Abstract
The present study assessed potential subclinical markers of amphotericin B (AmB)-related nephrotoxicity and infusion-related reactions (IRR). Subjects were pretreated with diphenhydramine and acetaminophen and received a 500-ml bolus infusion of 0.9% sodium chloride prior to each effective renal plasma flow (ERPF) assessment. ERPF was measured before and after administration of a single 0.25-mg/kg intravenous AmB dose using technetium-99m mercaptoacetyltriglycine. Blood was collected before and 3 h after AmB infusion for tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β) plasma concentrations. Overnight 12-h urine collections were performed before administration of AmB and for 2 nights after administration of AmB and analyzed for α and π glutathione-S-transferases (GSTα and GSTπ, respectively) and N-acetyl-β-d-glucosaminidase (NAG). Six men and six women with mean ± standard deviation (SD) ages of 24.8 ± 5.3 and 28.0 ± 8.5 years, respectively, were studied. Baseline serum creatinine values were within the normal range and were unaltered after administration of AmB. The mean ± SD decrease in ERPF after administration of AmB was significant (P < 0.05) in males (15.7 ± 8.1%) but not females (9.5 ± 14.0%). The GSTπ and GSTα indices increased significantly (P < 0.05) by two to fourfold and returned to baseline in males but were unaltered in females. NAG indices were unaffected by AmB. Six patients experienced an IRR that was associated with increased TNF-α (P < 0.05) but not IL-1β (P = 0.09). These results suggest a potential sex-related difference in AmB-induced nephrotoxicity and provide a rationale for use of ERPF, urine GST, and TNF-α as subclinical markers of polyene-induced toxicity.
Conventional amphotericin B desoxycholate (AmB) is an intravenously administered antifungal agent associated with a number of adverse drug reactions that include infusion-related reactions (IRR) and nephrotoxicity (12). The IRR consist of fever, chills, hypotension, tachypnea, nausea, and vomiting and occur in 50% to 70% of patients who receive this drug (12). These IRR are associated with the acute release of tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β) (1). An assessment of the expression of these cytokines has been performed in patients who have received AmB for the management of a documented fungal infection. However, TNF-α and IL-1β are proinflammatory cytokines that increase in concentration during an acute infection. Consequently, the direct effect of AmB on these cytokines and IRR in the absence of an acute infection in humans has not been characterized.
Nephrotoxicity is a serious, dose-limiting adverse event associated with the use of AmB (19). AmB-induced nephrotoxicity manifests itself as both glomerular and tubular dysfunction. The exact mechanisms involved in AmB-induced nephrotoxicity have not been elucidated (19). However, the short-term physiological effects of AmB include a reduction in the effective renal plasma flow (ERPF) and the glomerular filtration rate. Serum creatinine is the most common clinically used renal biomarker but remains insensitive to detection of low-level renal injury. Several urinary enzymes have been evaluated as potential biomarkers of renal injury (14, 22). The most notable include N-acetyl-β-d-glucosaminidase (NAG) and glutathione-S-transferases (GSTs). Assessment of ERPF can be performed using technetium-99m-mercaptoacetyltriglycine (99mTc-MAG3) and is currently used in approximately 250,000 renal scintigraphy procedures in the United States annually (5). This radiopharmaceutical has been shown to detect decreased ERPF in dogs receiving AmB (8).
To date, no study has been conducted to evaluate the effects of AmB on ERPF and enzymuria in humans. In addition, the expression of TNF-α and IL-1β in subjects with and without IRR is not known. The present study was designed to assess the use of ERPF, enzymuria, and cytokine expression as potential subclinical markers of AmB-related toxicity.
MATERIALS AND METHODS
Study protocol.
The study protocol was approved by the Human Research Review Committee and the Human Uses Subcommittee for Radiation Safety at the University of New Mexico Health Sciences Center. Healthy volunteers were informed in detail about the purpose, procedures, risks, and lack of direct benefits of participating in this research. All subjects provided signed informed consent prior to inclusion in the study. The study was performed at the University of New Mexico Health Sciences Center General Clinical Research Center (GCRC).
Inclusion criteria.
Subjects fulfilling the following criteria were eligible to participate: (i) healthy males and females, (ii) 18 to 65 years of age, (iii) nonsmoking volunteers, (iv) nonobese subjects defined as having a body mass index less than or equal to 30 kg/m2, and (v) female subjects of childbearing potential who were either previously surgically sterilized, using an effective barrier method of contraception (diaphragm, cervical cap, condom), or agreed to abstain from sex from the time of prestudy screening, during the entire study period, and for 8 weeks following the study period.
Exclusion criteria.
Subjects were not eligible if any of the following criteria were met: (i) a clinically significant chronic medical condition defined as any condition requiring pharmacologic or nonpharmacologic management; (ii) a history of adverse reactions to polyenes, radionuclides, acetaminophen, diphenhydramine, or meperidine; (iii) blood donation or participation in another study within 30 days of this study; (iv) being a pregnant or breastfeeding female; (v) unwillingness to abstain from all prescription medications, nonprescription medications, herbals, or vitamins during the study period; (vi) unwillingness to abstain from alcohol, cigarette smoking, and caffeine intake during the study period; (vii) a clinically significant abnormal physical examination, electrocardiogram, or laboratory study including a complete blood cell count, serum chemistry, transaminases, or bilirubin; or (viii) a calculated creatinine clearance of <100 ml/min. Eligible subjects performed study procedures within 2 weeks of the screening visit.
GCRC admission and procedures.
A urine sample was collected from female subjects to rule out pregnancy prior to receipt of the study medication. Eligible subjects were enrolled in the study between 5 and 6 p.m. A 12-h overnight urine collection was initiated for baseline enzymuria quantification. An overnight urine collection method was used to normalize for potential diurnal variations in proteinuria. A standardized caffeine-free meal was provided to each subject. The subjects were restricted from eating any meals until the following morning in order to obtain a fasting lipid and electrolyte profile. Elevated low-density lipoprotein concentrations have previously been reported to increase AmB-induced nephrotoxicity (24).
Baseline ERPF measurement.
The following morning, a peripheral intravenous catheter was inserted. Subjects received a 500-mg dose of acetaminophen and a 25-mg dose of diphenhydramine, followed by a 500-ml infusion of normal saline over 30 min immediately prior to the baseline ERPF study. A dose of 3 mCi of 99mTc-MAG3 was administered to each subject. Blood samples were collected at 12, 43, and 94 min, and radioactive counts per minute per milliliter were used to determine 99mTc-MAG3 clearance. The subjects then received a standardized caffeine-free meal for lunch.
ERPF and cytokine measurements during AmB administration.
The second ERPF assessment was performed at least 3 h after the baseline ERPF study, as 95% of 99mTc-MAG3 is cleared within 3 h (9). Subjects were again pretreated with a 500-mg dose of acetaminophen and a 25-mg dose of diphenhydramine, followed by a 500-ml infusion of normal saline over 30 min. Use of this pretreatment and normal saline was required by our Human Research Review Committee to minimize any potential toxicity to healthy volunteers who did not receive any direct benefits from participation. A blood sample was collected prior to the AmB infusion to determine baseline TNF-α and IL-1β concentrations. The AmB infusion of 0.25 mg/kg over 60 min was initiated after administration of the normal saline bolus. A single lot and formulation of AmB (Pharma-Tek, Huntington, NY) was used for this study. A dose of 3 mCi of 99mTc-MAG3 was administered to each subject 30 min (midpoint) into the AmB infusion. Timed blood sampling was performed as described above. The subject's heart rate, blood pressure, and temperature were measured every 20 min during the AmB infusion and then every 60 min for 3 h. A blood sample was collected and stored at the end of the 3-h monitoring phase after AmB infusion to determine TNF-α and IL-1β concentrations. All observed and volunteered adverse events were recorded.
Urine collection.
A second 12-h overnight urine collection was initiated within 30 min of the previous day's (baseline) urine collection start time. The subjects were maintained in the GCRC for an overnight stay for urine collection and observation. Aliquots from the urine collection were stored for analysis for NAG, GSTα, GSTπ, and creatinine concentrations. Subjects were interviewed for any potential adverse events, and an additional blood sample was collected to determine the serum chemistry. The subjects were discharged with a urine collection jug and instructed to begin a final 12-h overnight urine collection within 30 min of the previous day's collection time. The subjects returned the following day with their urine collection, and a final blood sample was collected for serum chemistry assessment.
Safety assessment and management.
Safety assessments were performed on each of the study days. All observed and volunteered adverse reactions were recorded in the study records regardless of association to study drugs. The records indicated the specific time of onset, severity, treatment, outcome, and duration of each episode. No additional medications were allowed during the study period except specified agents for symptomatic relief of AmB infusion-related side effects such as meperidine for rigors, acetaminophen for headache or fevers, and promethazine for nausea or vomiting. Aspirin or nonsteroidal anti-inflammatory agents such as ibuprofen, naproxen, and ketoprofen were not permitted during the study.
Sample analysis.
The blood samples collected during the ERPF studies were tested for radioactivity using a validated counting method (16). Urine was analyzed for NAG, GSTα, GSTπ, and creatinine concentrations. The urine samples were stored at 2 to 8°C, and all three samples were tested as a batch. These enzymes were assayed using commercially available kits for NAG (Roche Biochemicals, Indianapolis, IN) and GSTs (Biotrin International Ltd., Dublin, Ireland). The blood sample collected for cytokine analysis were transferred into cryotubes and stored at −85°C until processing. Commercially available immunoassay kits (R&D Systems Inc., Minneapolis, MN) were used to measure serum concentrations of TNF-α and IL-1β. These blood and urine samples were processed and analyzed according to the manufacturers instructions.
ERPF estimation.
99mTc-MAG3 modeling to estimate clearance was accomplished utilizing WinNonLin pharmacokinetic software. ERPF was calculated from 99mTc-MAG3 clearance values using a previously validated equation (ERPF = 1.86 × 99mTc-MAG3 clearance + 4.6) derived from the comparison of 99mTc-MAG3 to the standard, [123I]iodohippurate, and normalized for body surface area (11).
Statistical analysis.
Repeated-measures analysis of variance with time as the repeated factor was used as the overall analysis for comparison of the NAG index, GSTα, and GSTπ. Post-hoc comparisons of these enzymes was performed using Student's paired t test following Fisher's least-significant-difference method. Student's paired t test was used to compare baseline and post-AmB measurements of ERPF and cytokine concentrations. Student's t test was used to compare ERPF values between males and females. Fisher's exact test was used to compare the association of detectable cytokine concentrations to AmB IRR. For the power analysis, we considered data from previous healthy volunteer studies where the mean ± standard deviation (SD) ERPF was 420 ± 40 ml/min/1.73 m2 (17). Our sample size of 12 normal subjects was adequate to detect a 10% reduction in ERPF using a two-sided Student paired t test with a 90% power and α = 0.05. The assumption of a 10% reduction in ERPF was considered reasonable because data obtained with animals indicated that a 30 to 40% reduction in ERPF occurs during AmB infusions (18).
RESULTS
Thirteen subjects were screened for enrollment, and one subject was excluded secondary to an abnormal electrocardiogram. The demographic characteristics of the subjects are illustrated in Table 1. There was an equal distribution of males and females, with a median age (range) of 26 (18 to 43) years. A majority of subjects were Caucasian, and the median (range) body mass index was 24.5 (21.6 to 29.4) kg/m2. Males and females were comparable in height, weight, and body surface area. The mean ± SD dose of AmB received based on body surface area was not statistically significantly different in males (10.0 ± 0.6 mg/m2) and females (9.8 ± 0.5 mg/m2). Baseline serum chemistries, complete blood counts, and transaminases were within the normal ranges for all subjects and comparable between males and females. However, serum creatinine values were statistically significantly lower (P < 0.05) in females compared to males but calculated creatinine clearances were not significantly different. The median (range) fasting low-density lipoprotein concentration was 85 (72 to 133) mg/dl and was comparable in males and females.
TABLE 1.
Demographic characteristics of healthy volunteersa
| Variable | All | Male | Female |
|---|---|---|---|
| No. | 12 | 6 | 6 |
| Age (yr) | 26.4 ± 7.0 | 24.8 ± 5.3 | 28.0 ± 8.5 |
| Race | |||
| Caucasian | 9 | 4 | 5 |
| African American | 2 | 1 | 1 |
| East Indian | 1 | 1 | 0 |
| Ht (cm) | 175 ± 10.0 | 180 ± 6.6 | 170 ± 10.3 |
| Wt (kg) | 76.3 ± 10 | 80.2 ± 9.7 | 72.3 ± 9.3 |
| Body mass index (kg/m2) | 24.8 ± 2.4 | 24.7 ± 2.4 | 25.0 ± 2.4 |
| Blood urea nitrogen (mg/dl) | 12 ± 2 | 14 ± 2 | 11 ± 2 |
| Serum creatinine (mg/dl) | 0.9 ± 0.1 | 1.0 ± 0.1b | 0.8 ± 0.1 |
| Creatinine clearance (ml/min)c | 124 ± 11 | 128 ± 9 | 119 ± 11 |
| Total cholesterol (mg/dl) | 165 ± 27 | 159 ± 33 | 171 ± 20 |
| High-density lipoprotein (mg/dl) | 47 ± 11 | 47 ± 12 | 47 ± 10 |
| Low-density lipoprotein (mg/dl) | 91 ± 19 | 86 ± 23 | 96 ± 14 |
Values are presented as means ± standard deviations.
P < 0.01.
Creatinine clearances were calculated using the Cockroft-Gault equation.
ERPF measurements.
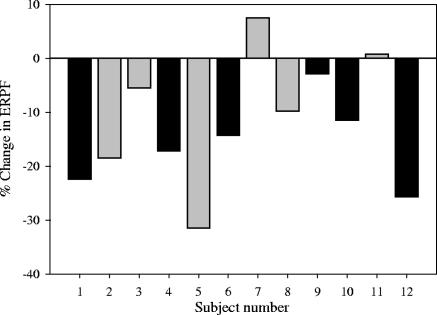
The baseline mean ± SD ERPF was 471 ± 76 ml/min/1.73 m2 for all subjects and was not significantly different between males (507 ± 86 ml/min/1.73 m2) and females (436 ± 48 ml/min/1.73 m2) (P = 0.11). The median (range) change in ERPF during AmB infusion was −12.9% (7.5, −31.5%), and eight subjects demonstrated at least a 10% reduction in ERPF. The specific change in ERPF for each individual subject is illustrated in Fig. 1 based on sex. This reduction in ERPF to 412 ± 89 ml/min/1.73 m2 during the AmB infusion was statistically significant (P = 0.002). Subgroup analysis revealed that this ERPF reduction occurred primarily in males compared to females. Males had a mean (95% confidence interval [CI]) percent change in ERPF of −15.7% (−9.2%, −22.2%), compared to −9.5% (1.7%, −20.7%) for females.
FIG. 1.
Graph illustrating percent changes in ERPF from the baseline during AmB administration in males (black bars) and females (gray bars).
Urine enzyme and creatinine concentrations.
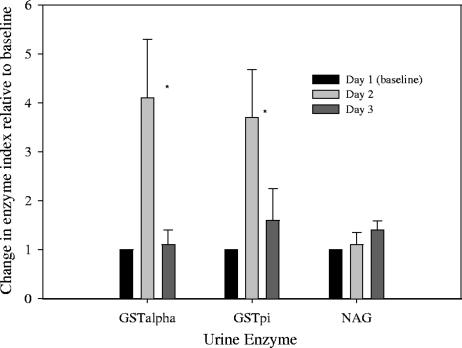
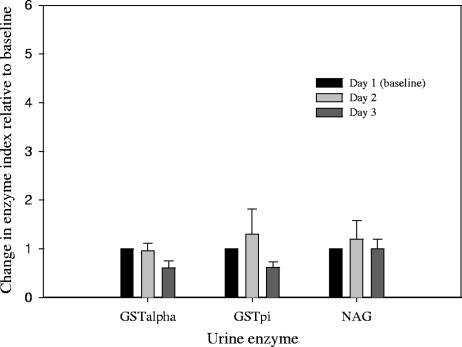
The mean ± SD urine concentrations of GSTα, GSTπ, NAG, and creatinine at baseline were 6.8 ± 2.5 μg/liter, 12.1 ± 3.0 μg/liter, 1.2 ± 1.0 μg/liter, and 67.9 ± 42.6 mg/dl, respectively. These urine enzyme values, including those obtained on day 2 and day 3 (after AmB infusion), were normalized to urine creatinine concentrations as previously described (22). This normalization was used to define each urine enzyme marker as an index value; for example, urine GSTα concentration divided by urine creatinine concentration equals GSTα index value. These results are illustrated in Fig. 2 and 3 according to sex and demonstrate a rise in GSTα and GSTπ indices in males only with no change in the NAG index in either sex. The mean ± SD GSTα indices (micrograms per millimole) on days 1, 2, and 3 in males were 0.4 ± 0.1, 2.0 ± 0.7, and 0.9 ± 0.6, respectively. The mean ± SD GSTα indices (micrograms per millimole) on days 1, 2, and 3 in females were 0.2 ± 0.1, 0.2 ± 0.1, and 0.1 ± 0.03, respectively. The mean ± SD change in the GSTα index on day 2 compared to day 1 was statistically significant in males but not in females (P = 0.001). The median (range) changes in GSTα in males and females were 3.3-fold (12-fold to 6.3-fold) and 0.9-fold (0.3-fold to 1.2-fold), respectively.
FIG. 2.
Mean (± the standard error of the mean) changes in GST and NAG indices relative to the baseline in male subjects. *, P < 0.05.
FIG. 3.
Mean (± the standard error of the mean) changes in GST and NAG indices relative to the baseline in female subjects.
Similarly, the change in the GSTπ index on day 2 compared to the baseline occurred to a statistically significant (P = 0.02) level in males but not females. The mean ± SD GSTπ indices (micrograms per millimole) on days 1, 2, and 3 in males were 1.5 ± 0.4, 3.4 ± 1.6, and 2.2 ± 1.9, respectively. The mean ± SD GSTπ indices (micrograms per millimole) on days 1, 2, and 3 in females were 1.5 ± 0.8, 2.6 ± 0.6, and 1.6 ± 0.6, respectively. The median (range) changes in GSTπ in males and females were 2.7-fold (1.3-fold to 6.2-fold) and 1.0-fold (0.6-fold to 3.7-fold) on day 2, respectively. The elevation in the GSTα and GSTπ indices on day 2 returned to near baseline values on day 3 for all male subjects and in one female (subject 7), who had a 3.7-fold increase in GSTπ. In comparison, NAG indices were not elevated to a significant degree in either males or females.
Safety and cytokine concentrations.
Seven subjects reported having an adverse reaction, six (50%) of which were considered to be AmB IRR. One subject (subject 2) reported to the study site a day after the end of the study with mild swelling at the site where the catheter had been maintained on day 2 of admission. This swelling was treated with an ice pack and a single 600-mg dose of ibuprofen. In general, all AmB IRR were minor in severity and consisted of chills (n = 5), fever (n = 1), headache (n = 1), and nausea and vomiting (n = 1). The subject with a headache was managed with a single dose of acetaminophen, and a single dose of promethazine was administered to the subject who experienced an episode of emesis. The symptoms of chills, fever, and headache occurred within 1 h of infusion and generally lasted 30 to 45 min. In contrast, the subject with nausea and vomiting developed these symptoms about 5 h after the administration of AmB. The subject number, sex, type of adverse reaction, and cytokine concentrations at baseline and 3 h are tabulated in Table 2. The concentrations of TNF-α were undetectable in all subjects at baseline and were measurable in seven subjects at 3 h. An increase in TNF-α concentrations was significantly associated with the presence of an AmB IRR (P = 0.007). In contrast, IL-1β concentrations were undetectable in 11 of the 12 subjects at baseline and detectable in 9 subjects after AmB infusion. However, a detectable IL-1β concentration was not associated with the presence of an AmB IRR (P = 0.09).
TABLE 2.
Comparison of AmB IRR to TNF-α and IL-1β concentrations in subjects 3 h post AmB infusion
| Subject no. | Sexc | Adverse reaction(s) | TNF-α (pg/ml)
|
IL-1β (pg/ml)
|
||
|---|---|---|---|---|---|---|
| Baseline | 3 h | Baseline | 3 h | |||
| 2 | F | None | UDa | UD | UDb | UD |
| 3 | F | None | UD | UD | UD | UD |
| 6 | M | None | UD | UD | UD | UD |
| 4 | M | None | UD | UD | UD | 1.2 |
| 9 | M | None | UD | UD | UD | 1.1 |
| 1 | M | None | UD | 52.9 | UD | 1.6 |
| 5 | F | Chills | UD | 40.6 | UD | 1.2 |
| 7 | F | Nausea and vomiting | UD | 161.1 | UD | 1.9 |
| 8 | F | Chills | UD | 26.2 | 2.2 | 3.2 |
| 11 | F | Chills | UD | 71.4 | UD | 1.2 |
| 10 | M | Chills and fever | UD | 53.2 | UD | 1.7 |
| 12 | M | Chills and headache | UD | 124.4 | 0 | 1.1 |
UD, undetectable TNF-α concentration (lower limit of detection, 1.6 pg/ml).
UD, undetectable IL-1β concentration (lower limit of detection, 0.7 pg/ml).
M, male; F, female.
DISCUSSION
The nephrotoxic potential of AmB has been described for more than 40 years. In addition, the potential mechanisms of nephrotoxicity have been characterized in vitro and in animal models. Validation of these mechanisms or assessment of the physiologic effects of amphotericin B in healthy volunteers has not been performed. The present study assessed the effects of AmB on the reduction of ERPF, enzymuria, and cytokine expression. This study demonstrated that the infusion of a 0.25-mg/kg dose of AmB was associated with at least a 10% reduction in ERPF in 8 of the 12 subjects studied. This change in ERPF occurred to a significant degree in males but not females. Similarly, direct renal tubular injury was measurable in male subjects but not female subjects. This potential sex-related difference in the nephrotoxic potential of AmB has not been reported in any prospective clinical trial to date. However, large retrospective trials have noted an association between male sex and the development of AmB-induced nephrotoxicity (2, 7). Bates et al. reviewed 643 admissions that included patients receiving amphotericin B and found that the hazard ratio (95% CI) for male sex and nephrotoxicity was 2.1 (1.4 to 3.1) by univariate analysis only (2). In contrast, Harbarth and colleagues demonstrated that the hazard ratio (95% CI) for male sex and risk of renal toxicity was 1.8 (1.3 to 2.6) by both univariate and multivariate analyses (7). A similar connection between male sex and the development of nephrotoxicity has been associated with the use of aminoglycosides (4). Although the underlying mechanism behind this observation is not known, progression of renal disease, for example, is consistently worse in males compared to females and could be a direct result of sex steroids (20). Differences in glomerular structure and hemodynamics, variations in the production of cytokines within the kidney, or a direct effect of sex steroids on cell membrane components may explain the observed increased nephrotoxic potential of AmB in males (20). The present study did not include postmenopausal women. Future studies should compare the predilection for AmB-induced nephrotoxicity among postmenopausal women, premenopausal women, and males. A similar risk of nephrotoxicity among postmenopausal women and age-matched males may support the hypothesis that sex steroids affect the nephrotoxic potential of AmB.
An increase in both cytoplasmic enzymes, namely, GSTα and GSTπ, was noted after administration of AmB in males but not females. In contrast, elevation of predominantly lysosomal NAG was not noted in any subject exposed to AmB. These data verify that AmB binds to the luminal membrane of tubular cells with corresponding increased permeability to cytosolic contents. These results are consistent with those noted in rats exposed to AmB, where a 30 to 40% reduction in ERPF and increased tubular permeability to inulin have been reported (6, 19). However, in rats enzymuria decreased with subsequent doses of AmB (19). We evaluated a single dose of AmB, and so the sensitivity of enzymuria as a marker of human renal tubular toxicity may be limited with repeated doses of AmB. Salt depletion in animals has also been shown to potentiate this fall in ERPF and is part of the rationale behind sodium loading of patients treated with AmB (18). The present study employed a sodium-loading protocol that led to administration of 150 to 200 meq of sodium prior to AmB infusion. The limited reduction in ERPF seen in this study compared to animal models may have been influenced by this factor. In addition, use of sodium loading prior to each ERPF assessment may have contributed to the differences noted between the sexes during AmB administration. Renal hemodynamics and tubular responses to sodium chloride can be influenced by female sex hormones (13). Given that the present study was performed with healthy volunteers for whom no direct benefits would be gained from this study, our protocol was designed to minimize risk and follow the standard of care utilized among patients treated with AmB. Future studies should include a subgroup of patients who do not receive sodium loading prior to AmB administration.
The incidence of AmB IRR in this study was consistent with those seen in two published healthy-volunteer trials (3, 10). These trials predominantly included males and were designed to assess the disposition of AmB. Consistent with this study, pretreatment with acetaminophen and diphenhydramine did not appear to have any effects on chills, nausea, and somatic discomfort (3). The mechanism behind the development of AmB IRR has been postulated to be a function of elevated proinflammatory cytokines TNF-α and IL-1β (1). These data have predominantly been generated through in vitro exposure of monocytes to AmB (15). In addition, induction of cytokine release from THP-1 leukemic monocytes to different amphotericin B formulations has yielded similar results (23). Antibody array analysis in this previous study revealed that AmB has the greatest effect on TNF-α with no effect on IL-1 (23). The development of AmB IRR in our study population correlated with an elevation of TNF-α and provides clinical evidence for this relationship. Also, a relationship between IL-1β concentrations and AmB IRR was not noted in this study. This disparity between the expressions of the proinflammatory cytokines TNF-α and IL-1β may have been a consequence of a single sample measurement after AmB administration, which prevented measurement of IL-1β during its optimal time window of expression (21). In addition, expression of IL-1β in monocytes is not induced at low concentrations of AmB and the low doses of AmB used in this study could also have contributed to this disparity (21). The role of other proinflammatory cytokines such as IL-6, IL-8, and growth-related protein was not evaluated in this study.
In summary, the infusion of a single low dose of AmB was associated with a reduction in ERPF and an elevation of urine GST indices to a greater extent in males compared to females. A larger clinical trial is necessary to validate whether these potential sex-related differences in AmB-induced nephrotoxicity truly exist. This alteration in urine and ERPF was not associated with any alteration in creatinine clearance and provides a basis for continued development as subclinical markers of AmB-induced nephrotoxicity. The difficulty in completing head-to-head trials between current amphotericin B formulations is evident. The disease states of the population being studied, differences in dose and duration of therapy, and concomitant use of nephrotoxic agents confound clinical trials. The continued development of subclinical markers of AmB-induced nephrotoxicity provides a system to evaluate potential differences between these compounds in healthy volunteers. In addition, measurement of TNF-α concentrations in healthy volunteers receiving these novel compounds may provide a system to compare their relative potential to cause IRR. Future studies evaluating nephrotoxic compounds should employ subclinical measurements of nephrotoxicity and IRR to limit unnecessary harm to healthy volunteers.
REFERENCES
- 1.Arning, M., K. O. Kliche, A. H. Heer-Sonderhoff, and A. Wehmeier. 1995. Infusion-related toxicity of three different amphotericin B formulations and its relation to cytokine plasma levels. Mycoses 38:459-465. [DOI] [PubMed] [Google Scholar]
- 2.Bates, D. W., L. Su, D. T. Yu, G. M. Chertow, D. L. Seger, D. R. Gomes, and R. Platt. 2001. Correlates of acute renal failure in patients receiving parenteral amphotericin B. Kidney Int. 60:1452-1459. [DOI] [PubMed] [Google Scholar]
- 3.Bekersky, I., R. M. Fielding, D. E. Dressler, J. W. Lee, D. N. Buell, and T. J. Walsh. 2002. Plasma protein binding of amphotericin B and pharmacokinetics of bound versus unbound amphotericin B after administration of intravenous liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate. Antimicrob. Agents Chemother. 46:834-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertino, J. S., Jr., L. A. Booker, P. A. Franck, P. L. Jenkins, K. R. Franck, and A. N. Nafziger. 1993. Incidence of and significant risk factors for aminoglycoside-associated nephrotoxicity in patients dosed by using individualized pharmacokinetic monitoring. J. Infect. Dis. 167:173-179. [DOI] [PubMed] [Google Scholar]
- 5.Bocher, M., Y. Shrem, A. Tappiser, M. Klein, D. Schechter, A. Taylor, and R. Chisin. 2001. Tc-99m mercaptoacetyltriglycine clearance: comparison of camera-assisted methods. Clin. Nucl. Med. 26:745-750. [DOI] [PubMed] [Google Scholar]
- 6.Cheng, J. T., R. T. Witty, R. R. Robinson, and W. E. Yarger. 1982. Amphotericin B nephrotoxicity: increased renal resistance and tubule permeability. Kidney Int. 22:626-633. [DOI] [PubMed] [Google Scholar]
- 7.Harbarth, S., S. L. Pestotnik, J. F. Lloyd, J. P. Burke, and M. H. Samore. 2001. The epidemiology of nephrotoxicity associated with conventional amphotericin B therapy. Am. J. Med. 111:528-534. [DOI] [PubMed] [Google Scholar]
- 8.Itkin, R. J., D. R. Krawiec, A. R. Twardock, H. B. Gelberg, and G. D. Koritz. 1994. Evaluation of the single-injection plasma disappearance of technetium-99m mercaptoacetyltriglycine method for determination of effective renal plasma flow in dogs with normal or abnormal renal function. Am. J. Vet. Res. 55:1652-1659. [PubMed] [Google Scholar]
- 9.Itoh, K. 2001. 99mTc-MAG3: review of pharmacokinetics, clinical application to renal diseases and quantification of renal function. Ann. Nucl. Med. 15:179-190. [DOI] [PubMed] [Google Scholar]
- 10.Kan, V. L., J. E. Bennett, M. A. Amantea, M. C. Smolskis, E. McManus, D. M. Grasela, and J. W. Sherman. 1991. Comparative safety, tolerance, and pharmacokinetics of amphotericin B lipid complex and amphotericin B desoxycholate in healthy male volunteers. J. Infect. Dis. 164:418-421. [DOI] [PubMed] [Google Scholar]
- 11.Muller-Suur, R., G. Magnusson, I. Bois-Svensson, and B. Jansson. 1991. Estimation of technetium 99m mercaptoacetyltriglycine plasma clearance by use of one single plasma sample. Eur. J. Nucl. Med. 18:28-31. [DOI] [PubMed] [Google Scholar]
- 12.Pathak, A., F. D. Pien, and L. Carvalho. 1998. Amphotericin B use in a community hospital, with special emphasis on side effects. Clin. Infect. Dis. 26:334-338. [DOI] [PubMed] [Google Scholar]
- 13.Pechere-Bertschi, A., and M. Burnier. 2004. Female sex hormones, salt, and blood pressure regulation. Am. J. Hypertens. 17:994-1001. [DOI] [PubMed] [Google Scholar]
- 14.Price, R. G. 1992. The role of NAG in the diagnosis of kidney disease including the monitoring of nephrotoxicity. Clin. Nephrol. 38(Suppl. 1):S14-S19. [PubMed] [Google Scholar]
- 15.Rogers, P. D., J. K. Jenkins, S. W. Chapman, K. Ndebele, B. A. Chapman, and J. D. Cleary. 1998. Amphotericin B activation of human genes encoding for cytokines. J. Infect. Dis. 178:1726-1733. [DOI] [PubMed] [Google Scholar]
- 16.Russell, C. D., A. Taylor, and D. Eshima. 1989. Estimation of technetium-99m-MAG3 plasma clearance in adults from one or two blood samples. J. Nucl. Med. 30:1955-1959. [PubMed] [Google Scholar]
- 17.Russell, C. D., B. L. Thorstad, M. V. Yester, M. Stutzman, and E. V. Dubovsky. 1988. Quantitation of renal function with technetium-99m MAG3. J. Nucl. Med. 29:1931-1933. [PubMed] [Google Scholar]
- 18.Sabra, R., K. Takahashi, R. A. Branch, and K. F. Badr. 1990. Mechanisms of amphotericin B-induced reduction of the glomerular filtration rate: a micropuncture study. J. Pharmacol. Exp. 253:34-37. [PubMed] [Google Scholar]
- 19.Sabra, R., and R. A. Branch. 1990. Amphotericin B nephrotoxicity. Drug Safety 5:94-108. [DOI] [PubMed] [Google Scholar]
- 20.Silbiger, S. R., and J. Neugarten. 2003. The role of gender in the progression of renal disease. Adv. Ren. Replace. Ther. 10:3-14. [DOI] [PubMed] [Google Scholar]
- 21.Simitsopoulou, M., E. Roilides, J. Dotis, M. Dalakiouridou, F. Dudkova, E. Andreadou, and T. J. Walsh. 2005. Differential expression of cytokines and chemokines in human monocytes induced by lipid formulations of amphotericin B. Antimicrob. Agents Chemother. 49:1397-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sundberg, A. G. M. 1995. Glutathione transferases in the urine: sensitive methods for detection of kidney damage induced by nephrotoxic agents in humans. Environ. Health Perspect. 102(Suppl. 3):293-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turtinen, L. W., D. N. Prall, L. A. Bremer, R. E. Nauss, and S. C. Hartsel. 2004. Antibody array-generated profiles of cytokine release from THP-1 leukemic monocytes exposed to different amphotericin B formulations. Antimicrob. Agents Chemother. 48:396-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wasan, K. M., and J. S. Conklin. 1997. Enhanced amphotericin B nephrotoxicity in intensive care patients with elevated levels of low-density lipoprotein. Clin. Infect. Dis. 24:78-80. [DOI] [PubMed] [Google Scholar]