Abstract
The waxy cell walls of mycobacteria provide intrinsic tolerance to a broad range of antibiotics, and this effect is augmented by specific resistance determinants. The inducible determinant erm(38) in the nontuberculous species Mycobacterium smegmatis confers high resistance to lincosamides and some macrolides, without increasing resistance to streptogramin B antibiotics. This is an uncharacteristic resistance pattern falling between the type I and type II macrolide, lincosamide, and streptogramin B (MLSB) phenotypes that are conferred, respectively, by Erm monomethyltransferases and dimethyltransferases. Erm dimethyltransferases are typically found in pathogenic bacteria and confer resistance to all MLSB drugs by addition of two methyl groups to nucleotide A2058 in 23S rRNA. We show here by mass spectrometry analysis of the mycobacterial rRNA that Erm(38) is indeed an A2058-specific dimethyltransferase. The activity of Erm(38) is lethargic, however, and only a meager proportion of the rRNA molecules become dimethylated in M. smegmatis, while most of the rRNAs are either monomethylated or remain unmethylated. The methylation pattern produced by Erm(38) clarifies the phenotype of M. smegmatis, as it is adequate to confer resistance to lincosamides and 14-member ring macrolides such as erythromycin, but it is insufficient to raise the level of resistance to streptogramin B drugs above the already high intrinsic tolerance displayed by this species.
Mycobacteria are the causative agents of several human diseases, the most serious being leprosy and tuberculosis, while other nontuberculous members including Mycobacterium smegmatis and Mycobacterium fortuitum are potential human pathogens associated primarily with wound or lung infections (3, 23, 30). Antimicrobial treatment of nontuberculous mycobacteria is often challenging, as drug uptake is hampered by their relatively impervious cell wall, and this effect is often augmented by the presence of specific resistance determinants (2, 7). Such determinants are erm(38) in M. smegmatis and the closely related erm(39) in M. fortuitum, both of which confer resistance to macrolide and lincosamide drugs (18, 19).
These determinants are members of the erm family of genes that are found in a diverse range of pathogenic and drug-producing bacteria (24, 25, 31). All erm genes encode methyltransferases that specifically target the N6 position of nucleotide A2058 in 23S rRNA (Escherichia coli numbering) (28) but differ as to whether they monomethylate or dimethylate this nucleotide (31, 33). Erm monomethyltransferases confer the so-called macrolide, lincosamide, and streptogramin B (MLSB) type I phenotype with high resistance to lincosamides and low to moderate resistance to macrolide and streptogramin B antibiotics; Erm dimethyltransferases confer the type II phenotype with high resistance to all the MLSB antibiotics (25, 31, 33). Dimethylation at 23S rRNA nucleotide A2058 is the common MLSB resistance mechanism in pathogenic bacteria (24). Erm(38) and Erm(39) confer high resistance to macrolides and lincosamide but no resistance to streptogramin B drugs (18, 19). This resistance phenotype falls somewhere between the typical MLSB type I and type II patterns and could reflect that either the activities of Erm(38) and Erm(39) differ from other Erm mono- and dimethyltransferases or that there are anomalies in the Mycobacterium ribosomes so that ML drugs target a site other than streptogramin B (18).
With a view to explaining the molecular mechanism of the resistance conferred by Erm(38), we determined the methylation status of M. smegmatis rRNA. The masses of each nucleotide in the sequence around A2058 of M. smegmatis 23S rRNA were accurately determined using matrix-assisted laser desorption ionization (MALDI)-time of flight tandem mass spectrometry (1); rRNA methylation was verified by using a primer extension assay with reverse transcriptase. The methylation by Erm(38) was compared with the well-characterized monomethyltransferase Erm(O) (SrmA from Streptomyces ambofaciens) and the dimethyltransferase Erm(E) (from Saccharopolyspora erythraea). When constitutively expressed in the M. smegmatis host, Erm(O) and Erm(E) retain their specificities and exclusively modify nucleotide A2058, adding one and two methyl groups, respectively. Expression of Erm(38) from its natural promoter requires prior induction with subinhibitory concentrations of the macrolide erythromycin. Erm(38) is also specific for nucleotide A2058 although, in comparison with the Erm(O) and Erm(E) methyltransferases, Erm(38) is lethargic in its activity and gives rise to a mixture of unmethylated, monomethylated, and dimethylated products. The data provide clear evidence that Erm(38) is indeed an A2058 dimethyltransferase, although the small proportion of dimethylated product is insufficient to confer high resistance to streptogramin B drugs.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The M. smegmatis strain and plasmids used here are listed in Table 1. Cells were grown at 37°C in Middlebrook 7H9 (Difco) liquid broth containing 0.2% (vol/vol) glycerol or on solid Middlebrook 7H10 medium (Difco) containing 0.5% (vol/vol) glycerol. Kanamycin was added at 15 μg/ml to maintain the plasmids. The erm(38) gene was induced by overnight incubation in liquid medium containing a subinhibitory level (6.4 μg/ml) of erythromycin (Sigma) followed by dilution and growth to late log phase in the same medium for ribosome preparations. The efficiency of erm(38) induction was tested by measuring MICs on agar plates containing stepwise twofold dilutions of the lincosamide clindamycin, the macrolide erythromycin (Sigma), the streptogramin B quinupristin (a pristinamycin derivative), and the ketolide telithromycin (Sanofi-Aventis). The erm(O) and erm(E) genes were constitutively expressed from plasmids; rRNA preparations and MIC measurements for the erm(O) and erm(E) strains were carried out under conditions where the chromosome-encoded erm(38) was silent.
TABLE 1.
M. smegmatis strain and plasmids used in this study
| Strain or plasmid | Description | Reference(s) |
|---|---|---|
| M. smegmatis mc2155 | No plasmid | 29 |
| M. smegmatis mc2155/pMIP12 | Strain with empty pMIP12 plasmid encoding kanamycin resistance | 4,12 |
| M. smegmatis mc2 155/pOMV30 | Strain with pMIP12 plasmid containing erm(O) (formerly srmA) from Streptomyces ambofaciens | 4 |
| M. smegmatis mc2155/pOMV20 | Strain with pMIP12 plasmid containing erm(E) from Saccharopolyspora erythraea | 4 |
Preparation of ribosomes and RNA purification.
M. smegmatis cells were grown to late log phase in 100 ml of medium and were harvested by centrifugation. Cells were washed by being resuspended twice in 100 ml buffer A (10 mM Tris-Cl, pH 7.2, 4 mM MgCl2, 10 mM NH4Cl, 100 mM KCl) and pelleted by centrifugation. The following steps were carried out at 4°C: cells were lysed by sonication in 10 ml buffer A, debris was removed by centrifugation at 16,000 rpm for 20 min, and ribosomes were recovered by centrifugation at 18,000 rpm for 19 h. Ribosomes were resuspended in 200 μl of buffer A, proteins were removed by phenol-chloroform extraction, and the rRNA was redissolved in 30 μl of H2O.
MALDI mass spectrometry analysis of rRNAs.
The 23S rRNA region around A2058 was isolated by a hybridization method (1). A mixture of 33 pmol of M. smegmatis rRNA and 330 pmol of an oligodeoxynucleotide complementary to the nucleotide sequence G2035 to G2087 (Fig. 1) in 66 μl of 80 mM HEPES (pH 7.0) and 180 mM KCl was heated for 5 min at 90°C, followed by slow cooling to 45°C. Mung bean nuclease (3 U; New England Biolabs) and RNase A (1.5 μg) were added in 25 μl of 50 mM sodium acetate (pH 5.0), 30 mM NaCl, and 1 mM ZnCl2, followed by further incubation for 50 min at 35°C, before the reaction was stopped by extraction with phenol-chloroform. The RNA-DNA hybrid was recovered by ethanol precipitation and run on a denaturing 13% polyacrylamide gel to release the protected rRNA fragment (approximately 53 nucleotides), which was then excised and extracted from the gel.
FIG. 1.
Secondary structure of the 3′ half of 23S rRNA (based on data from the Gutell laboratory comparative RNA site at http://www.rna.icmb.utexas.edu). The Mycobacterium sequence shown around the Erm methyltransferase target at nucleotide A2058 was selected by hybridization for mass spectrometry analysis. The E. coli numbering system for rRNA nucleotides (8, 20) is used throughout; nucleotide A2058 corresponds to A2283 in M. smegmatis rRNA numbering.
Samples of the 53-mer rRNA fragment (2.5 pmol in 1 μl) were mixed with 0.5 μl 3-hydroxypicolinic acid (0.5 M in 50% acetonitrile), 2.0 μl RNase T1 (0.5 μg/μl; Sigma-Aldrich), and 1.5 μl H2O and were digested for 2.5 h at 37°C. The oligonucleotides produced by RNase T1 digestion were analyzed by MALDI mass spectrometry as previously described (1, 14, 16).
Primer extension.
Dimethylation of the rRNA was quantified by an adapted primer extension procedure with reverse transcriptase (13, 27). Briefly, a 5′-32P-labeled deoxynucleotide primer complementary to the region spanning C2063 to C2080 of Mycobacterium 23S rRNA (Fig. 1) was extended with 1 U of reverse transcriptase (Life Sciences) and 1 mM dTTP, 1 mM dCTP and 5 mM dideoxyguanosine triphosphate, with 1.5 pmol of intact rRNA as the template. Extension products were run on a 13% polyacrylamide-7 M urea gel alongside dideoxy sequencing reactions.
Erm alignments.
Representative erm mono- and dimethyltransferase genes (http://faculty.washington.edu/marilynr/) together with their upstream sequences were obtained from the National Center for Biotechnology Information website (http://www.ncbi.nih.gov/) and compared using the MultAlin online website (http://prodes.toulouse.inra.fr/multalin/multalin.html) (5). Secondary structural elements within the erm(38) leader mRNA were predicted with the help of the Mfold program (http://bioweb.pasteur.fr/seqanal/interfaces/mfold-simple.html).
RESULTS
Resistance phenotypes.
Prior to induction of the erm(38) gene, the growth of M. smegmatis was inhibited by erythromycin at 32 μg/ml, fitting well with observations from other studies (4, 18, 21, 22). Preincubation of cells with subinhibitory amounts of erythromycin led to induction of erm(38) expression and rRNA methylation (see below) and increased the MIC for erythromycin to 256 μg/ml (Table 2). The extent of cell growth on clindamycin and telithromycin is indicative of MLSB type I and type II phenotypes (13), and these phenotypes tallied here with the MICs for M. smegmatis strains expressing erm(O) and erm(E), respectively (Table 2).
TABLE 2.
Expression of erm genes in Mycobacterium smegmatis
| erm gene M. smegmatis plasmida | Induction of erm(38) | MIC (μg/ml)b
|
A2058 dimethylationc (%) | |||
|---|---|---|---|---|---|---|
| Clindamycin | Erythromycin | Telithromycin | Quinupristin | |||
| Empty plasmid | No | 32 | 32 | 4 | 64 | 0 (± 3) |
| Empty plasmid | Yesd | 1,024 | 256 | 32 | 64 | 8 (± 4) |
| erm(O) | No | 1,024 | 256 | 32 | 64 | 0 (± 3) |
| erm(E) | No | 1,024 | 2,048 | 2,048 | 512 | 98 (± 5) |
The plasmid constructions are listed in Table 1.
The MICs are the minimal concentrations of antibiotics that prevent cell growth on plates incubated up to 48 h. Sections of the data overlap and are consistent with previous reports of M. smegmatis MICs for clindamycin, erythromycin, and quinupristin (18) and for telithromycin (21) after induction of erm(38) and for clindamycin, erythromycin, and pristinomycin when M. smegmatis expresses erm(O) and erm(E) (4).
Dimethylation at A2058 was quantified by reverse transcriptase primer extension as a percentage of the total 23S rRNA molecules per strain; standard deviations of the means of three measurements are shown in brackets. Primer extension does not detect the appreciable amounts of A2058 monomethylation in the strains expressing erm(38) and erm(O) (see text).
The chromosome-encoded erm(38) gene was induced by preincubation with low conconcentrations of erythromycin as described in Materials and Methods.
Mass spectrometry analysis of the A2058 region.
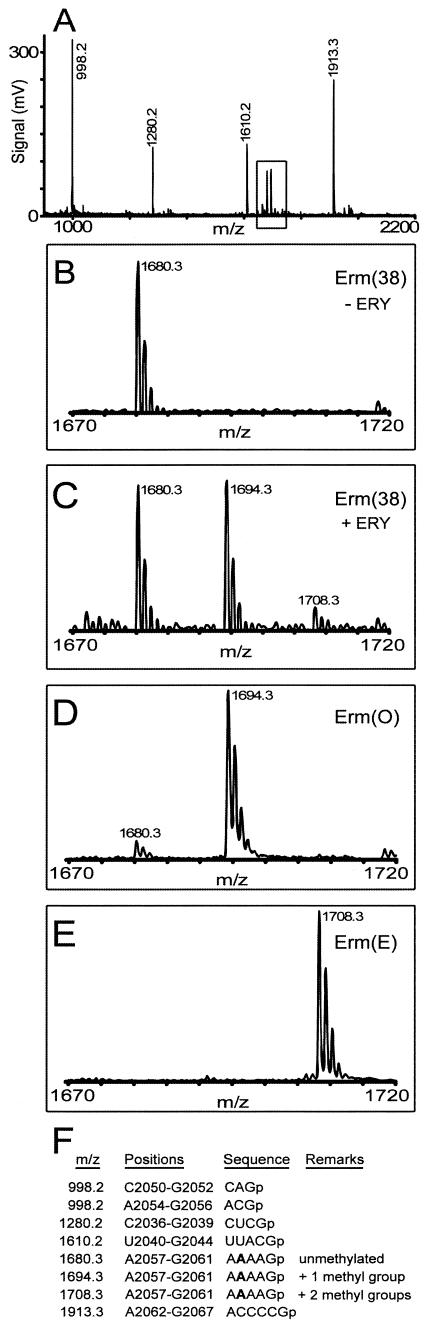
MALDI mass spectrometry was used to determine the methylation status of the mycobacterial 23S rRNA. This technique measures the masses of RNA oligonucleotides to within 0.1 Da (10), and thus the presence of one methyl group (plus 14 Da) or two methyl groups (plus 28 Da) is readily detected. For technical reasons, oligonucleotides in the range of 3 to 10 nucleotides in length are easiest to identify, and intact rRNAs are too large to be directly analyzed by this method. Fragments of the rRNA with predictable and suitable sizes can be formed by digestion with the nucleotide-specific RNases A and T1, although many fragments have similar or identical masses and produce overlapping signals (14). For instance, after digestion of the rRNA with RNase T1, nucleotide A2058 resides in a pentamer fragment of m/z 1680 together with five other rRNA fragments. This problem was overcome by a hybridization method (1) that enabled us to isolate the 23S rRNA sequence G2035 to G2087 (Fig. 1) and derive an unambiguous oligonucleotide containing A2058.
The target of Erm(38).
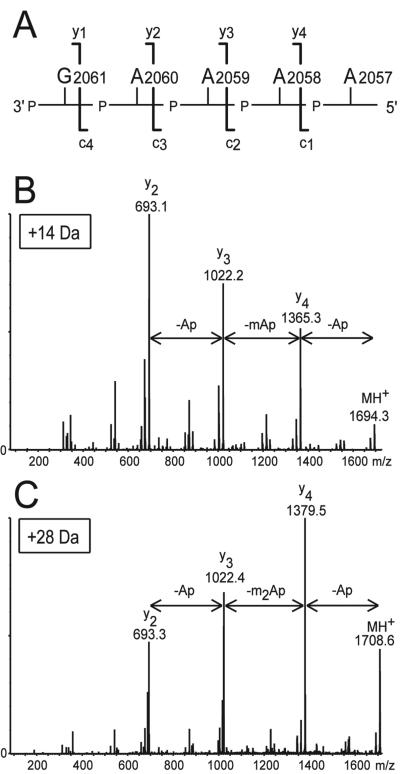
The Erm methyltransferase target at nucleotide A2058 is contained in the RNase T1 fragment, 5′-AAAAGp at m/z 1680.3, which is a unique signal under the analytical conditions used here (Fig. 2). Without expression of erm(38), no methylated fragments were observed (Fig. 2B). Induction of erm(38) expression resulted in new spectral signals at m/z 1694.3 and 1708.3 (Fig. 2C), corresponding to the addition of one methyl group (plus 14 Da) or two methyl groups (plus 28 Da). To determine the precise location of the methyl groups, the m/z 1694.3 and 1708.3 ions were analyzed further by MALDI quadrupole-time of flight tandem mass spectrometry. This technique was used to select and fragment ions to yield a degradation pattern from which the mass of each nucleotide could be deduced (Fig. 3A). The 14-Da and 28-Da mass increases were both shown to reside at nucleotide A2058 (Fig. 3B and C).
FIG.2.
(A) MALDI mass spectra of the M. smegmatis rRNA sequence spanning G2035 to G2087 after digestion with RNase T1. The spectral region around the pentanucleotide containing A2058 (5′-AAAAGp; m/z 1680.3) is enlarged on the m/z axis to show the methylated ions at m/z 1694.3 and 1708.3. (B) Control rRNA from cells without erythromycin induction (−ERY). (C) Sample from cells grown in presence of 6.4 μg/ml erythromycin (+ERY). (D and E) Samples from cells without erythromycin induction, but constitutively expressing Erm(O) and Erm(E) from plasmids. The spectra have been electronically smoothed by using the Proteometrics, Inc., m/z software program. (F) The calculated singly protonated masses of the RNase T1 fragments from the G2035 to G2087 sequence; A2058 is indicated in boldface type. The theoretical masses match the experimentally measured m/z values in panels A and B to within 0.1 Da.
FIG. 3.
Tandem mass spectra of single protonated 5′-AAAAGp methylation signals selected to within 1 Da of the main peak. (A) Backbone cleavage of the pentanucleotide 5′-AAAAGp (A2057 to G2061) results in a, b, c, and d ions from the 5′ end, and w, x, y, and z ions from the 3′ end (15). In the instrumentation setup used here, the y ions predominate (11); for the sake of simplicity, only these are indicated. The location of the single methyl group (in the m/z 1694.3 ion) and the two methyl groups (m/z 1708.6) can be seen from the shift in the y ion peaks. Loss of an unmodified adenosine nucleotide corresponds to a difference of 329 Da, a singly methylated adenosine is 343 Da, and a double methylated adenosine is 357 Da. (B) The mass difference between the parent ion (m/z 1694.3), the y4 ion (m/z 1365.3), the y3 ion (m/z 1022.2), and the y2 ion (m/z 693.1) clearly shows that the single methyl group is located on the second adenosine (A2058). (C) The fragmentation pattern of the m/z 1708.6 ion is equally unambiguous and shows that two methyl groups are attached to A2058. The spectra were smoothed with MassLynx software.
Similar mass spectrometry analyses were carried out for the rRNAs from M. smegmatis strains constitutively expressing erm(O) and erm(E). In cells with erm(O), the predominant AAAAGp fragment was at 1694.3 m/z (Fig. 2D); and in cells with erm(E), this fragment was exclusively at 1708.3 m/z (Fig. 2E). Analyses of the 1694.3 and 1708.3 m/z ions by quadrupole mass spectrometry established that Erm(O) and Erm(E) had, respectively, monomethylated and dimethylated nucleotide A2058. The qualitative data derived from the MALDI mass spectrometry were backed up with a quantitative assay using primer extension. N6,N6-dimethylation of adenosines halts the progress of reverse transcriptase, giving rise to a gel band that can be quantified, but the enzyme reads past unmethylated or N6-monomethylated adenines (13). Consistent with the mass spectrometry data, primer extension detected no dimethylation in rRNA from the erm(O) strain; expression of erm(38) gave rise to a minor amount of A2058 dimethylation; and A2058 dimethylation was stoichiometric in cells expressing erm(E) (Table 2).
DISCUSSION
In this report, we have characterized the rRNA methylation pattern produced in M. smegmatis by the resistance methyltransferase Erm(38). There was no evidence of erm(38) expression in the absence of inducer (Fig. 2B), and methyltransferase expression required prior incubation with noninhibitory amounts of an antibiotic such as erythromycin (18). A short leader sequence is located immediately upstream of the erm(38) cistron and encodes a peptide of 19 amino acids (Fig. 4). The structure of the leader sequence is reminiscent of the leader prefixing erm(C), which is inducible in a manner similar to that of erm(38) (9, 32). The upstream sequence of the erm(C) mRNA can fold into (at least) two conformations, and equivalent structures are evident in the corresponding region of the erm(38) mRNA. The more stable conformation (Fig. 4A) sequesters the start of the erm cistron, preventing its translation, whereas in an alternative conformation (Fig. 4B) this sequence is accessible for ribosomes to initiate translation of erm. Small amounts of inducers such as erythromycin presumably cause ribosomes to pause on the leader sequence and interfere with the folding of the mRNA, so that it adopts the conformation in Fig. 4B and erm(38) can be expressed.
FIG. 4.
Putative secondary structures of the erm(38) leader mRNA. (A) The more stable ground state structure in which the base-paired segments 1:2, 3:4, 5:8, and 6:7 are formed. Translation of erm(38) is prevented, as its ribosome binding site (Shine-Dalgarno 2 [SD2]) and initiation codon are sequestered in the secondary structure formed by segments 5:8. (B) Erythromycin-induced pausing of ribosomes on the leader is proposed to occupy the regions 1 to 3 and leave sequences 4 and 5 free to interact resulting in a rearranged conformation in which SD2 becomes accessible for translational initiation at the erm(38) cistron. In contrast to SD2, the SD1 sequence is a poor match with the consensus Shine-Dalgarno sequence, and initiation could also occur from an alternative sequence (SD1*, which is equally poor) using the in-frame methionine at codon 6. This would not alter the principle of the induction mechanism proposed here.
Induction of erm(38) expression in M. smegmatis led to an appreciable increase in the minimal concentration of erythromycin that was required to inhibit cell growth (Table 2). This change in growth pattern on erythromycin correlated with a change in the methylation status at 23S rRNA nucleotide A2058. No methylation at A2058 was observed prior to erm(38) induction, whereas a mixture of unmethylated, monomethylated, and dimethylated rRNA was evident after induction. Monomethylated A2058 has previously been shown to confer high resistance to lincosamides and moderate resistance to 14-member ring macrolide drugs such as erythromycin (13, 31, 33). The predominant modification signal (Fig. 2C) corresponded to monomethylated A2058, fitting the resistance pattern observed previously for Erm(38) expression in M. smegmatis (18). The small amount of dimethylation at A2058 would marginally enhance erythromycin resistance without further improving lincosamide resistance (13, 31, 33).
M. smegmatis is not particularly permeable to streptogramin B compounds; consequently, this species has a relatively high intrinsic tolerance towards quinupristin (MIC, 64 μg/ml). Higher resistance to quinupristin is conferred by dimethylation at A2058, with monomethylation having no detectable effect (Table 2). It follows that the methylation pattern in M. smegmatis rRNA after erm(38) induction (Fig. 2C) would hardly be expected to increase quinupristin tolerance beyond the ground state. Thus, we conclude that the resistance phenotypes conferred against streptogramin B and other drugs (Table 2) (18) fit well with the methylation activity of the Erm(38) enzyme. We cannot of course rule out that M. smegmatis ribosomes bind streptogramin B drugs in an anomalous manner (18), but there is no necessity to invoke such an explanation on the basis of the present data.
RNA modification by Erm dimethyltransferases is thought to follow the same reaction pathway seen for Erm(C) (6). The Erm(C) dimethyltransferase modifies 23S rRNA via a monomethylated intermediate, and the kinetics of the second methyl group addition is highly dependent on the concentration of the cofactor, S-adenosylmethionine (6). Whether an Erm enzyme adds a second methyl group to nucleotide A2058 is probably determined by the structure of the catalytic site and by how A2058 is accommodated there (26); however, alignments and comparison of the different Erm sequences give very few clear clues to distinguish between the mono- and dimethyltransferases. The empirical data presented here show that Erm(38) is indeed capable of dimethylating the rRNA and thus belongs to the dimethyltransferase branch of this enzyme family.
Erm(38) is, however, a rather lazy family member. The erythromycin induction conditions used here resulted in only a small proportion of the rRNA becoming dimethylated at nucleotide A2058. Possibly, Erm(38) activity could be boosted by raising the erythromycin concentration step-wise or by using other erm inducers (17). However, the increase in erythromycin resistance seen here is consistent (albeit 1 dilution less) with a previous report of erm(38) induction with the erythromycin derivative clarithromycin (18), which is markedly more effective at penetrating the M. smegmatis cell wall (4, 18, 21). The other two methyltransferases, Erm(O) and Erm(E), stoichiometrically methylated A2058, suggesting that the sluggish activity of Erm(38) is not due to physiological factors within M. smegmatis cells, such as limiting amounts of S-adenosylmethionine. The homologue erm(39) in M. fortuitum confers a ML resistance phenotype similar to that of erm(38) in M. smegmatis (19), presumably also by producing incompletely methylated rRNA. It remains to be determined whether the lackluster activity of the erm resistance determinants in nontuberculous mycobacteria is due to restrained gene expression or is an intrinsic property of the enzyme structure.
Acknowledgments
We thank Jean-Luc Pernodet for providing the M. smegmatis strains, as well as for scientific discussions. Jacob Poehlsgaard, Jens Øbro, and Rikke Lind Jensen are thanked for discussions and critical comments on the manuscript.
Support from the Danish Research Agency (FNU-rammebevilling 21-04-0520) and the Nucleic Acid Center of the Danish Grundforskningsfond is gratefully acknowledged.
REFERENCES
- 1.Andersen, T. E., B. T. Porse, and F. Kirpekar. 2004. A novel partial modification at 2501 in Escherichia coli 23S ribosomal RNA. RNA 10:907-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosne-David, S., V. Barros, S. C. Verde, C. Portugal, and H. L. David. 2000. Intrinsic resistance of Mycobacterium tuberculosis to clarithromycin is effectively reversed by subinhibitory concentrations of cell wall inhibitors. J. Antimicrob. Chemother. 46:391-395. [DOI] [PubMed] [Google Scholar]
- 3.Brown-Elliot, B. A., and R. J. J. Wallace. 2002. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin. Microbiol. Rev. 15:716-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buriankova, K., F. Doucet-Populaire, O. Dorson, A. Gondran, J.-C. Ghnassia, J. Weiser, and J.-L. Pernodet. 2004. Molecular basis of intrinsic macrolide resistance in the Mycobacterium tuberculosis complex. Antimicrob. Agents Chemother. 48:143-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corpet, F. 1988. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 16:10881-10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denoya, C., and D. Dubnau. 1989. Mono- and dimethylating activities and kinetic studies of the ermC 23S rRNA methyltransferase. J. Biol. Chem. 264:2615-2624. [PubMed] [Google Scholar]
- 7.Doucet-Populaire, F., K. Buriankova, J. Weiser, and J.-L. Pernodet. 2002. Natural and acquired macrolide resistance in mycobacteria. Curr. Drug Targets Infect. Disord. 2:355-370. [DOI] [PubMed] [Google Scholar]
- 8.Gutell, R. R., N. Larsen, and C. R. Woese. 1994. Lessons from an evolving rRNA: 16S and 23S rRNA structures from a comparative perspective. Microbiol. Rev. 58:10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horinouchi, S., and B. Weisblum. 1980. Posttranscriptional modification of mRNA conformation: mechanism that regulates erythromycin-induced resistance. Proc. Natl. Acad. Sci. USA 77:7079-7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirpekar, F., S. Douthwaite, and P. Roepstorff. 2000. Mapping posttranscriptional modifications in 5S ribosomal RNA by MALDI mass spectrometry. RNA 6:296-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirpekar, F., and T. N. Krogh. 2001. RNA fragmentation studied in a matrix-assisted laser desorption/ionisation tandem quadropole/orthogonal time-of-flight mass spectrometer. Rapid Commun. Mass Spectrom. 15:8-14. [DOI] [PubMed] [Google Scholar]
- 12.Le Dantec, C., N. Winter, B. Gicquel, V. Vincent, and M. Picardeau. 2001. Genomic sequence and transcriptional analysis of a 23-kilobase mycobacterial linear plasmid: evidence for horizontal transfer and identification of plasmid maintenance systems. J. Bacteriol. 183:2157-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu, M., and S. Douthwaite. 2002. Activity of the ketolide antibiotic telithromycin is refractory to Erm monomethylation of bacterial rRNA. Antimicrob. Agents Chemother. 46:1629-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madsen, C. T., J. Mengel-Jorgensen, F. Kirpekar, and S. Douthwaite. 2003. Identifying the methyltransferases for m5U747 and m5U1939 in 23S rRNA using MALDI mass spectrometry. Nucleic Acids Res. 31:4738-4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLuckey, S. A., G. J. Van Berkel, and G. L. Glish. 1992. Tandem mass spectrometry of small multiply charged oligonucleotides. J. Am. Soc. Mass Spectrom. 3:60-70. [DOI] [PubMed] [Google Scholar]
- 16.Mengel-Jorgensen, J., and F. Kirpekar. 2002. Detection of pseudouridine and other modifications in tRNA by cyanoethylation and MALDI mass spectrometry. Nucleic Acids Res. 30:E135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Min, Y.-H., J.-H. Jeong, Y.-J. Choi, H.-J. Yun, K. Lee, M.-J. Shim, J.-H. Kwak, and E.-C. Choi. 2003. Heterogeneity of macrolide-lincosamide-streptogramin B resistance phenotypes in enterococci. Antimicrob. Agents Chemother. 47:3415-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nash, K. A. 2003. Intrinsic macrolide resistance in Mycobacterium smegmatis is conferred by a novel erm gene, erm(38). Antimicrob. Agents Chemother. 47:3053-3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nash, K. A., Y. Zhang, B. A. Brown-Elliott, and R. J. Wallace, Jr. 2005. Molecular basis of intrinsic macrolide resistance in clinical isolates of Mycobacterium fortuitum. J. Antimicrob. Chemother. 55:170-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noller, H. F. 1984. Structure of ribosomal RNA. Annu. Rev. Biochem. 53:119-162. [DOI] [PubMed] [Google Scholar]
- 21.Pfister, P., N. Corti, S. Hobbie, C. Bruell, R. Zarivach, A. Yonath, and E. C. Böttger. 2005. 23S rRNA base pair 2057-2611 determines ketolide susceptibility and fitness cost of the macrolide resistance mutation 2058A→G. Proc. Natl. Acad. Sci. USA 102:5180-5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfister, P., S. Jenni, J. Poehlsgaard, A. Thomas, S. Douthwaite, N. Ban, and E. C. Böttger. 2004. The structural basis of macrolide-ribosome binding assessed using mutagenesis of 23S rRNA positions 2058 and 2059. J. Mol. Biol. 342:1569-1581. [DOI] [PubMed] [Google Scholar]
- 23.Pierre-Audigier, C., E. Jouanguy, S. Lamhamedi, F. Altare, J. Rauzier, V. Vincent, D. Canioni, J. F. Emile, A. Fischer, S. Blanche, J. L. Gaillard, and J. L. Casanova. 1997. Fatal disseminated Mycobacterium smegmatis infection in a child with inherited interferon gamma receptor deficiency. Clin. Infect. Dis. 24:982-984. [DOI] [PubMed] [Google Scholar]
- 24.Roberts, M. C. 2004. Resistance to macrolide, lincosamide, streptogramin, ketolide, and oxazolidinone antibiotics. Mol. Biotechnol. 28:47-62. [DOI] [PubMed] [Google Scholar]
- 25.Roberts, M. C., J. Sutcliffe, P. Courvalin, L. B. Jensen, J. Rood, and H. Seppala. 1999. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob. Agents Chemother. 43:2823-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schluckebier, G., P. Zhong, K. D. Stewart, T. J. Kavanaugh, and C. Abad-Zapatero. 1999. The 2.2 Å structure of the rRNA methyltransferase ErmC′ and its complexes with cofactor and cofactor analogs: implications for the reaction mechanism. J. Mol. Biol. 289:277-291. [DOI] [PubMed] [Google Scholar]
- 27.Sigmund, C. D., M. Ettayebi, A. Borden, and E. A. Morgan. 1988. Antibiotic resistance mutations in ribosomal RNA genes of Escherichia coli. Methods Enzymol. 164:673-690. [DOI] [PubMed] [Google Scholar]
- 28.Skinner, R., E. Cundliffe, and F. Schmidt. 1983. Site of action of a ribosomal RNA methylase responsible for resistance to erythromycin and other antibiotics. J. Biol. Chem. 258:12702-12706. [PubMed] [Google Scholar]
- 29.Thompson, C. J., R. H. Skinner, J. Thompson, J. M. Ward, D. A. Hopwood, and E. Cundliffe. 1982. Biochemical characterization of resistance determinants cloned from antibiotic-producing streptomycetes. J. Bacteriol. 151:678-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagner, D., and L. S. Young. 2004. Nontuberculous mycobacterial infections: a clinical review. Infection 32:257-270. [DOI] [PubMed] [Google Scholar]
- 31.Weisblum, B. 1995. Erythromycin resistance by ribosome modification. Antimicrob. Agents Chemother. 39:577-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weisblum, B. 1995. Insights into erythromycin action from studies of its activity as inducer of resistance. Antimicrob. Agents Chemother. 39:797-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zalacain, M., and E. Cundliffe. 1990. Methylation of 23S ribosomal RNA due to carB, an antibiotic-resistance determinant from the carbomycin producer, Streptomyces thermotolerans. Eur. J. Biochem. 189:67-72. [DOI] [PubMed] [Google Scholar]