Acute hepatitis C virus (HCV) infection has been identified as an important cause of fulminant hepatic failure (FHF), characterized by rapid deterioration of liver function from massive hepatic necrosis leading to encephalopathy and multiorgan failure with a high mortality rate of 65 to 92% (2, 3). Alpha interferon-ribavirin is effective in treating acute and chronic HCV infections (4, 8). We report the details of successful antiviral therapy for a patient with HCV-associated FHF, accompanied by HCV eradication and prevention of chronicity.
The present study was approved by the ethics committee of Kaohsiung Municipal HsiaoKang Hospital. Informed consent was obtained from the patient's next of kin.
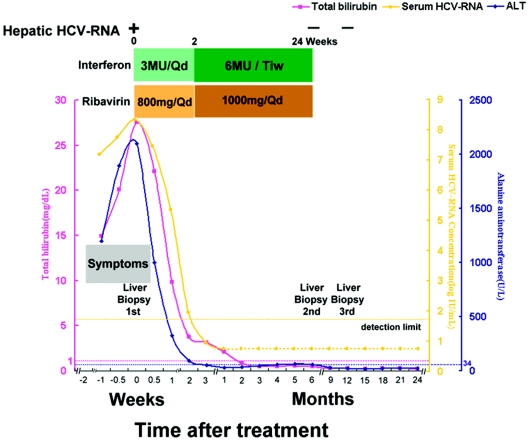
A 49-year-old Taiwanese woman presented in March 2002 with a 7-day history of anorexia and tea-colored urine. She had received an illegal percutaneous administration of folk remedies 2 months ago and had no experience with interferon-ribavirin previously. Rapid deterioration of liver function with coagulopathy preceding an irritable mood and mental disturbance developed at day 4. Seroconversion of antibodies to HCV with HCV genotype 1b infection was detected. She had serum levels of bilirubin of 14.95, 20.12, and 27.57 mg/dl at days 0, 3, and 7, respectively; alanine aminotransferase levels of 1,198, 1,896, and 2,100 U/liter, respectively; and HCV RNA levels of 7.22, 7.85, and 8.37 logs (IU/ml), respectively (Fig. 1). Based on the assessment of clinical and virologic measurements, HCV-associated FHF was diagnosed (2). Other conditions predisposing to fulminant hepatitis, including hepatitis A, B, and E viruses, cytomegalovirus, Epstein-Barr virus, and autoimmune or alcohol- or drug-related causes were excluded. Serologic markers of human immunodeficiency virus were negative.
FIG. 1.
Clinical course and biochemical and molecular profiles of a patient with fulminant hepatitis C treated with alpha interferon and ribavirin combination therapy. Serum HCV RNA was determined by using a quantitative branched DNA assay (VERSANT HCV RNA 3.0 assay; Bayer Diagnostics) and a qualitative PCR assay (COBAS AMPLICOR Hepatitis C Virus Test, version 2.0; Roche). The levels of serum HCV RNA are presented as logarithmic transformations of original values; the horizontal dotted lines indicate the upper limits of the normal ranges. A dotted line indicates PCR negativity for serum HCV RNA, and a solid line indicates PCR positivity. Qd, daily; Tiw, three times a week; ALT, alanine aminotransferase.
Because of the rapid hepatic decompensation and the low probability of an available liver donor, we conducted a treatment protocol at day 7 with recombinant alpha interferon 2b (3 × 106 U given intramuscularly daily) plus 800 mg of oral ribavirin daily for 2 weeks, followed by alpha interferon 2b (6 × 106 U thrice weekly) plus ribavirin (1,000 mg/day) for 22 weeks. Her psychoneurological and gastrointestinal manifestations were reversed, her liver biochemistry normalized, and seronegativity of HCV RNA developed 1 month later and was sustained throughout the 2-year follow-up period. Only mild and self-limited side effects, such as hemolytic anemia, were noted. Liver histopathology revealed acute submassive necrosis at 10 days after antiviral therapy, minimal necroinflammatory activity at the end of treatment, and complete remission to normal liver histology at 6 months after the end of treatment. Hepatic HCV RNA was undetectable in the latter two specimens.
Orthotopic liver transplantation, an established treatment option for FHF, is associated with a number of problems, including donor shortages, the risk of death while waiting for a transplant, postoperative mortality, universal HCV recurrence, and rapid progression of HCV-related disease in liver transplant recipients (1, 2). Our patient was characterized by continuous viral replication and high HCV RNA levels, and the extent of liver damage correlated with the magnitude of viral replication (3), implying that viral factors are important in the pathogenesis of HCV-related FHF. Effective antiviral therapy, which quickly lowers the viral loads (6), may rescue patients with HCV-associated FHF from rapid, extensive destruction of hepatocytes and provide an opportunity for adequate regeneration of native liver. However, alpha interferon is not recommended for patients with hepatitis B-related hepatic decompensation because of the risk of developing overt liver failure (5, 7). Physicians should be careful when treating FHF patients with interferon.
Acknowledgments
There are no conflicts of interest associated with this study.
REFERENCES
- 1.Berenguer, M. 2002. Natural history of recurrent hepatitis C. Liver Transplant. 8:S14-S18. [DOI] [PubMed] [Google Scholar]
- 2.Bernuau, J., and J. P. Benhamou. 1999. Fulminant and subfulminant liver failure, p. 1341-1374. In J. Bircher, J. P. Benhamou, N. McIntyre, M. Rizzetto, and J. Rodes (ed.), Oxford textbook of clinical hepatology, second ed., vol. 2. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 3.Farci, P., H. J. Alter, A. Shimoda, S. Govindarajan, L. C. Cheung, J. C. Melpolder, R. A. Sacher, J. W. Shih, and R. H. Purcell. 1996. Hepatitis C virus-associated fulminant hepatic failure. N. Engl. J. Med. 335:631-634. [DOI] [PubMed] [Google Scholar]
- 4.Jaeckel, E., M. Cornberg, H. Wedemeyer, T. Santantonio, J. Mayer, M. Zankel, G. Pastore, M. Dietrich, C. Trautwein, and M. P. Manns. 2001. Treatment of acute hepatitis C with interferon alfa-2b. N. Engl. J. Med. 345:1452-1457. [DOI] [PubMed] [Google Scholar]
- 5.Liaw, Y.-F., N. Leung, R. Guan, G. K. Lau, and I. Merican. 2003. Asian-Pacific consensus statement on the management of chronic hepatitis B: an update. J. Gastroenterol. Hepatol. 18:239-245. [DOI] [PubMed] [Google Scholar]
- 6.Neumann, A. U., N. P. Lam, H. Dahari, D. R. Gretch, T. E. Wiley, T. J. Layden, and A. S. Perelson. 1998. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science 282:103-107. [DOI] [PubMed] [Google Scholar]
- 7.Perrillo, R., C. Tamburro, F. Regenstein, L. Balart, H. Bodenheimer, M. Silva, E. Schiff, C. Bodicky, B. Miller, C. Denham, et al. 1995. Low-dose, titratable interferon alfa in decompensated liver disease caused by chronic infection with hepatitis B virus. Gastroenterology 109:908-916. [DOI] [PubMed] [Google Scholar]
- 8.Poynard, T., M. F. Yuen, V. Ratziu, and C. L. Lai. 2003. Viral hepatitis C. Lancet 362:2095-2100. [DOI] [PubMed] [Google Scholar]