Abstract
Nucleobase and nucleoside transporters play central roles in the biochemistry of parasitic protozoa, as they lack the ability to synthesize purines de novo and are absolutely reliant upon purine salvage from their hosts. Furthermore, such transporters are potentially critical to the pharmacology of these important human pathogens, because they mediate the uptake of purine analogues, as well as some nonpurine drugs, that can be selectively cytotoxic to the parasites. We here report the first identification and characterization of a purine nucleobase transporter in Leishmania amastigotes. Uptake of [3H]hypoxanthine by Leishmania mexicana amastigotes was mediated by a single high-affinity transporter, LmexNBT1, with a Km of 1.6 ± 0.4 μM and high affinity for adenine, guanine, and xanthine but low affinity for nucleosides and pyrimidine nucleobases. Allopurinol, an antileishmanial hypoxanthine analogue, was apparently taken up by the same transporter. Using [3H]allopurinol, a Km value of 33.6 ± 6.0 μM was obtained. All evidence was compatible with a model of a single purine nucleobase transporter being expressed in amastigotes. Using various purine nucleobase analogues, a model for the interactions between hypoxanthine and the transporter's permeant binding site was constructed. The binding interactions were compared with those of the LmajNBT1 transporter in Leishmania major promastigotes and found to be very similar.
Parasitic protozoa of the genus Leishmania are the etiological agents of leishmaniasis. Some 12 million people throughout the world suffer from leishmaniasis, ranging from the disfiguring cutaneous form to often-fatal visceral leishmaniasis. Leishmania species are sandfly-transmitted protozoan parasites. The life cycle is divided into promastigotes and metacyclics in the insect vectors and amastigotes in their mammalian hosts, which are responsible for all clinical manifestations.
Most of the currently available antileishmanial drugs have been discovered empirically, as until recently insufficient information was known about the biochemistry, physiology, and molecular biology of these parasites and the interactions with their hosts (23). The limitations of the current treatment for leishmaniasis, as a result of drug resistance and the severe side effects of most of the existing therapeutic agents, and the urgent need for new therapeutic approaches, are well documented (12, 56, 60).
The development of a rational therapeutic strategy for the treatment and prevention of parasitic disease depends on exploitation of fundamental biochemical disparities between parasite and host, such as the inability of protozoan parasites to synthesize purines de novo (5). Current antiprotozoal agents often derive selectivity from selective accumulation by the parasite rather than the host cell (15). The selectivity and the efficacy of purine antimetabolites can be achieved by the cell surface transporters that mediate access to the cell, as substrate recognition by nucleobase transporters is strikingly different in humans and kinetoplastids such as Trypanosoma brucei (58) and Leishmania major promastigotes (2). Purine and pyrimidine antimetabolites have been highly successful against many viral infections as well as malignancies (27) and show great promise against protozoal infections as well (reviewed in references 14, 17, and 23). Allopurinol, a purine nucleobase analogue, is clinically used against various manifestations of leishmaniasis (1, 13, 39).
Whereas purine transporters in promastigotes have now been studied in detail (see references 17 and 29 for recent reviews), very little is known about such transporters in the infective amastigote forms. The only study of purine transporters in amastigotes demonstrated that there are at least two high-affinity adenosine transporters in Leishmania donovani amastigotes, T1 and T2 (24). Purine nucleobase transport in this intracellular form has yet to be described, despite a clear interest from physiological and pharmacological perspectives.
In a previous study, we have shown that allopurinol is efficiently taken up by a high-affinity purine nucleobase transporter in L. major promastigotes, LmajNBT1 (2). We now report the first characterization of nucleobase transport in Leishmania mexicana amastigotes, including the transport of allopurinol. Our data are consistent with a single broad-specificity nucleobase transporter mediating the uptake of all natural purine nucleobases and allopurinol. The functional characterization of this transporter, LmexNBT1, showed it to be virtually identical to LmajNBT1.
MATERIALS AND METHODS
Materials.
Nonradioactive nucleobases and nucleosides were obtained as indicated in the footnotes to Table 1. [2, 8-3H]adenine (1.19 TBq/mmol) was bought from NEN (Boston, MA), [8-3H]hypoxanthine (1.04 TBq/mmol) and [2-3H]adenosine (0.92 TBq/mmol) were purchased from Amersham Pharmacia Biotech (Buckinghamshire, United Kingdom), and [3H(G)]allopurinol (44.4 GBq/mmol) was obtained from Moravek (Brea, CA).
TABLE 1.
Kinetic constants of purine nucleobase uptake in L. mexicana amastigotes and L. major promastigotesa
| Parameter (unit) | Value, mean ± SE (n) for:
|
|||
|---|---|---|---|---|
|
L. mexicana amastigotes
|
L. major promastigotes
|
|||
| [3H]hypoxanthine | [3H]allopurinol | [3H]adenine | [3H]allopurinol | |
| Km (μM) | 1.6 ± 0.4 (4) | 33.6 ± 6.0 (3) | 4.6 ± 0.9 (3) | 54.3 ± 2.9 (3) |
| Vmax (pmol 107 cells−1 s−1) | 0.092 ± 0.057 (4) | 0.051 ± 0.017 (3) | 3.2 ± 0.3 (3) | 0.24 ± 0.06 (3) |
| Ki (μM) with: | ||||
| Adenineb | 4.2 ± 0.8 (5) | |||
| Hypoxanthineb | 3.8 ± 0.6 (3) | 1.3 ± 0.3 (3) | 0.30 ± 0.09 (3) | |
| Guanineb | 1.7 ± 0.1 (4) | 2.8 ± 0.7 (4) | ||
| Xanthineb | 13 ± 2 (3) | 23 ± 8 (3) | ||
| Allopurinolb | 39 ± 6 (3) | 56 ± 1.5 (3) | ||
| Aminopurinolc | 170 ± 20 (4) | NDd | ||
| Purineb | 3.4 ± 0.2 (3) | 6.7 ± 0.4 (3) | ||
| 1-Deazapurinec | 54 ± 5 (3) | 26 ± 4.1 (3) | ||
| 3-Deazaguanine | 130 ± 14 (3) | 48 ± 5.0 (3) | ||
| 6-Thioguanineb | 10 ± 1.2 (3) | 6.2 ± 0.8 (3) | ||
| 7-Deazaguanine | >1,000 (3) | 430 ± 140 (3) | ||
| 9-Deazaguaninee | 204 ± 28 (4) | 204 ± 4 (3) | ||
| Adenosineb | 950 ± 240 (3) | 5,150 ± 550 (3) | ||
| Inosineb | 380 ± 26 (3) | 125 ± 15 (3) | ||
| Guanosineb | 210 ± 48 (3) | 68 ± 17 (4) | ||
Kinetic parameters were determined through competitive inhibition of [3H]hypoxanthine (amastigotes) or [3H]adenine (promastigotes). In a few cases, extrapolation was required due to limitations of solubility of the inhibitor and based on the assumption of a Hill slope of −1 and eventual 100% inhibition. Extrapolation was not attempted when inhibition at the highest inhibitor concentration was <50%. Permeant concentrations were 2 μM (allopurinol) or 0.1 to 0.5 μM (hypoxanthine), except for determinations of Km. Data for L. major promastigotes are from reference 2.
Obtained from Sigma.
Obtained from Aldrich.
ND, not determined.
Gift from Howard Cottam (University of California, San Diego).
Leishmania culture.
Axenic cultures of the MNYC/BZ/62/M379 strain of Leishmania mexicana were used throughout. Amastigotes were maintained by twice-weekly serial passage in Schneider's Drosophila medium (Gibco), including 20% heat-inactivated fetal calf serum (Gibco) and 0.3% gentamicin. The pH of the medium was adjusted to 5.5 with 1.0 M HCl. Cultures were maintained at 33°C in a CO2 incubator. Amastigotes were harvested by centrifugation (10 min, 2,500 rpm), washed twice with a modified CBSS buffer (33 mM MES [morpholineethanesulfonic acid], 98 mM NaCl, 4.6 mM KCl, 0.3 mM CaCl2, 0.07 mM MgSO4, 5.8 mM NaH2PO4, 0.3 mM MgCl2, 14 mM d-glucose, pH 6.0), and resuspended at 108 cells/ml in the same buffer (24).
Transport assays.
Transport of radiolabeled purine nucleosides and nucleobases (adenosine, adenine, hypoxanthine, and allopurinol) into L. mexicana amastigotes was performed using the oil stop technique. The amastigotes were harvested by centrifugation (2,500 × g, 10 min) and washed twice in modified CBSS buffer. Cells were washed and resuspended in the same buffer at 32°C and used at approximately 107 cells/ml. Transport was measured at 32°C. One hundred microliters of cell suspension was added to a microcentrifuge tube containing 200 μl oil (7:1 [vol/vol] dibutyl-phthalate/mineral oil; d = 1.018 g/ml) and 100 μl [3H]adenine, [3H]hypoxanthine, [3H]adenosine, or [3H]allopurinol (concentrations as indicated below) and incubated for the indicated times. Incubations were terminated by adding 1 ml ice-cold 4 mM unlabeled permeant in CBSS buffer, and cells were separated from extracellular label by centrifugation through the oil (12,000 × g, 30 s). The tubes were frozen in liquid nitrogen and the tips containing the cell pellet cut off. The pellet was dissolved in 250 μl 2% sodium dodecyl sulfate. Scintillation fluid (3 ml) (Optiphase HiSafe III; Perkin-Elmer) was added, and radioactivity was determined in a 1450 MicroBeta Trilax scintillation counter. Zero-uptake values were obtained in the presence of 1 mM unlabeled permeant at 0°C. Nonmediated transport was determined in the presence of 1 mM unlabeled permeant at 32°C. Transport values were calculated after subtraction of the zero-uptake values. In inhibition studies, test compounds were mixed with 3H-labeled permeant prior to addition of cells.
Data analysis.
The Prism 3 software package (GraphPad Software, San Diego, CA) was used to calculate the kinetic parameters, given as means and standard errors, by using nonlinear regression. Inhibition data were plotted to a sigmoid curve with a variable slope and used to determine 50% inhibitory concentrations (IC50s) (based on a minimum of six points in triplicate). Ki values were then obtained using the Cheng-Prusoff equation (10), Ki = IC50/[1 + (L/Km)], where L is the permeant concentration and Km is the value obtained for this permeant. The Gibbs free energy of the transporter-ligand interaction was then calculated from ΔG° = −RTln(Ki), in which R is the gas constant and T the absolute temperature. Errors given in tables and shown as bars in figures are standard errors. It should be noted that these equations are valid only for competitive inhibition, as described previously (19, 58).
Toxicity test.
The drug sensitivity assay with Alamar blue was performed as described for T. brucei and Leishmania (38, 51) in 96-well plates, with some modifications. Briefly, 100 μl of Schneider's Drosophila medium (Gibco), used for culturing amastigotes, was added to wells, leaving the first column empty. Two hundred microliters of different test compounds was added, at double the final concentration, to the first column. Doubling dilutions were performed, leaving one well drug free as negative control. L. mexicana amastigotes were inoculated into each well at a final density of 1 × 106/ml culture medium. Twenty microliters of Alamar blue reagent (TREK Diagnostic Systems, Ltd.) was added after 48 h and fluorescence development determined after a further incubation time of 72 h. The plates were read using a LS55 luminescence spectrometer (Perkin-Elmer) at 530-nm excitation and 590-nm emission wavelengths.
RESULTS
Purine transport in axenic amastigotes of Leishmania.
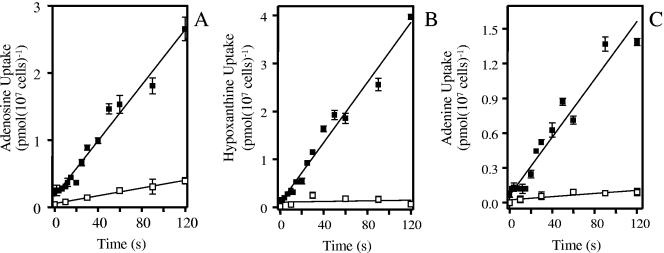
Total uptake of adenosine in axenic amastigote forms of L. mexicana was determined using a rapid stop oil spin protocol adapted from similar protocols with various other protozoa (2, 16, 18-20). Figure 1A demonstrates that the transport of 1 μM [3H]adenosine was linear for at least 120 s at 0.021 ± 0.001 pmol 107 cells−1 s−1 (linear regression; r2 = 0.97). The addition of 1 mM unlabeled adenosine inhibited the uptake of 1 μM [3H] adenosine by 87% (r2 = 0.99), indicating that the vast majority of adenosine transport occurs via a mediated pathway (Fig. 1A). These observations are consistent with an earlier report for adenosine transport in amastigote forms of L. donovani (24).
FIG. 1.
Linear purine transport in axenic amastigotes of Leishmania mexicana. A. Time course of 1 μM [3H]adenosine uptake by L. mexicana amastigotes in the presence (□) or absence (▪) of 1 mM unlabeled adenosine. B. Uptake of 1 μM [3H]hypoxanthine was linear over 120 s (r2 = 0.97), as calculated by linear regression, in the presence (□) or the absence (▪) of 1 mM unlabeled hypoxanthine. C. Uptake of 1 μM [3H]adenine was linear over 120 s (r2 = 0.94), as calculated by linear regression, in the presence (□) or the absence (▪) of 1 mM unlabeled adenine.
Uptake of [3H]hypoxanthine at 1 μM by the axenic amastigotes of L. mexicana was linear for up to 120 s (Fig. 1B) and faster than [3H]adenosine uptake, at 0.031 ± 0.001 pmol 107 cells−1 s−1 (r2 = 0.98). In the presence of 1 mM unlabeled hypoxanthine, [3H]hypoxanthine transport was not significantly different from zero (P = 0.7, F test), indicating high-affinity transport that is completely saturated at 1 mM. [3H]adenine uptake (1 μM) was likewise linear over 120 s, and this was the least efficiently accumulated of the three purines tested, with a rate of 0.012 ± 0.001 pmol 107 cells−1 s−1, which was almost 95% inhibited by 1 mM unlabeled adenine (Fig. 1C). Subsequent experiments were performed with [3H]hypoxanthine and 60-s incubations, which are well within the linear phase of uptake and therefore measure true initial rates of transport.
A high-affinity purine nucleobase transporter in amastigotes.
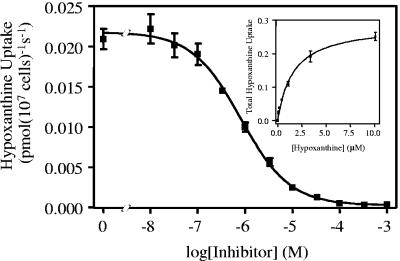
[3H]hypoxanthine transport (0.1 μM) measured in the presence of up to 1 mM of unlabeled hypoxanthine was monophasic and complied with simple Michaelis-Menten kinetics, yielding a Km of 1.6 ± 0.4 μM and a Vmax of 0.092 ± 0.057 pmol 107 parasites−1 s−1 (n = 4) (Fig. 2). The transport of [3H]hypoxanthine was inhibited by a range of purine nucleobases and nucleosides (Table 1) but not significantly by various pyrimidines at up to 1 mM (P > 0.05 by a paired Student's t test against a no-inhibitor control; n = 3). The hypoxanthine transporter, designated L. mexicana nucleobase transporter 1 (LmexNBT1), appears to be mildly selective for oxopurines over aminopurines, judging from the Ki values for guanine and adenine (1.7 ± 0.1 and 4.2 ± 0.8 μM, respectively). None of the inhibition plots suggested the presence of more than one hypoxanthine transporter in L. mexicana amastigotes, as Hill slopes were consistently near −1 and complete inhibition to control values (transport at 0°C, 0 s) was generally observed.
FIG. 2.
Transport of 0.1 μM [3H]hypoxanthine over 60 s was inhibited by the indicated concentrations of unlabeled hypoxanthine, with an IC50 of 0.81 μM for this experiment. The inset depicts the conversion of the hypoxanthine inhibition data to a Michaelis-Menten plot of total hypoxanthine uptake, with a Km of 1.6 μM and a Vmax of 0.29 pmol 107 cells−1 s−1 for this experiment, showing total hypoxanthine transport as opposed to [3H]hypoxanthine transport only.
[3H]allopurinol transport by L. mexicana amastigotes.
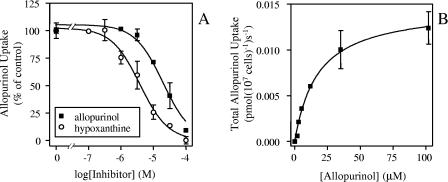
Allopurinol is a close structural analogue of hypoxanthine and in L. major promastigotes is indeed accumulated through the hypoxanthine transporter LmajNBT1 (2). We therefore investigated whether a similar situation exists in amastigotes. As L. major amastigotes cannot be kept in axenic culture, we used amastigotes from L. mexicana. Using these cells, transport of allopurinol was clearly less efficient than that of hypoxanthine, with a rate for 5 μM [3H]allopurinol of 0.0061 ± 0.0002 pmol 107 cells−1 s−1 (linear regression of six points over 4 min; r2 = 0.99), which was completely inhibited by 1 mM hypoxanthine (uptake not significantly different from zero; P = 0.33 by F test). Transport of [3H]allopurinol (5 μM) was still detectable, however, in the presence of 1 mM unlabeled allopurinol (0.00052 ± 0.00014 pmol 107 cells−1 s−1) (linear regression of five points over 10 min; P = 0.03 by F test), although it was greatly reduced. These results indicate that the allopurinol transporter has a higher affinity for hypoxanthine than for allopurinol.
Competition experiments showed that transport of 2 μM [3H]allopurinol over 120 s was indeed inhibited more strongly by hypoxanthine than by allopurinol, with a Ki of 3.8 ± 0.6 μM for hypoxanthine and a Km of 33.6 ± 6.0 μM for allopurinol (n = 3) (Fig. 3). These data are all consistent with a single high-affinity transporter for purine nucleobases, including allopurinol. While the Vmax values of LmexNBT1 for hypoxanthine and allopurinol are similar (Table 1), the efficiency of uptake, expressed as Km/Vmax, is considerably higher for hypoxanthine.
FIG. 3.
Transport of [3H]allopurinol by L. mexicana amastigotes. A. Inhibition of 2 μM [3H]allopurinol transport over 120 s by up to 100 μM of unlabeled allopurinol (▪) or hypoxanthine (○). B. Michaelis-Menten plot of allopurinol uptake, derived from the data in panel A.
Structure-activity relationships of purine analogues and the LmexNBT1 transporter.
The inhibitory effects of purine analogues for LmexNBT1 were tested by measuring initial uptake rates of 0.1 μM [3H]hypoxanthine in the presence of various concentrations of the test compound. Results were plotted as log inhibitor concentration versus hypoxanthine uptake, and Ki values, calculated from the IC50s, were used to calculate the estimated Gibbs free energy of binding, ΔG° (reviewed in reference 17). The results, summarized in Tables 1 and 2, show that the LmexNBT1 transporter shows broad specificity for purine nucleobases but displays low affinity for the corresponding nucleosides.
TABLE 2.
Gibbs free energies of substrates interacting with the LmexNBT1 transportera
| Substrate | ΔG° (kJ/mol) | δ(ΔG°) (kJ/mol) | Control |
|---|---|---|---|
| Hypoxanthine | −34.4 | ||
| Adenine | −31.9 | 2.5 | HX |
| Allopurinol | −26.2 | 8.2 | HX |
| Aminopurinol | −22.3 | 9.6 | Adenine |
| Xanthine | −28.9 | 5.5 | HX |
| Guanine | −34.2 | 0.2 | HX |
| Inosine | −20.3 | 14.1 | HX |
| Adenosine | −17.9 | 14.0 | Adenine |
| Guanosine | −21.9 | 12.3 | Guanine |
| Purine | −32.5 | −0.6 | Adenine |
| 1-Deazapurine | −25.3 | 7.2 | Purine |
| 3-Deazaguanine | −23.0 | 11.2 | Guanine |
| 6-Thioguanine | −29.7 | 4.5 | Guanine |
| 7-Deazaguanine | >−18 | 16.2 | Guanine |
| 9-Deazaguanine | −21.9 | 12.3 | Guanine |
The Gibbs free energy of substrate-transporter interaction was calculated from the Km and Ki values listed in Table 1, using the Nernst equation as described previously (19,58). The difference between the value and that with a control compound (either hypoxanthine [HX] as the highest-affinity compound, the corresponding physiological nucleobase [in the case of chemical analogues], or the corresponding nucleobase [in the case of nucleosides]) yielded the δ(ΔG°), the loss in binding energy relative to the control compound.
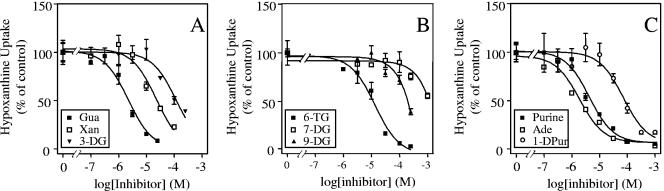
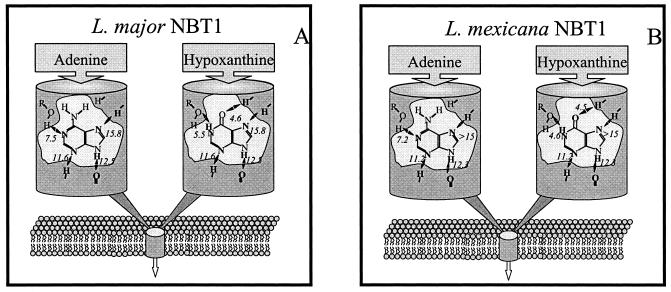
LmexNBT1 displayed equal affinity for guanine (Fig. 4A) and hypoxanthine (Fig. 2), showing that the 2-amino group of guanine did not contribute to binding or pose a steric hindrance to binding. However, affinity for xanthine (Fig. 4A) was markedly reduced [δ(ΔG°) = 5.5 kJ/mol compared to hypoxanthine]. As this is unlikely to be the result of steric hindrance at position 2, this must be the result of protonation of the pyrimidine nitrogen at position 3 of the purine ring that occurs in xanthine. A comparison between guanine and 3-deazaguanine (Fig. 4A) shows that the unprotonated N-3 contributes 11.2 kJ/mol to the binding energy of guanine (Table 2). As only 5.5 kJ/mol is lost in xanthine, it can be postulated that weak hydrogen bonds totaling ∼6 kJ/mol can be established between the LmexNBT1 binding pocket and N(3)H and/or the 2-keto group of xanthine. From inhibition plots with 9-deazaguanine (Fig. 4B), the Gibbs free energy of H bonds with N(9)H was similarly estimated at −12.3 kJ/mol (Table 2). The Ki for 7-deazaguanine could not be clearly determined, due to very low affinity and solubility limitations, but was shown to be over 1 mM, which would indicate a δ(ΔG°) of >15 kJ/mol for N-7. Furthermore, the substitution of the 6-keto group for a thione group (6-thioguanine) reduced the ΔG° by 4.5 kJ/mol (6-thioguanine versus guanine) (Fig. 4B). This could be an indication of a hydrogen bond interacting with the keto group, as thiones are much weaker H bond acceptors (50), although the possibility of a steric effect by the larger sulfur atom provides an alternative explanation. Indeed, the δ(ΔG°) between hypoxanthine and purine, which lacks both the 6-keto and lactam hydrogen on N-1, is only 1.9 kJ/mol (Table 2). However, this needs to take into account the strong hydrogen bond with the pyrimidine N-1, estimated at 7.2 kJ/mol from the comparison between purine and 1-deazapurine (Fig. 4C), bringing the actual contribution of the 6-keto/N(1)H combination to 9.1 kJ/mol, of which 4.5 kJ/mol could be tentatively assigned to the keto group from the δ(ΔG°) of 6-thioguanine. Finally, adenine (Fig. 4C) and purine proved to have the same affinity for LmexNBT1 (Table 1), confirming the preference for a hydrogen bond acceptor at position 6 of the purine ring. The above observations were combined into a model for the binding of purine substrates by LmexNBT1 and compared to the model for substrate binding by the equivalent transporter of L. major (Fig. 5).
FIG. 4.
Inhibition of 0.1 μM [3H]hypoxanthine uptake in axenic amastigotes of L. mexicana by guanine (Gua), xanthine (Xan), and 3-deazaguanine (3-DG) (A); by 6-thioguanine (6-TG), 7-deazaguanine, and 9-deazaguanine (B); and by purine, adenine (Ade), and 1-deazapurine (1-DPur) (C). Data are expressed as percentages of control values (uptake in the absence of any inhibitor, typically 0.005 to 0.02 pmol/107 cells/s).
FIG. 5.
Schematic representation of the LmajNBT1 (A) and LmexNBT1 (B) transporters and the interactions with their permeants, adenine and hypoxanthine. Estimated Gibbs free energies for proposed bonds are indicated, in negative kilojoules per mole. The model for LmajNBT1 was adapted from reference 2, with permission.
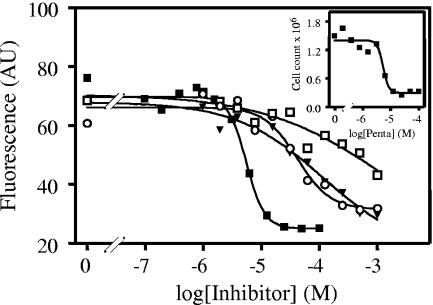
Antileishmanial activity of some nucleobase analogues.
Allopurinol is well known to possess antileishmanial activity, but we previously noted that, at least in promastigotes, aminopurinol and the pyrimidine nucleobase 5-fluorouracil displayed substantially higher activity (46). We therefore tested the same compounds on the human-infective amastigote stage, using pentamidine as a positive control and the same technique based on the fluorophore Alamar blue (Fig. 6). The method was validated using cell counts in parallel to the fluorescence assay, yielding the same IC50s (Fig. 6, inset). As with L. major promastigotes, aminopurinol and 5-fluorouracil were 10- to 20-fold more active against axenic L. mexicana amastigotes than allopurinol. IC50s (in micromolar; n = 3) were 5.6 ± 1.0, 630 ± 87, 65 ± 5, and 36 ± 6 for pentamidine, allopurinol, aminopurinol, and 5-fluorouracil, respectively.
FIG. 6.
Effects of various drugs on L. mexicana amastigotes in axenic culture. Amastigotes (2 × 105) were incubated with doubling dilutions of pentamidine (▪), allopurinol (□), aminopurinol (▾), and 5-fluorouracil (○) for 72 h and for an additional 48 h in the presence of Alamar blue reagent as described in Materials and Methods. Fifty percent effective doses for pentamidine as determined by fluorescence or cell counts (inset) were identical.
DISCUSSION
Purine and pyrimidine antimetabolites are widely used to combat a variety of infectious diseases and other pathologies (14). Current evidence indicates that purine analogues alone or in combination with other chemotherapeutic agents are very effective against almost any fast-growing cells (22, 41). Allopurinol, a purine analogue, has been used to treat leishmaniasis alone (34, 48) or combined with other drugs (21, 35, 36, 39, 47). It should be pointed out, however, that the efficacy of allopurinol monotherapy remains unclear. A large-scale randomized trial sponsored by the World Health Organization showed no difference between allopurinol- and placebo-treated groups (57). It may be that the value of allopurinol in the treatment of leishmaniasis is solely by synergistically enhancing the action of other treatments, and there is much, although largely anecdotal, evidence for this. One such report, by Chunge et al. (11), describes the treatment of five patients with visceral leishmaniasis unresponsive to sodium stibogluconate who were cured by a combination of the same drug plus additional allopurinol. In vitro as well, allopurinol has been reported to augment the antileishmanial effects of pentavalent antimonials (7). Other pyrazolopyrimidines, such as 9-deazainosine, displayed favorable therapeutic indexes in L. donovani-infected hamsters (6) and also offer promise as potential chemotherapeutic agents (32). However, it is widely acknowledged that the potential for damaging side effects can pose a challenge to the development of purine-based drugs (23).
Selectivity and efficacy of purine antimetabolites can be achieved through the enzymes of the purine metabolic pathways that convert the prodrug to the cytotoxic metabolite, usually a nucleotide analogue, and understanding of the substrate specificities of the enzymes involved is important in both drug efficacy and selectivity. The metabolism of allopurinol in Leishmania species to the active metabolite 4-aminopyrazolo(3,4-d)pyrimidine ribonucleoside triphosphate, which is incorporated into RNA, has been well studied (30, 31, 33, 43, 45). The drug is selective, because mammalian cells do not show this conversion or incorporation (44).
In addition, many antiprotozoal drugs derive their selective activity through uptake by cell surface transporters on the target cell rather than the host's cells (15). The main human nucleobase transporter, hFNT1, displays very low affinity for allopurinol (52, 58), whereas allopurinol uptake in Trypanosoma brucei was mediated by high-affinity nucleobase transporters (18, 42). We have demonstrated that a strategy of purine-based chemotherapy, designed for specific uptake by protozoan parasites, is feasible and depends on detailed knowledge of the substrate recognition motifs of the respective transporters (17, 58, 59).
We recently reported the characterization of [3H]hypoxanthine, [3H]allopurinol, and [3H]uracil transport in L. major promastigotes (2, 46), but the presence of nucleobase transporters in amastigotes has not yet been reported. The issue is important in understanding the selectivity of the drug as well as the potential for the development of resistance, as allopurinol-resistant Leishmania lines have been described (9, 26) and resistance appears to be easily inducible (K. Soteriadou, personal communication). We consider that this resistance might be related to loss of transporter function, as allopurinol was taken up by a single nucleobase transporter in L. major promastigotes (2), in sharp contrast to the situation in T. brucei brucei, where the presence of multiple allopurinol transporters appears to preclude transport-related resistance (42). We have therefore conducted a study of purine nucleobase uptake in Leishmania mexicana amastigotes, with particular emphasis on allopurinol uptake. We found that hypoxanthine and allopurinol are taken up by a single plasma membrane transporter, LmexNBT1, and we characterized its substrate profile in detail.
LmexNBT1 proved to be extremely similar to LmajNBT1. A comparison of their substrate binding models (Fig. 5) reveals that the positions and even the relative strengths of the hydrogen bonds are completely conserved between the two transporters, suggesting very similar architectures for the two transporters. This level of conservation is remarkable in that it signifies conservation both throughout the life cycle and between Leishmania species from different continents. Furthermore, the binding site architecture of these transporters appears to be very similar to that of the H2 hypoxanthine/allopurinol transporter in bloodstream T. brucei brucei but very different from the binding motif of hFNT1 (2, 58). This suggests that these related kinetoplastids would accumulate similar purine antimetabolites, which could be designed to be excluded from human cells. At present, none of the genes for these involved transporters have been identified with any certainty, precluding an assessment of whether the high level of functional conservation between the various T. brucei and Leishmania transporters is matched by genetic conservation. However, all protozoan purine transporter genes cloned to date are members of the equilibrative nucleoside transporter family (17), and the percent identity between the T. brucei brucei TbNBT1/H4 or TbNT8.1 nucleobase transporters and the L. major LmNT3 nucleobase transporter is only 50% (8, 25, 53).
LmexNBT1 showed only moderate affinity for allopurinol and aminopurinol. Yet, it seems unlikely that transport rates are a limiting factor in the efficacy of pyrazolopyrimidines against leishmaniasis, the conversion to the aminopurinol riboside triphosphate being a more likely bottleneck: aminopurinol, despite having lower affinity for LmexNBT1, was 10-fold more active against axenic amastigotes in culture. Nelson and coworkers reported the direct incorporation of aminopurinol into the aminopurine nucleotide pool, and subsequently into the RNA, of both Leishmania and Trypanosoma cruzi (33, 43) by means of phosphoribosylation, a conversion that does not happen in humans (44).
This begs the question as to why aminopurinol has not been used against leishmaniasis, particularly as it has been long known that aminopurinol is more potent than allopurinol against Leishmania promastigotes in vitro (4) as well as against T. cruzi in mice (3). In mammals, aminopurinol is used as an experimental drug, which reduces the release of lipoproteins by the liver, lowering plasma cholesterol levels (55). Chronic use, however, induces a “fatty liver” (40) and adrenal cell hypertrophy by depleting adrenal cholesterol (28, 37). Aminopurinol given to mice for 10 days at 1 mg/kg induced no toxic effect, but an increase to 10 mg/kg caused high mortality rates and hepatomegaly (3). A toxicological study with rodents, dogs, and cats found tolerance to high single doses of aminopurinol but mouse 50% lethal doses of 25 mg/kg when five consecutive daily doses were given (49). The hepatotoxic effects led to the abandonment of aminopurinol as a potential antileukemia drug (54), but levels active against T. cruzi in mice (3) were found to be nontoxic to humans (54).
In summary, we have undertaken the first study of nucleobase transport in Leishmania amastigotes and identified a high-affinity nucleobase transporter that is responsible for the uptake of allopurinol and other purine bases. As the evidence is consistent with a model for a single hypoxanthine/allopurinol transporter, the potential for the rapid development of allopurinol resistance by loss of such a transporter would appear to be a potential concern. However, the use of purine analogues in combination chemotherapy with another antileishmanial agent may well be synergistic and prevent or even overcome early onset of resistance to either drug.
Acknowledgments
M.I.A. was supported by a personal studentship from the Libyan government.
REFERENCES
- 1.Abrishami, M., M. Soheilian, A. Farahi, and Y. Dowlati. 2002. Successful treatment of ocular leishmaniasis. Eur. J. Dermatol. 12:88-89. [PubMed] [Google Scholar]
- 2.Al Salabi, M. I., L. J. Wallace, and H. P. de Koning. 2003. A Leishmania major nucleobase transporter responsible for allopurinol uptake is a functional homolog of the Trypanosoma brucei H2 transporter. Mol. Pharmacol. 63:814-820. [DOI] [PubMed] [Google Scholar]
- 3.Avila, J. L., A. Avila, E. Munoz, and H. Monzon. 1983. Trypanosoma cruzi: 4-aminopyrazolopyrimidine in the treatment of experimental Chagas' disease. Exp. Parasitol. 56:236-240. [DOI] [PubMed] [Google Scholar]
- 4.Avila, J. L., and M. A. Casanova. 1982. Comparative effects of 4-aminopyrazolopyrimidine, its 2′-deoxyriboside derivative, and allopurinol on in vitro growth of American Leishmania species. Antimicrob. Agents Chemother. 22:380-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berens, R. L., E. C. Krug, and J. J. Marr. 1995. Purine and pyrimidine metabolism, p. 89-117. In J. J. Marr and M. Muller (ed.), Biochemistry and molecular biology of parasites. Harcourt Brace & Company, London, United Kingdom.
- 6.Berman, J. D., W. L. Hanson, J. K. Lovelace, V. B. Waits, J. E. Jackson, W. L. Chapman, Jr., and R. S. Klein. 1987. Activity of purine analogs against Leishmania donovani in vivo. Antimicrob. Agents Chemother. 31:111-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berman, J. D., and L. S. Lee. 1983. Activity of oral drugs against Leishmania tropica in human macrophages in vitro. Am. J. Trop. Med. Hyg. 32:947-951. [DOI] [PubMed] [Google Scholar]
- 8.Burchmore, R. J., L. J. Wallace, D. Candlish, M. I. Al Salabi, P. R. Beal, M. P. Barrett, S. A. Baldwin, and H. P. de Koning. 2003. Cloning, heterologous expression, and in situ characterization of the first high affinity nucleobase transporter from a protozoan. J. Biol. Chem. 278:23502-23507. [DOI] [PubMed] [Google Scholar]
- 9.Cavaliero, T., P. Arnold, A. Mathis, T. Glaus, R. Hofmann-Lehmann, and P. Deplazes. 1999. Clinical, serologic, and parasitologic follow-up after long-term allopurinol therapy of dogs naturally infected with Leishmania infantum. J. Vet. Intern. Med. 13:330-334. [DOI] [PubMed] [Google Scholar]
- 10.Cheng, Y., and W. H. Prusoff. 1973. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 22:3099-3108. [DOI] [PubMed] [Google Scholar]
- 11.Chunge, C. N., G. Gachihi, R. Muigai, K. Wasunna, J. R. Rashid, J. D. Chulay, G. Anabwani, C. N. Oster, and A. D. Bryceson. 1985. Visceral leishmaniasis unresponsive to antimonial drugs. III. Successful treatment using a combination of sodium stibogluconate plus allopurinol. Trans. R. Soc. Trop. Med. Hyg. 79:715-718. [DOI] [PubMed] [Google Scholar]
- 12.Croft, S. L. 2001. Monitoring drug resistance in leishmaniasis. Trop. Med. Int. Health 6:899-905. [DOI] [PubMed] [Google Scholar]
- 13.Das, V. N., A. Ranjan, A. N. Sinha, N. Verma, C. S. Lal, A. K. Gupta, N. A. Siddiqui, and S. K. Kar. 2001. A randomized clinical trial of low dosage combination of pentamidine and allopurinol in the treatment of antimony unresponsive cases of visceral leishmaniasis. J. Assoc. Physicians India 49:609-613. [PubMed] [Google Scholar]
- 14.De Koning, H., and G. Diallinas. 2000. Nucleobase transporters. Mol. Membr. Biol. 17:75-94. [DOI] [PubMed] [Google Scholar]
- 15.De Koning, H. P. 2001. Transporters in African trypanosomes: role in drug action and resistance. Int. J. Parasitol. 31:512-522. [DOI] [PubMed] [Google Scholar]
- 16.De Koning, H. P., M. I. Al Salabi, A. M. Cohen, G. H. Coombs, and J. M. Wastling. 2003. Identification and characterisation of high affinity nucleoside and nucleobase transporters in Toxoplasma gondii. Int. J. Parasitol. 33:821-831. [DOI] [PubMed] [Google Scholar]
- 17.De Koning, H. P., D. J. Bridges, and R. J. Burchmore. Purine and pyrimidine transport in pathogenic protozoa: from biology to therapy. FEMS Microbiol. Rev., in press. [DOI] [PubMed]
- 18.De Koning, H. P., and S. M. Jarvis. 1997. Hypoxanthine uptake through a purine-selective nucleobase transporter in Trypanosoma brucei brucei procyclic cells is driven by protonmotive force. Eur. J. Biochem. 247:1102-1110. [DOI] [PubMed] [Google Scholar]
- 19.De Koning, H. P., and S. M. Jarvis. 1999. Adenosine transporters in bloodstream forms of Trypanosoma brucei brucei: substrate recognition motifs and affinity for trypanocidal drugs. Mol. Pharmacol. 56:1162-1170. [DOI] [PubMed] [Google Scholar]
- 20.De Koning, H. P., C. J. Watson, L. Sutcliffe, and S. M. Jarvis. 2000. Differential regulation of nucleoside and nucleobase transporters in Crithidia fasciculata and Trypanosoma brucei brucei. Mol. Biochem. Parasitol. 106:93-107. [DOI] [PubMed] [Google Scholar]
- 21.Denerolle, P., and G. Bourdoiseau. 1999. Combination allopurinol and antimony treatment versus antimony alone and allopurinol alone in the treatment of canine leishmaniasis (96 cases). J. Vet. Intern. Med. 13:413-415. [DOI] [PubMed] [Google Scholar]
- 22.El Farrash, M. A., J. M. Youssef, and S. E. El Mongy. 2003. Allopurinol as a potential therapeutic agent for recurrent herpes labialis. J. Med. Dent. Sci. 50:147-154. [PubMed] [Google Scholar]
- 23.El Kouni, M. H. 2003. Potential chemotherapeutic targets in the purine metabolism of parasites. Pharmacol. Ther. 99:283-309. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh, M., and T. Mukherjee. 2000. Stage-specific development of a novel adenosine transporter in Leishmania donovani amastigotes. Mol. Biochem. Parasitol. 108:93-99. [DOI] [PubMed] [Google Scholar]
- 25.Henriques, C., M. A. Sanchez, R. Tryon, and S. M. Landfear. 2003. Molecular and functional characterization of the first nucleobase transporter gene from African trypanosomes. Mol. Biochem. Parasitol. 130:101-110. [DOI] [PubMed] [Google Scholar]
- 26.Kamau, S. W., M. Hurtado, U. U. Muller-Doblies, F. Grimm, and R. Nunez. 2000. Flow cytometric assessment of allopurinol susceptibility in Leishmania infantum promastigote. Cytometry 40:353-360. [PubMed] [Google Scholar]
- 27.Kolb, V. M. 1997. Novel and unusual nucleosides as drugs. Prog. Drug Res. 48:195-232. [DOI] [PubMed] [Google Scholar]
- 28.Kovanen, P. T., J. L. Goldstein, D. A. Chappell, and M. S. Brown. 1980. Regulation of low density lipoprotein receptors by adrenocorticotropin in the adrenal gland of mice and rats in vivo. J. Biol. Chem. 255:5591-5598. [PubMed] [Google Scholar]
- 29.Landfear, S. M., B. Ullman, N. S. Carter, and M. A. Sanchez. 2004. Nucleoside and nucleobase transporters in parasitic protozoa. Eukaryot. Cell 3:245-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marr, J. J. 1983. Pyrazolopyrimidine metabolism in Leishmania and trypanosomes: significant differences between host and parasite. J. Cell. Biochem. 22:187-196. [DOI] [PubMed] [Google Scholar]
- 31.Marr, J. J., and R. L. Berens. 1983. Pyrazolopyrimidine metabolism in the pathogenic trypanosomatidae. Mol. Biochem. Parasitol. 7:339-356. [DOI] [PubMed] [Google Scholar]
- 32.Marr, J. J., R. L. Berens, N. K. Cohn, D. J. Nelson, and R. S. Klein. 1984. Biological action of inosine analogs in Leishmania and Trypanosoma spp. Antimicrob. Agents Chemother. 25:292-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marr, J. J., R. L. Berens, and D. J. Nelson. 1978. Antitrypanosomal effect of allopurinol: conversion in vivo to aminopyrazolopyrimidine nucleotides by Trypanosoma cruzi. Science 201:1018-1020. [DOI] [PubMed] [Google Scholar]
- 34.Marsden, P. D., C. C. Cuba, and A. C. Barreto. 1984. Allopurinol treatment in human Leishmania braziliensis braziliensis infections. Trans. R. Soc. Trop. Med. Hyg. 78:701. [DOI] [PubMed] [Google Scholar]
- 35.Martinez, S., M. Gonzalez, and M. E. Vernaza. 1997. Treatment of cutaneous leishmaniasis with allopurinol and stibogluconate. Clin. Infect. Dis. 24:165-169. [DOI] [PubMed] [Google Scholar]
- 36.Martinez, S., and J. J. Marr. 1992. Allopurinol in the treatment of American cutaneous leishmaniasis. N. Engl. J. Med. 326:741-744. [DOI] [PubMed] [Google Scholar]
- 37.Mazzocchi, G., P. Rebuffat, A. S. Belloni, G. Gottardo, V. Meneghelli, and G. G. Nussdorfer. 1988. Effects of mevinolin, an inhibitor of cholesterol synthesis, on the morphological and functional responses of rat adrenal zona fasciculata to a prolonged treatment with 4-aminopyrazolo-pyrimidine. Anat. Rec. 221:700-706. [DOI] [PubMed] [Google Scholar]
- 38.Mikus, J., and D. Steverding. 2000. A simple colorimetric method to screen drug cytotoxicity against Leishmania using the dye Alamar Blue. Parasitol. Int. 48:265-269. [DOI] [PubMed] [Google Scholar]
- 39.Momeni, A. Z., M. R. Reiszadae, and M. Aminjavaheri. 2002. Treatment of cutaneous leishmaniasis with a combination of allopurinol and low-dose meglumine antimoniate. Int. J. Dermatol. 41:441-443. [DOI] [PubMed] [Google Scholar]
- 40.Murakoshi, M., Y. Osamura, and K. Watanabe. 1985. Ultrastructural studies in 4-aminopyrazolopyrimidine (4-APP)-induced fatty liver. Tokai J. Exp. Clin. Med. 10:5-12. [PubMed] [Google Scholar]
- 41.Namazi, M. R. 2004. Cetirizine and allopurinol as novel weapons against cellular autoimmune disorders. Int. Immunopharmacol. 4:349-353. [DOI] [PubMed] [Google Scholar]
- 42.Natto, M. J., L. J. Wallace, D. Candlish, M. I. Al Salabi, S. E. Coutts, and H. P. de Koning. 2005. Trypanosoma brucei: expression of multiple purine transporters prevents the development of allopurinol resistance. Exp. Parasitol. 109:80-86. [DOI] [PubMed] [Google Scholar]
- 43.Nelson, D. J., C. J. Bugge, G. B. Elion, R. L. Berens, and J. J. Marr. 1979. Metabolism of pyrazolo(3,4-d)pyrimidines in Leishmania braziliensis and Leishmania donovani. Allopurinol, oxipurinol, and 4-aminopyrazolo(3,4-d)pyrimidine. J. Biol. Chem. 254:3959-3964. [PubMed] [Google Scholar]
- 44.Nelson, D. J., C. J. Bugge, H. C. Krasny, and G. B. Elion. 1973. Formation of nucleotides of (6-14C)allopurinol and (6-14C)oxipurinol in rat tissues and effects on uridine nucleotide pools. Biochem. Pharmacol. 22:2003-2022. [DOI] [PubMed] [Google Scholar]
- 45.Nelson, D. J., S. W. LaFon, J. V. Tuttle, W. H. Miller, R. L. Miller, T. A. Krenitsky, G. B. Elion, R. L. Berens, and J. J. Marr. 1979. Allopurinol ribonucleoside as an antileishmanial agent. Biological effects, metabolism, and enzymatic phosphorylation. J. Biol. Chem. 254:11544-11549. [PubMed] [Google Scholar]
- 46.Papageorgiou, I. G., L. Yakob, M. I. Al Salabi, G. Diallinas, K. P. Soteriadou, and H. P. de Koning. 2005. Identification of the first pyrimidine nucleobase transporter in Leishmania: similarities with the Trypanosoma brucei U1 transporter and antileishmanial activity of uracil analogues. Parasitology 130:275-283. [DOI] [PubMed] [Google Scholar]
- 47.Pasa, S., S. O. Toz, H. Voyvoda, and Y. Ozbel. 2005. Clinical and serological follow-up in dogs with visceral leishmaniosis treated with allopurinol and sodium stibogluconate. Vet. Parasitol. 128:243-249. [DOI] [PubMed] [Google Scholar]
- 48.Pfaller, M. A., and J. J. Marr. 1974. Antileishmanial effect of allopurinol. Antimicrob. Agents Chemother. 5:469-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Philips, F. S., J. Scholler, and S. S. Sterberg. 1956. Production of fatty livers by 4-aminopyrazolo-(3,4-d)-pyrimidine; toxicological and pathological studies. Proc. Soc. Exp. Biol. Med. 93:398-402. [DOI] [PubMed] [Google Scholar]
- 50.Pitha, J., and K. H. Scheit. 1975. Hydrogen bonding abilities of 2,4-dithiouridine derivatives. Biochemistry 14:554-557. [DOI] [PubMed] [Google Scholar]
- 51.Raz, B., M. Iten, Y. Grether-Buhler, R. Kaminsky, and R. Brun. 1997. The Alamar Blue assay to determine drug sensitivity of African trypanosomes (T.b. rhodesiense and T.b. gambiense) in vitro. Acta Trop. 68:139-147. [DOI] [PubMed] [Google Scholar]
- 52.Razavi, M., M. Kraupp, and R. Marz. 1993. Allopurinol transport in human erythrocytes. Biochem. Pharmacol. 45:893-897. [DOI] [PubMed] [Google Scholar]
- 53.Sanchez, M. A., R. Tryon, S. Pierce, G. Vasudevan, and S. M. Landfear. 2004. Functional expression and characterization of a purine nucleobase transporter gene from Leishmania major. Mol. Membr. Biol. 21:11-18. [DOI] [PubMed] [Google Scholar]
- 54.Shaw, R. K., R. N. Shulman, J. D. Davidson, D. P. Rall, and E. Frei III. 1960. Studies with the experimental antitumor agent 4-aminopyrazolo[3, 4-d]pyrimidine. Cancer 13:482-489. [DOI] [PubMed] [Google Scholar]
- 55.Shiff, T. S., P. S. Roheim, and H. A. Eder. 1971. Effects of high sucrose diets and 4-aminopyrazolopyrimidine on serum lipids and lipoproteins in the rat. J. Lipid Res. 12:596-603. [PubMed] [Google Scholar]
- 56.Sundar, S. 2001. Treatment of visceral leishmaniasis. Med. Microbiol. Immunol. (Berlin) 190:89-92. [DOI] [PubMed] [Google Scholar]
- 57.Velez, I., S. Agudelo, E. Hendrickx, J. Puerta, M. Grogl, F. Modabber, and J. Berman. 1997. Inefficacy of allopurinol as monotherapy for Colombian cutaneous leishmaniasis. A randomized, controlled trial. Ann. Intern. Med. 126:232-236. [DOI] [PubMed] [Google Scholar]
- 58.Wallace, L. J., D. Candlish, and H. P. de Koning. 2002. Different substrate recognition motifs of human and trypanosome nucleobase transporters. Selective uptake of purine antimetabolites. J. Biol. Chem. 277:26149-26156. [DOI] [PubMed] [Google Scholar]
- 59.Wallace, L. J., D. Candlish, A. Hagos, K. L. Seley, and H. P. de Koning. 2004. Selective transport of a new class of purine antimetabolites by the protozoan parasite Trypanosoma brucei. Nucleosides Nucleotides Nucleic Acids 23:1441-1444. [DOI] [PubMed] [Google Scholar]
- 60.Yardley, V., and S. L. Croft. 2000. A comparison of the activities of three amphotericin B lipid formulations against experimental visceral and cutaneous leishmaniasis. Int. J. Antimicrob. Agents 13:243-248. [DOI] [PubMed] [Google Scholar]