo, DNA gyrase is the primary target and topo IV is the secondary target for the coumarins. It would be interesting to determine whether the priority of target is reserved for other bacteria, as is the case with quinolones. Hardy and Cozzarelli (9) also tried, without success, to isolate a high-level novobiocin-resistant parE mutant by plating an E. coli strain on increased levels of novobiocin. The reason for this is unclear.
It is necessary to develop new antibacterial agents that are also active against existing antibiotic-resistant strains of bacteria. DNA gyrase is a clinically validated target, and coumarins are also potent against quinolone-resistant strains. Hence, several pharmaceutical companies have been researching a GyrB inhibitor. Because ParE is the secondary target for coumarins and the first-step mutants that have mutations only in GyrB are commonly isolated, a compound that inhibits both GyrB and ParE at the same level should be explored. It is expected that the emergence of resistant mutants is rare when the compound is used clinically.
Acknowledgments
We thank Hiroaki Yoshida for critical reading of the manuscript and useful discussion.
REFERENCES
- 1.Alovero, F. L., X.-S. Pan, J. E. Morris, R. H. Manzo, and L. M. Fisher. 2000. Engineering the specificity of antibacterial fluoroquinolones: benzenesulfonamide modifications at C-7 of ciprofloxacin change its primary target in Streptococcus pneumoniae from topoisomarase IV to gyrase. Antimicrob. Agents Chemother. 44:320-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Battisti, J. M., L. S. Smitherman, D. S. Samuels, and M. F. Minnick. 1998. Mutations in Bartonella bacilliformis gyrB confer resistance to coumermycin A1. Antimicrob. Agents Chemother. 42:2906-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanche, F., B. Cameron, F.-X. Bernard, L. Maton, B. Manse, L. Ferrero, N. Ratet, C. Lecoq, A. Goniot, D. Bisch, and J. Crouzet. 1996. Differential behaviors of Staphylococcus aureus and Escherichia coli type II topoisomerases. Antimicrob. Agents Chemother. 40:2714-2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breines, D. M., S. Ouabdesselam, E. Y. Ng, J. Tankovic, S. Shah, C. J. Soussy, and D. C. Hooper. 1997. Quinolone resistance locus nfxD of Escherichia coli is a mutant allele of the parE gene encoding a subunit of topoisomerase IV. Antimicrob. Agents Chemother. 41:175-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrero, L., B. Cameron, and J. Crouzet. 1995. Analysis of gyrA and grlA mutations in stepwise-selected ciprofloxacin-resistant mutants of Staphylococcus aureus. Antimicrob. Agents Chemother. 39:1554-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrero, L., B. Cameron, B. Manse, D. Lagneaux, J. Crouzet, A. Famechon, and F. Blanche. 1994. Cloning and primary structure of Staphylococcus aureus DNA topoisomerase IV: a primary target of fluoroquinolones. Mol. Microbiol. 13:641-653. [DOI] [PubMed] [Google Scholar]
- 7.Fournier, B., and D. C. Hooper. 1998. Mutations in topoisomerase IV and DNA gyrase of Staphylococcus aureus: novel pleiotropic effects on quinolone and coumarin activity. Antimicrob. Agents Chemother. 42:121-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gellert, M., M. H. O'Dea, T. Itoh, and J. Tomizawa. 1976. Novobiocin and coumermycin inhibit DNA supercoiling catalyzed by DNA gyrase. Proc. Natl. Acad. Sci. USA 73:4474-4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardy, C. D., and N. R. Cozzarelli. 2003. Alteration of Escherichia coli topoisomerase IV to novobiocin resistance. Antimicrob. Agents Chemother. 47:941-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heisig, P. 1996. Genetic evidence for a role of parC mutations in development of high-level fluoroquinolone resistance in Escherichia coli. Antimicrob. Agents Chemother. 40:879-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiasa, H., D. O. Yousef, and K. J. Marians. 1996. DNA strand cleavage is required for replication fork arrest by a frozen topoisomerase-quinolone-DNA ternary complex. J. Biol. Chem. 271:26424-26429. [DOI] [PubMed] [Google Scholar]
- 12.Hudson, M. C., and R. Curtiss III. 1990. Regulation of expression of Streptococcus mutans genes important to virulence. Infect. Immun. 58:464-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito, H., H. Yoshida, M. B.-Shonai, T. Niga, H. Hattori, and S. Nakamura. 1994. Quinolone resistance mutations in the DNA gyrase gyrA and gyrB genes of Staphylococcus aureus. Antimicrob. Agents Chemother. 38:2014-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khodursky, A. B., B. J. Peter, M. B. Schmid, J. Derisi, D. Botstein, P. O. Brown, and N. R. Cozzarelli. 2000. Analysis of topoisomerase function in bacterial replication fork movement: use of DNA microarrays. Proc. Natl. Acad. Sci. USA 97:9419-9424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levine, C., H. Hiasa, and K. J. Marians. 1998. DNA gyrase and topoisomerase IV: biochemical activities, physiological roles during chromosome replication, and drug sensitivities. Biochim. Biophys. Acta 1400:29-43. [DOI] [PubMed] [Google Scholar]
- 16.Lewis, R. J., O. M. P. Singh, C. V. Smith, T. Skarzynski, A. Maxwell, A. J. Wonacott, and D. B. Wigley. 1996. The nature of inhibition of DNA gyrase by the coumarins and the cyclothialidines revealed by X-ray crystallography. EMBO J. 15:1412-1420. [PMC free article] [PubMed] [Google Scholar]
- 17.Maxwell, A. 1993. The interaction between coumarin drugs and DNA gyrase. Mol. Microbiol. 9:681-686. [DOI] [PubMed] [Google Scholar]
- 18.Muñoz, R., M. Bustamante, and A. G. de la Campa. 1995. Ser-127-to-Leu substitution in the DNA gyrase B subunit of Streptococcus pneumoniae is implicated in novobiocin resistance. J. Bacteriol. 177:4166-4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 20.Pan, X.-S., J. Ambler, S. Mehtar, and L. M. Fisher. 1996. Involvement of topoisomerase IV and DNA gyrase as ciprofloxacin targets in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 40:2321-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng, H., and K. J. Marians. 1993. Escherichia coli topoisomerase IV. Purification, characterization, subunit structure, and subunit interactions. J. Biol. Chem. 268:24481-24490. [PubMed] [Google Scholar]
- 22.Stieger, M., P. Angehrn, B. Wohlgensinger, and H. Gmünder. 1996. GyrB mutations in Staphylococcus aureus strains resistant to cyclothialidine, coumermycin, and novobiocin. Antimicrob. Agents Chemother. 40:1060-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugino, A., C. L. Peebles, K. N. Kreuzer, and N. R. Cozzarelli. 1977. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc. Natl. Acad. Sci. USA 74:4767-4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takei, M., H. Fukuda, R. Kishii, and M. Hosaka. 2001. Target preference of 15 quinolones against Staphylococcus aureus, based on antibacterial activities and target inhibition. Antimicrob. Agents Chemother. 45:3544-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang, J. C. 1996. DNA topoisomerases. Annu. Rev. Biochem. 65:635-692. [DOI] [PubMed] [Google Scholar]
- 26.Wigley, D. B., G. J. Davies, E. J. Dodson, A. Maxwell, and G. Dodson. 1991. Crystal structure of an N-terminal fragment of the DNA gyrase B protein. Nature 351:624-629. [DOI] [PubMed] [Google Scholar]
- 27.Yamagishi, J.-I., T. Kojima, Y. Oyamada, K. Fujimoto, H. Hattori, S. Nakamura, and M. Inoue. 1996. Alterations in the DNA topoisomerase IV grlA gene responsible for quinolone resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 40:1157-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamagishi, J.-I., H. Yoshida, M. Yamayoshi, and S. Nakamura. 1986. Nalidixic acid-resistant mutations of the gyrB gene of Escherichia coli. Mol. Gen. Genet. 204:367-373. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida, H., M. Bogaki, M. Nakamura, and S. Nakamura. 1990. Quinolone resistance-determining region in the DNA gyrase gyrA gene of Escherichia coli. Antimicrob. Agents Chemother. 34:1271-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshida, H., M. Bogaki, M. Nakamura, L. M. Yamanaka, and S. Nakamura. 1991. Quinolone resistance-determining region in the DNA gyrase gyrB gene of Escherichia coli. Antimicrob. Agents Chemother. 35:1647-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zechiedrich, E. L., and N. R. Cozzarelli. 1995. Roles of topoisomerase IV and DNA gyrase in DNA unlinking during replication in Escherichia coli. Genes Dev. 9:2859-2869. [DOI] [PubMed] [Google Scholar]