Abstract
We evaluated the efficacy of azithromycin therapy given as a single high dose or divided over 5 days for the treatment of mild experimental Mycoplasma pneumoniae pneumonia. Although both azithromycin regimens significantly reduced quantitative cultures, lung histopathology, and pulmonary cytokines and chemokines, there were no significant differences between the two regimens.
It is unknown if antimycoplasmal therapy can be optimized utilizing high-dose azithromycin therapy. The purpose of the present study was to evaluate the microbiologic and immunologic efficacy of azithromycin therapy given as a single high-dose compared with divided doses over 5 days in our murine Mycoplasma pneumoniae pneumonia model (6, 7, 10). We compared the activities of these two regimens on the clearance of M. pneumoniae from the respiratory tract and assessed their impact on the pulmonary immune response as measured by bronchoalveolar lavage cytokines and chemokines and by histologic inflammation.
As previously described in full detail, 2-month-old female BALB/c mice (Charles River) were intranasally inoculated once (day 0) with 107 to 108 CFU of M. pneumoniae (ATCC 29342) in 50 μl SP4 broth (4, 7, 10). Methoxyflurane, an inhaled anesthetic, was used for inoculum sedation. Animal guidelines were followed in accordance with the Institutional Animal Care and Research Advisory Committee. Azithromycin (Pfizer) for injection was dissolved in sterile water as per container instructions. Azithromycin was given 24 h after M. pneumoniae inoculation and administered subcutaneously at 50 mg/kg of body weight for only 1 day (dosage, 1 mg in 0.1 ml per mouse) or 10 mg/kg once daily for 5 days (dosage, 0.2 mg in 0.1 ml per mouse). The placebo group received sterile water for 5 days starting at 24 h after inoculation. We used 10 mg/kg as the conventional azithromycin dosage in this study as it has been used in other mouse studies and has been shown to be comparable to an oral dose of 500 mg in humans (3, 9, 14). The mycoplasma inoculum was sent to the Diagnostic Mycoplasma Laboratory (Birmingham, AL) for susceptibility testing; the MIC for azithromycin was 0.000125 μg/ml. Bronchoalveolar lavage (BAL) quantitative mycoplasma culture, lung histopathologic score (HPS) determination, BAL cytokine and chemokine concentration determination by a mouse cytokine multiplex antibody bead kit (Biosource International, Luminex), and whole-body, unrestrained plethysmography (Buxco, Troy, NY) before and after aerosolized methacholine (50 mg/ml) exposure (baseline plethysmography = airway obstruction; postmethacholine plethysmography = airway hyperreactivity) were performed as previously described (4). The one-way analysis of variance test was used to compare outcome variables of different treatment regimens at the same time point, if the data were normally distributed. In instances where the data were not normally distributed, the analysis of variance on ranks test was used for comparisons.
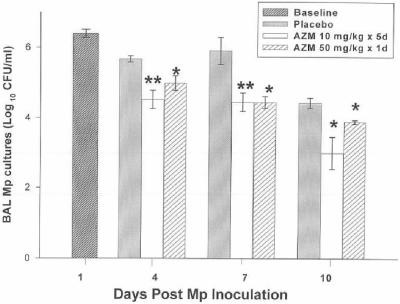
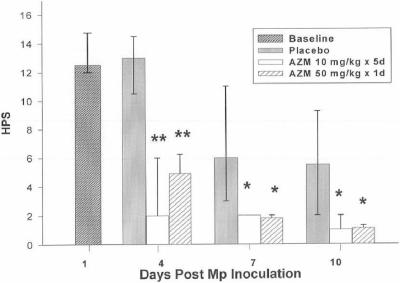
The effects of the two azithromycin regimens on quantitative BAL cultures, lung HPS, and BAL cytokines and chemokines are shown in Fig. 1, 2, and 3. BAL interleukin 1β (IL-1β), IL-2, IL-4, IL-6, IL-10, granulocyte-macrophage colony-stimulating factor (GM-CSF), and gamma interferon (IFN-γ) concentrations were not significantly modified by either azithromycin regimen. No significant differences were demonstrated between the azithromycin regimens for BAL cultures, lung HPS, or BAL cytokines and chemokines. Of note, cytokines and chemokines were measured at day 4 of infection, when the daily treatment group had received a total of only 30 mg/kg of azithromycin while the single high-dose group had received 50 mg/kg. Airway obstruction, as defined by baseline enhanced pause (Penh), was not significantly affected by either regimen. Airway hyperreactivity, defined by Penh values after exposure to methacholine, was significantly reduced in mice treated with the daily-dose regimen of azithromycin only on day 7 (P < 0.05). No differences in Penh values were observed between the azithromycin regimens.
FIG. 1.
Mean CFU of M. pneumoniae (Mp) in BAL cultures of infected mice treated with subcutaneous azithromycin (AZM) (10 mg/kg/day for 5 days or 50 mg/kg for 1 day) or placebo (therapy was started 1 day after inoculation). Error bars represent standard deviations. *, P < 0.05; **, P < 0.005 (compared with placebo); 5 to 10 mice per treatment regimen at each time point. Values are results from two independent experiments.
FIG. 2.
Median HPS of mice infected with M. pneumoniae (Mp) and treated with azithromycin (AZM) (10 mg/kg/day for 5 days or 50 mg/kg for 1 day) or placebo. Error bars represent 25th to 75th percentiles. *, P < 0.05; **, P < 0.001 (compared with placebo); 5 to 10 mice per treatment regimen at each time point. Values are results from two independent experiments.
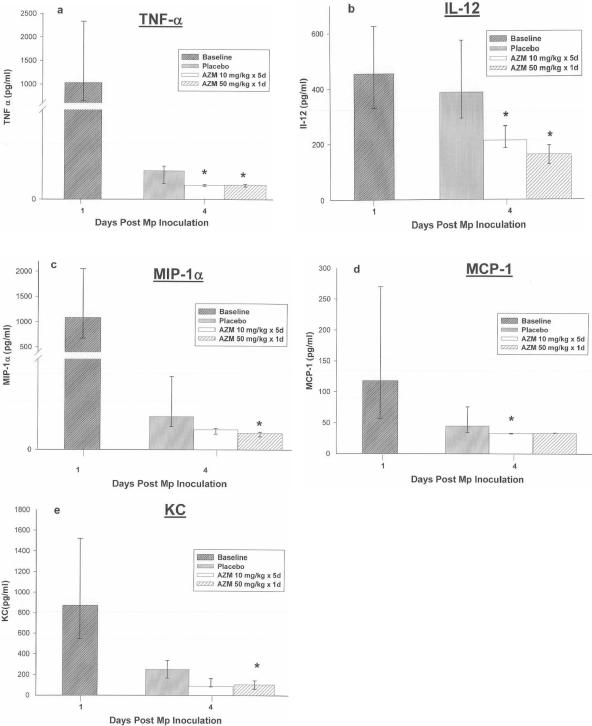
FIG. 3.
BAL cytokine and chemokine concentrations in mice infected with M. pneumoniae (Mp) and treated with azithromycin (AZM) (10 mg/kg/day for 5 days or 50 mg/kg for 1 day) or placebo. Data are expressed as means or medians as described in the text. Error bars represent standard deviations when variables had a normal distribution and 25th to 75th percentiles when variables did not have a normal distribution. *, P < 0.05 (compared with placebo); 5 to 10 mice per treatment regimen at each time point. Values are results from two independent experiments. Of note, cytokines and chemokines were measured at day 4 of infection, when the daily treatment group had received a total of only 30 mg/kg of azithromycin while the single-high-dose group had received 50 mg/kg.
Both azithromycin regimens had a significant but limited effect on pulmonary cytokines and chemokines, with significant reductions demonstrated for concentrations of the proinflammatory (tumor necrosis factor alpha [TNF-α]), TH-1 (IL-12), and chemotactic (MIP-1α, MCP-1, and KC) cytokines without any significant effect on the concentrations of IL-1β, IL-2, IL-4, IL-6, IL-10, IFN-γ, and GM-CSF. TNF-α, IL-12, and the chemokines (MCP-1, MIP-1α, and KC) have consistently been associated with disease severity in our model (4, 6). These cytokines (TNF-α, IL-12, MCP-1, MIP-1α, and KC) are also among the cytokines that have been shown previously to be significantly reduced in response to antibiotic regimens effective against M. pneumoniae, which may help explain the improvement in the lung inflammation in response to therapy despite the lack of M. pneumoniae eradication (5, 7, 10).
In contrast, while azithromycin therapy had no effect on concentrations of IL-6 and IFN-γ, significant reductions in these cytokines have been demonstrated with clarithromycin (a macrolide), cethromycin (a ketolide), and NVP-PDF713 (a peptide deformylase inhibitor) therapy in this model (5, 7, 10). In addition, these cytokines (IL-6 and IFN-γ) are associated with disease severity in our model (4, 6). The mechanism for the difference in cytokine modulation by azithromycin compared with other antimicrobials investigated in this model is unknown; however, azithromycin may interact with mycoplasma virulence properties (such as protein production) in a different manner than these other inhibitors of protein synthesis, as antimicrobial structural differences are known to produce differential inhibitory effects on protein synthesis and 50S ribosomal subunit assembly (8). The beneficial clinical effect of macrolide or azalide therapy on Pseudomonas aeruginosa respiratory infections is often postulated to be due to inhibition of protein synthesis with resultant suppression of pseudomonal virulence factors (11, 12, 13, 15). Similarly, the relationship between these antimicrobials and M. pneumoniae may be analogous to that proposed for clindamycin and Streptococcus pyogenes virulence properties (1). Azithromycin is also speculated to have host immunomodulatory activity independent of antibacterial activity (2). However, we have previously demonstrated in our model that while clarithromycin had an immunomodulatory effect on pulmonary inflammation induced by live M. pneumoniae, clarithromycin had no host immunomodulatory effect on pulmonary inflammation induced by dead M. pneumoniae (7).
The two azithromycin regimens did not significantly reduce airway obstruction, while a significant effect was observed with clarithromycin, cethromycin, and NVP-PDF713 in previous investigations in this model (5, 7, 10). From an immunopathogenic perspective, the lack of effect of azithromycin on airway obstruction may be a functional consequence of the lack of significant reduction in pulmonary concentrations of IL-6 and/or IFN-γ with azithromycin therapy. This will have to be validated in other studies.
Therapy with azithromycin either given as a single high-dose or divided over 5 days resulted in significant improvement in microbiologic, histologic, and immunologic markers of disease in experimental M. pneumoniae pneumonia. No significant differences were demonstrated between the single high-dose and daily-dose regimens of azithromycin for the outcome variables evaluated.
Acknowledgments
This study was funded in part by a research grant from Pfizer Laboratories.
REFERENCES
- 1.American Academy of Pediatrics, Committee on Infectious Diseases. 1998. Severe invasive group A streptococcal infections: a subject review. Pediatrics 101:136-140. [PubMed] [Google Scholar]
- 2.Amsden, G. W. 2005. Anti-inflammatory effects of macrolides—an underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions? J. Antimicrob. Chemother. 55:10-21. [DOI] [PubMed] [Google Scholar]
- 3.Bin, X. X., K. Wolf, T. Schaffner, and R. Malinverni. 2000. Effect of azithromycin plus rifampin versus amoxicillin alone on eradication and inflammation in the chronic course of Chlamydia pneumoniae pneumonitis in mice. Antimicrob. Agents Chemother. 44:1761-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fonseca-Aten, M., A. M. Rios, A. Mejias, S. Chavez-Bueno, K. Katz, A. M. Gomez, G. H. McCracken, Jr., and R. D. Hardy. 2005. Mycoplasma pneumoniae induces host-dependent pulmonary inflammation and airway obstruction in mice. Am. J. Respir. Cell Mol. Biol. 32:201-210. [DOI] [PubMed] [Google Scholar]
- 5.Fonseca-Aten, M., C. Salvatore, A. Mejías, A. M. Ríos, S. Chávez-Bueno, K. Katz, A. M. Gómez, G. H. McCracken, Jr., and R. D. Hardy. Evaluation of LBM415 (NVP PDF-713), a novel peptide deformylase inhibitor, for the treatment of experimental Mycoplasma pneumoniae pneumonia. Antimicrob. Agents Chemother., in press. [DOI] [PMC free article] [PubMed]
- 6.Hardy, R. D., H. S. Jafri, K. Olsen, M. Wordemann, J. Hatfield, B. B. Rogers, P. Patel, L. Duffy, G. Cassell, G. H. McCracken, and O. Ramilo. 2001. Elevated cytokine and chemokine levels and prolonged pulmonary airflow resistance in a murine Mycoplasma pneumoniae pneumonia model: a microbiologic, histologic, immunologic, and respiratory plethysmographic profile. Infect. Immun. 69:3869-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardy, R. D., A. M. Rios, S. Chavez-Bueno, H. S. Jafri, J. Hatfield, B. B. Rogers, G. H. McCracken, and O. Ramilo. 2003. Antimicrobial and immunologic activities of clarithromycin in a murine model of Mycoplasma pneumoniae-induced pneumonia. Antimicrob. Agents Chemother. 47:1614-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mabe, S., J. Eller, and W. S. Champney. 2004. Structure-activity relationships for three macrolide antibiotics in Haemophilus influenzae. Curr. Microbiol. 49:248-254. [DOI] [PubMed] [Google Scholar]
- 9.Malinverni, R., C. C. Kuo, L. A. Campbell, A. Lee, and J. T. Grayston. 1995. Effects of two antibiotic regimens on course and persistence of experimental Chlamydia pneumoniae TWAR pneumonitis. Antimicrob. Agents Chemother. 39:45-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rios, A. M., A. Mejias, S. Chavez-Bueno, M. Fonseca-Aten, K. Katz, J. Hatfield, A. M. Gomez, H. S. Jafri, G. H. McCracken, Jr., O. Ramilo, and R. D. Hardy. 2004. Impact of cethromycin (ABT-773) therapy on microbiological, histologic, immunologic, and respiratory indices in a murine model of Mycoplasma pneumoniae lower respiratory infection. Antimicrob. Agents Chemother. 48:2897-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubin, B. K., and M. O. Henke. 2004. Immunomodulatory activity and effectiveness of macrolides in chronic airway disease. Chest 125:70S-78S. [DOI] [PubMed] [Google Scholar]
- 12.Tateda, K., Y. Ishii, T. Matsumoto, N. Furuya, M. Nagashima, T. Matsunaga, A. Ohno, S. Miyazaki, and K. Yamaguchi. 1996. Direct evidence for antipseudomonal activity of macrolides: exposure-dependent bactericidal activity and inhibition of protein synthesis by erythromycin, clarithromycin, and azithromycin. Antimicrob. Agents Chemother. 40:2271-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tateda, K., Y. Ishii, T. Matsumoto, T. Kobayashi, S. Miyazaki, and K. Yamaguchi. 2000. Potential of macrolide antibiotics to inhibit protein synthesis of Pseudomonas aeruginosa: suppression of virulence factors and stress response. J. Infect. Chemother. 6:1-7. [DOI] [PubMed] [Google Scholar]
- 14.Wolf, K., and R. Malinverni. 1999. Effect of azithromycin plus rifampin versus that of azithromycin alone on the eradication of Chlamydia pneumoniae from lung tissue in experimental pneumonitis. Antimicrob. Agents Chemother. 43:1491-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wozniak, D. J., and R. Keyser. 2004. Effects of subinhibitory concentrations of macrolide antibiotics on Pseudomonas aeruginosa. Chest 125:62S-69S. [DOI] [PubMed] [Google Scholar]