Abstract
The caspofungin clinical trial database offers an opportunity to assess susceptibility results for Candida pathogens obtained from patients with candidiasis and allows for correlations between efficacy outcomes and MICs. Candida isolates have been identified from patients enrolled in four studies of esophageal candidiasis and two studies of invasive candidiasis. The MICs of caspofungin for all baseline isolates were measured at a central laboratory using NCCLS criteria (document M-27A); MICs for caspofungin were defined as the lowest concentration inhibiting prominent growth at 24 h. MICs were then compared to clinical and microbiological outcomes across the two diseases. Susceptibility testing for caspofungin was performed on 515 unique baseline isolates of Candida spp. obtained from patients with esophageal candidiasis. MICs for caspofungin ranged from 0.008 to 4 μg/ml; the MIC50 and MIC90 were 0.5 and 1.0 μg/ml, respectively. Susceptibility testing was also performed on 231 unique baseline isolates of Candida spp. from patients with invasive candidiasis. The majority (∼96%) of MICs were between 0.125 and 2 μg/ml, with MIC50 and MIC90 for caspofungin being 0.5 and 2.0 μg/ml, respectively. Overall, caspofungin demonstrated potent in vitro activity against clinical isolates of Candida species. A relationship between MIC for caspofungin and treatment outcome was not seen for patients with either esophageal candidiasis or invasive candidiasis. Patients with isolates for which the MICs were highest (>2 μg/ml) had better outcomes than patients with isolates for which the MICs were lower (<1 μg/ml). Additionally, no correlation between MIC and outcome was identified for specific Candida species.
Caspofungin is a parenteral echinocandin antifungal with known fungicidal activity against Candida species (1, 2, 5, 8, 9). As caspofungin specifically targets the fungal cell wall as opposed to the fungal cell membrane, this echinocandin retains activity against clinical Candida isolates with documented resistance to either the azoles or the polyenes (4, 14, 17, 29). In several phase II/III trials, caspofungin has exhibited efficacy outcomes comparable to those of amphotericin B deoxycholate or fluconazole for the treatment of esophageal candidiasis (3, 30, 31). Similarly, in a large multicenter study, caspofungin was as effective as amphotericin B deoxycholate in the primary treatment of invasive candidiasis (candidemia and other Candida infections) (12, 15). In all of these studies, caspofungin has demonstrated an excellent safety profile with few serious drug-related adverse events and few therapy discontinuations resulting from toxicity (3, 12, 15, 30, 31).
Despite its favorable clinical profile against Candida infections, the utility of in vitro susceptibility testing for caspofungin has not been fully established. Recent work has focused on the development of a standard, reproducible method of susceptibility testing for caspofungin. This study, involving 17 different microbiology laboratories, assessed the interlaboratory reproducibility of caspofungin microdilution susceptibility testing against a panel of 30 isolates of various Candida species. Consistent MIC data were obtained using the broth microdilution method (M-27A) set forth by the National Committee for Clinical Laboratory Standards (NCCLS); the greatest reproducibility was generated with the visual “prominent growth reduction” endpoint (MIC2) measured at 24 h in RPMI-1640 medium (18). Surveillance studies employing this method have demonstrated excellent in vitro potency and a wide spectrum of caspofungin activity against a variety of clinical isolates of Candida species (20-22).
The caspofungin clinical trial database provides an additional opportunity to assess in vitro susceptibility results for caspofungin against Candida isolates. All Candida isolates collected from patients enrolled in the caspofungin studies involving either esophageal candidiasis or invasive candidiasis were evaluated by using the susceptibility endpoint that equates to a prominent inhibition of growth. Herein we describe the MIC results for caspofungin against all unique baseline isolates from these studies. The following report also provides correlations of MIC data to efficacy outcomes for the indications of esophageal candidiasis and invasive candidiasis in an attempt to identify caspofungin susceptibility breakpoints for Candida spp.
MATERIALS AND METHODS
Overview of clinical studies of esophageal candidiasis. (i) Description of studies.
Four phase II and III studies have evaluated the efficacy of caspofungin against esophageal candidiasis, including three phase II studies (protocols 003, 004, and 007) and a pivotal phase III study (protocol 020). The first two phase II trials (protocols 003 and 004) were randomized, double-blind studies comparing various doses of caspofungin (35, 50, or 70 mg/day) to intravenous amphotericin B deoxycholate (0.5 mg/kg of body weight/day) (3, 30). The third phase II study (protocol 007) was a small, noncomparative study primarily performed to evaluate the pharmacokinetics and efficacy of caspofungin for patients with esophageal candidiasis. The pivotal phase III study (protocol 020) was a double-blind (with in-house blinding), randomized trial which compared caspofungin (50 mg/day intravenously) to intravenous fluconazole (200 mg/day) for the treatment of documented esophageal candidiasis (31). Overall, a total of 236 patients with documented esophageal candidiasis received caspofungin in these four studies.
All four studies of esophageal candidiasis shared common design elements. In each of these four studies, patients had to be at least 18 years of age with symptoms and microbiological documentation of esophageal candidiasis. At study entry, patients underwent esophageal endoscopy, with photographic documentation and biopsy, as symptoms and/or protocol dictated. In all studies, specimens from brushings and biopsies were collected for culturing and subsequent susceptibility testing. Severity of disease was graded according to a four-point scale for patients with esophageal candidiasis, as per prior studies (10-12).
(ii) Efficacy assessment.
In all four esophageal candidiasis studies, the primary clinical endpoint was the combined evaluation of symptoms and endoscopic lesions. A favorable clinical response required the complete resolution of symptoms and a substantial reduction in the number of endoscopic lesions. For all four studies, an endoscopy was performed at an early follow-up visit for all patients. A favorable microbiological response, defined as eradication (no Candida isolated on culture) or presumptive eradication (no culture performed because no visible lesions were identified on the follow-up endoscopy), was considered a secondary efficacy endpoint, because the eradication of Candida species is difficult to achieve in such severely immunocompromised patients.
Overview of clinical studies of invasive candidiasis. (i) Description of studies.
Two separate studies have evaluated the efficacy of caspofungin in the treatment of documented invasive Candida infections. The pivotal study (protocol 014) was a randomized, double-blind (with in-house blinding), comparative study for patients with documented invasive Candida infections (15). In this study, patients with clinical and microbiological evidence of invasive candidiasis received caspofungin (50 mg daily, following a 70-mg loading dose on day 1) or amphotericin B deoxycholate (0.5 to 1.0 mg/kg daily). A second study (protocol 026) also included a limited number of patients with invasive Candida infections at study entry (32). This empirical therapy study (protocol 026) was a double-blind (with in-house blinding), randomized, comparative study to evaluate the safety, tolerability, and efficacy of caspofungin (50 mg/day following a 70-mg loading dose) versus liposomal amphotericin B (3 mg/kg/day; AmBisome) in the treatment of presumed fungal infections in patients with persistent fever and neutropenia. Of the 1,111 patients enrolled in this study, a total of 24 patients had invasive candidiasis (12 in each treatment group) at study entry. Across these two studies, a total of 127 patients received caspofungin for documented invasive candidiasis.
The two studies shared certain common elements with regard to the patients enrolled with invasive candidiasis. In both studies, all patients had clinical and microbiological evidence at study entry. For patients with candidemia, blood cultures were collected daily until cultures were negative for Candida for up to 48 h. Similarly, based on the site of the underlying infection, follow-up cultures and/or radiographic studies were collected at these otherwise sterile sites of infection to confirm eradication and radiographic resolution, respectively.
(ii) Efficacy assessment.
The primary efficacy parameter for the patients with invasive Candida infections in each of the two studies (protocols 014 and 026) was the overall response. A favorable overall response necessitated that the patient have a favorable clinical response and a favorable microbiological response at the end of study therapy. The two studies implemented very similar definitions of a favorable overall response. Slight differences were noted in the definition of a favorable clinical response. Whereas a favorable clinical response in the study of invasive candidiasis (protocol 014) required the resolution of all signs and symptoms related to Candida infection, a favorable clinical response in the subset of patients with invasive candidiasis enrolled in the empirical therapy study (protocol 026) included all patients who had resolution or clinically meaningful improvement in all attributable signs and symptoms.
The microbiological outcome was a component of the overall response. In both studies, a favorable microbiological response was limited to eradication or presumptive eradication. Eradication signified cultures negative for Candida spp. at the end of study therapy or demonstrated at a prior on-therapy time point with no clinical evidence of infection. Infections that required an invasive procedure for the documentation of negative cultures were considered to have an evaluation of presumptive eradication if there was no apparent evidence of residual infection from symptoms, physical examination, or appropriate noninvasive studies. Note that the classification of presumptive eradication was limited to patients with nonblood sites of infection.
Overview of efficacy analyses.
In the studies of both esophageal candidiasis and invasive candidiasis, efficacy results were analyzed using a modified-intention-to-treat (MITT) approach and an evaluable-patients (EP) approach. The MITT population, which was the primary subject of analysis, included all randomized patients who met the definition of documented Candida infection and received at least one dose of caspofungin therapy. The EP analysis included all randomized patients who met the MITT criteria, received at least 5 days of caspofungin study therapy, and had appropriate end-of-therapy evaluations.
Isolates and antifungal susceptibility testing.
At study entry, specimens were collected for the culturing of Candida species during endoscopy (for esophageal candidiasis) or from the sterile site of infection (for invasive candidiasis). CHROMagar (Paris, France) and differential medium were provided to all sites to assist in the preliminary isolation and identification of Candida spp. Subcultures of stock cultures were prepared on Sabouraud dextrose agar slants and sent at appropriate intervals to the Merck Clinical Microbiology Laboratory (Rahway, NJ) for definitive identification and antifungal susceptibility testing. At Merck, all Candida isolates were identified to the species level by a combination of the Dade MicroScan Rapid Yeast Identification assay (West Sacramento, CA), conventional microscopy, and culturing on CHROMagar (Paris, France). Broth microdilution MICs for caspofungin were determined by using the NCCLS M-27A methodology (16). Assays were performed using buffered RPMI 1640 medium, inocula of 103 CFU/ml, and an incubation temperature of 35°C for 24 h. For Candida isolates, the MICs for caspofungin were defined as the visual endpoint that equates to prominent inhibition of growth (18).
Following identification and susceptibility testing, a unique baseline isolate(s) from each patient was identified in an effort to allow for these assessments of outcomes per pathogen and per MIC. The unique baseline isolate(s) for each patient consisted of only the isolate(s) obtained on the date closest to the initiation of study therapy; on that day, only one isolate from each identified Candida species was included, unless the multiple isolates from the same Candida species had widely differing MIC findings (>2-fold difference in MICs collected from the RPMI 1640 medium). If the patient had multiple sterile sites of infection at study entry (i.e., blood and peritoneal infection), only one of the samples was included in the unique baseline isolates, unless the species differed or MICs at the two sites were widely different.
Where possible, the Candida isolates from the site of infection were also collected from those patients with unfavorable microbiological responses to caspofungin. Coupled with the unique baseline isolates, such subsequent isolates were used to ascertain whether an increase in MIC for the Candida isolate following caspofungin therapy may have correlated with the eventual failure.
RESULTS
Esophageal candidiasis. (i) MIC distribution.
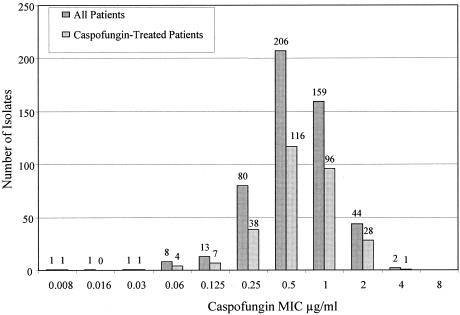
Susceptibility testing for caspofungin was performed on 515 unique baseline isolates from all patients enrolled in the four phase II or III studies of esophageal candidiasis (Fig. 1). Of the 515 isolates, 292 were obtained from patients who were subsequently treated with caspofungin. The distribution of MIC results for caspofungin among these 292 isolates is generally consistent with the pattern for all isolates (Fig. 1). Overall, MICs for caspofungin ranged from 0.008 to 4 μg/ml, with a majority (∼95%) of values between 0.25 and 2 μg/ml. In fact, the MIC50 and MIC90 for caspofungin across all Candida species was 0.5 and 1.0 μg/ml, respectively (Table 1).
FIG. 1.
Display of the distribution of all unique baseline isolates from patients enrolled in the four esophageal candidiasis studies. Graph includes all 515 unique baseline isolates from these four studies and a subset of 292 unique baseline isolates from caspofungin-treated patients. The number of isolates in each category is shown above the corresponding bar.
TABLE 1.
Caspofungin MICs for Candida isolates
| Species | Result for:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Esophageal candidiasis isolates
|
Invasive candidiasis isolates
|
|||||||
| n | MIC50 (μg/ml) | MIC90 (μg/ml) | Range (μg/ml) | n | MIC50 (μg/ml) | MIC90 (μg/ml) | Range (μg/ml) | |
| All Candida spp. | 515 | 0.5 | 1 | 0.008-4 | 231 | 0.5 | 2 | 0.06-8 |
| C. albicans | 416 | 0.5 | 1 | 0.008-2 | 107 | 0.5 | 1 | 0.06-1 |
| C. glabrata | 44 | 1 | 2 | 0.5-2 | 27 | 1 | 1 | 0.5-1 |
| C. tropicalis | 14 | 0.5 | 1 | 0.25-1 | 39 | 0.5 | 1 | 0.25-2 |
| C. parapsilosis | 7 | 2 | 4 | 1-4 | 43 | 2 | 2 | 0.5-4 |
| C. guilliermondii | 21 | 2 | 2 | 1-4 | 5 | 2 | 4 | 1-4 |
| C. krusei | 10 | 2 | 2 | 1-2 | 7 | 2 | 2 | 1-2 |
| C. lipolytica | 1 | 2 | 2 | 2 | 1 | 0.5 | 0.5 | 0.5 |
| C. lusitaniae | 1 | 2 | 2 | 2 | 1 | 0.5 | 0.5 | 0.5 |
| C. kefyr | 1 | 0.5 | 0.5 | 0.5 | 0 | |||
| C. rugosa | 0 | 1 | 8 | 8 | 8 | |||
(ii) Correlation of MIC to outcome.
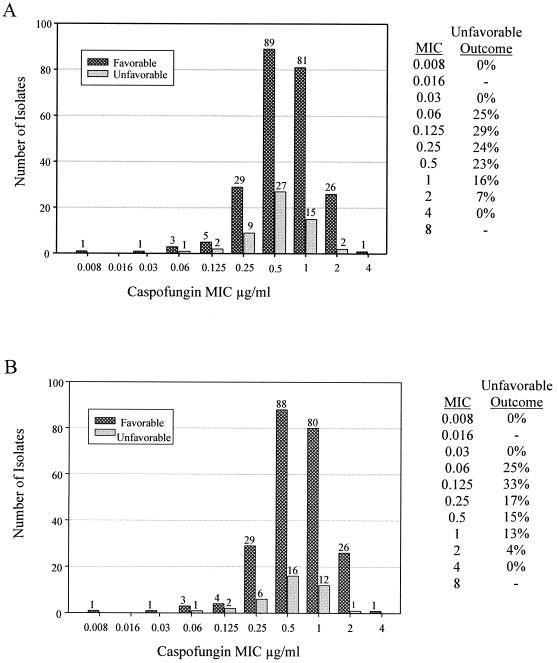
The correlation of caspofungin MICs to clinical outcomes for the patients with esophageal candidiasis is shown for the MITT population and the EP population in Fig. 2A and B, respectively. For both efficacy populations, there was no clear correlation between MIC for caspofungin and therapeutic outcome for caspofungin-treated patients. Notably, patients with isolates for which MICs were higher (1 to 2 μg/ml) tended to have better outcomes than patients with isolates for which MICs were lower (<0.5 μg/ml). Similar trends were noted among the four specific studies (protocols 003, 004, 007, and 020) and across the three specific caspofungin doses (35, 50, and 70 mg/day). Similar patterns were also noted in the relationship of MICs for caspofungin to microbiological outcomes (data not shown).
FIG. 2.
The correlations of caspofungin MICs to clinical outcomes for the patients with esophageal candidiasis. Displayed are the results from the MITT (A) and EP (B) populations. These correlations are displayed graphically and in tabular format based on the proportion of patients with unfavorable outcomes per each MIC for caspofungin. The number of isolates in each category is shown above the corresponding bar.
C. albicans was the sole pathogen identified in most patients (>150) with esophageal candidiasis. Approximately 44 patients had mixed infections with C. albicans and a non-C. albicans Candida species (including, in descending order, C. glabrata, C. guilliermondii, C. krusei, C. tropicalis, and C. parapsilosis). As C. albicans likely represents the predominant cause of the infection for patients with mixed infections, the correlations of MICs of caspofungin to clinical outcomes are based on the MIC for the C. albicans isolate. The majority of patients (93%) with C. albicans infections had MICs of 0.25, 0.5, or 1.0 μg/ml, and no clear correlation between outcome and the MIC for C. albicans was identified across this range (success in 77%, 77%, and 83%, respectively). There were only seven isolates obtained from patients who did not also have a coinfection with C. albicans, and each had a favorable clinical outcome.
In 41 patients with unfavorable microbiological outcomes following caspofungin treatment, susceptibility testing was also performed on a subsequent Candida isolate collected from the esophagus. Comparisons of the MICs of these subsequent isolates to the original baseline isolates failed to demonstrate any instance where a ≥2-fold increase in MIC for caspofungin was noted for the subsequent isolate. In fact, the MIC of the subsequent isolate from one patient actually decreased by twofold dilutions relative to the baseline isolate (1.0 μg/ml to 0.25 μg/ml).
Invasive candidiasis. (i) MIC distribution.
Susceptibility testing for caspofungin was performed on 231 unique baseline isolates from all patients enrolled in the two studies of invasive candidiasis. Of the 231 isolates, 114 were obtained from patients who were subsequently treated with caspofungin. The distributions of MIC results for caspofungin for isolates obtained from all patients and for isolates obtained from caspofungin-treated patients are similar (Fig. 3). The distribution of MIC results for caspofungin among these 114 isolates is generally consistent with the overall pattern from the esophageal studies (Fig. 1). Overall, MICs for caspofungin ranged from 0.06 to 8 μg/ml, with a majority (∼96%) of values between 0.125 and 2 μg/ml. In fact, the MIC50 and MIC90 for caspofungin across all Candida species were 0.5 and 2.0 μg/ml, respectively (Table 1).
FIG. 3.
Display of the distribution of all unique baseline isolates from patients enrolled in the two studies of invasive candidiasis. The graph includes all 231 unique baseline isolates from these two studies and the subset of 114 unique baseline isolates from caspofungin-treated patients. The number of isolates in each category is shown above the corresponding bar.
(ii) Correlation of MIC to outcome.
The correlation of caspofungin MICs to overall outcomes for the patients with invasive candidiasis is displayed for the MITT population and the EP population in Fig. 4A and B, respectively. Similar to what was found for esophageal candidiasis, there was no clear correlation between the MIC for caspofungin and the efficacy response seen in caspofungin-treated patients with invasive candidiasis. There was a greater proportion of favorable outcomes for patients with isolates for which MICs were higher (>2 μg/ml) than for patients with isolates for which MICs were lower (<1 μg/ml).
FIG. 4.
The correlations of caspofungin MICs to overall outcomes for the patients with invasive candidiasis. Displayed are the results from the MITT population (A) and EP population (B). These correlations are displayed graphically and in tabular format based on the proportion of patients with unfavorable outcomes per each MIC for caspofungin. The number of isolates in each category is shown above the corresponding bar.
In the caspofungin-treated patients, a wide array of Candida species was isolated from the patients with invasive candidiasis. The most common species isolated was C. albicans (43 isolates), followed by C. tropicalis (23 isolates), C. parapsilosis (22 isolates), and C. glabrata (15 isolates). The correlations between caspofungin MICs and overall outcomes are shown for each of these species in Table 2. The correlation of MIC to overall outcome for other, less common Candida species, including C. krusei (five isolates), C. guilliermondii (four isolates), C. lipolytica (one isolate), and C. rugosa (one isolate), are grouped together in Table 2. No clear correlation between MIC and outcome for the specific Candida pathogen(s) was identified. Notably, similar trends were identified by looking at the relationship of the MIC for caspofungin to the microbiological response (data not shown).
TABLE 2.
Correlation of caspofungin MICs to overall outcomes (MITT population) in patients with invasive candidiasis caused by various Candida pathogens
| Pathogen | Caspo- fungin MIC (μg/ml) | Total no. of isolates | Overall outcome
|
|||
|---|---|---|---|---|---|---|
| Favorable
|
Unfavorable
|
|||||
| No. of isolates | % Success | No. of isolates | % Failure | |||
| C. albicans | 0.008 | |||||
| 0.016 | ||||||
| 0.03 | ||||||
| 0.06 | ||||||
| 0.125 | 3 | 2 | 66.7 | 1 | 33.3 | |
| 0.25 | 18 | 11 | 61.1 | 7 | 38.9 | |
| 0.5 | 17 | 14 | 82.4 | 3 | 17.6 | |
| 1 | 5 | 3 | 60.0 | 2 | 40.0 | |
| 2 | ||||||
| 4 | ||||||
| 8 | ||||||
| C. tropicalis | 0.008 | |||||
| 0.016 | ||||||
| 0.03 | ||||||
| 0.06 | ||||||
| 0.125 | ||||||
| 0.25 | 5 | 4 | 80.0 | 1 | 20.0 | |
| 0.5 | 11 | 9 | 81.8 | 2 | 18.2 | |
| 1 | 5 | 5 | 100 | 0 | 0.0 | |
| 2 | 2 | 1 | 50.0 | 1 | 50.0 | |
| 4 | ||||||
| 8 | ||||||
| C. parapsilosis | 0.008 | |||||
| 0.016 | ||||||
| 0.03 | ||||||
| 0.06 | ||||||
| 0.125 | ||||||
| 0.25 | ||||||
| 0.5 | 1 | 1 | 100 | 0 | 0.0 | |
| 1 | 5 | 1 | 20.0 | 4 | 80.0 | |
| 2 | 15 | 13 | 86.7 | 2 | 13.3 | |
| 4 | 1 | 1 | 100 | 0 | 0.0 | |
| 8 | ||||||
| C. glabrata | 0.008 | |||||
| 0.016 | ||||||
| 0.03 | ||||||
| 0.06 | ||||||
| 0.125 | ||||||
| 0.25 | ||||||
| 0.5 | 5 | 4 | 80.0 | 1 | 20.0 | |
| 1 | 10 | 8 | 80.0 | 2 | 20.0 | |
| 2 | ||||||
| 4 | ||||||
| 8 | ||||||
| Other Candida | 0.008 | |||||
| speciesa | 0.016 | |||||
| 0.03 | ||||||
| 0.06 | ||||||
| 0.125 | ||||||
| 0.25 | ||||||
| 0.5 | 1 | 0 | 0.0 | 1 | 100 | |
| 1 | 3 | 2 | 66.7 | 1 | 33.3 | |
| 2 | 6 | 6 | 100 | 0 | 0.0 | |
| 4 | ||||||
| 8 | 1 | 1 | 100 | 0 | 0.0 | |
Includes C. krusei (five isolates), C. guilliermondii (four isolates), C. lipolytica (one isolate), and C. rugosa (one isolate).
In 20 patients with unfavorable microbiological outcomes following treatment with caspofungin, susceptibility testing was also performed on a subsequent Candida isolate collected from the site of invasive infection. Comparisons of the MICs of the paired isolates (original baseline isolate and subsequent isolate) from these specific patients failed to demonstrate any instance where a ≥2-fold increase in the MIC of caspofungin was noted for the subsequent isolate.
DISCUSSION
In this report, we present the susceptibility results for caspofungin and their correlation to outcomes for 746 clinical isolates of Candida spp. All yeast isolates were collected between 1997 and 2003 from patients with documented evidence of either esophageal or invasive candidiasis. These pretreatment isolates were uniformly tested in a single microbiological laboratory in an effort to ensure accurate identification and consistent MIC readings for the echinocandin. In our estimation, this review represents one of the largest evaluations of caspofungin susceptibility of contemporary yeast isolates using the method recently described by Odds and his colleagues (18). Importantly, it also signifies the first attempt to correlate the MIC for an echinocandin to clinical outcomes for patients with documented Candida infections. The entry criteria, outcome definitions, and primary evaluation time points were similar across the studies within each of the two indications (3, 15, 30-32), and, as a result, such correlations of susceptibility results to efficacy responses could be readily combined across all protocols.
This review highlights two interesting findings that warrant further discussion. First, the results demonstrate potent in vitro activity of caspofungin against a variety of strains of Candida. Across all Candida species, the MIC50 and MIC90 for caspofungin were 0.5 and 2 μg/ml, respectively (24, 25, 27, 28). The lowest MICs were noted for C. albicans, C. glabrata, and C. tropicalis, which accounted for over 85% of the baseline isolates from these patients. A trend towards higher MICs relative to those of the other species was noted for C. parapsilosis, C. krusei, and C. guilliermondii. The pattern of susceptibility results is consistent with other surveillance studies involving clinical isolates of Candida species (20-22). However, in these studies, the higher MICs did not equate to poorer efficacy responses in patients infected with these strains; in fact, clinical success following caspofungin therapy was seen in 77% of patients with invasive candidiasis resulting from any one of these three pathogens (73%, 100%, and 75% for C. parapsilosis, C. krusei, and C. guilliermondii, respectively). In comparison, favorable outcomes were noted for 75% of the patients with invasive candidiasis resulting from C. albicans, C. glabrata, or C. tropicalis (70%, 80%, and 83%, respectively).
Second, the data from these clinical trials fail to demonstrate a correlation between higher MICs for caspofungin and clinical or microbiological outcomes for patients treated with this echinocandin. Combining the results across the two disease states helps to better elucidate this finding. Among the 746 unique baseline isolates identified from the six studies of esophageal and invasive candidiasis, 406 identified from patients treated with caspofungin are available for MIC outcome assessments. The vast majority of these isolates (386 isolates; 95%) had MICs ranging between 0.25 and 2 μg/ml. Across this range, the MIC pattern was not related to outcome (MICs of 0.25, 0.5, 1, and 2 μg/ml were associated with success rates of 71%, 77%, 81%, and 91%, respectively). Additionally, all three isolates with caspofungin MICs of >2 μg/ml responded favorably to this antifungal. Taken together, a caspofungin success rate of 83% (149 of 178) was demonstrated for all isolates with MICs of >1 μg/ml of caspofungin.
The lack of correlation with higher MICs is not an unexpected finding, given previous experience with both antibacterial (10, 27, 28) and antifungal susceptibility testing (24, 25). The finding is likely a result of several factors. For one thing, antifungal susceptibility testing provides only a static assessment of the potential effect of an antimicrobial agent on a particular pathogen. The antifungal activity of caspofungin may indeed be augmented by a variety of pharmacological conditions, including the achieved pharmacokinetic concentration for the patient at the particular site of infection and the potentiated effect of serum or other compounds. In addition, host factors, such as the patient's underlying medical condition or immunological status, or specific medical practices may significantly impact the outcome for the patient, irrespective of the inherent antimicrobial effect described in the laboratory (24). In particular, the APACHE (acute physiology and chronic health evaluation) score and vascular catheter exchanges have repeatedly been shown to play a major role in the outcomes of patients in clinical trials of invasive candidiasis (15, 23, 26). Another reason for the lack of correlation between susceptibility results and outcomes may relate to the paucity of isolates included in these clinical trials with known resistance to echinocandin. Although isolates of Candida spp. with reduced susceptibility to caspofungin have been generated in the laboratory (7, 13, 19) and have been reported on rare occasions in the clinical arena (11; see also caspofungin acetate [CANCIDAS] U.S. product insert, 2004, Merck & Co., Inc.), the strains included in these studies were collected from patients receiving a finite course of therapy for a documented infection. Furthermore, most isolates were collected from patients enrolled prior to the licensure of marketed caspofungin (3, 15, 30-32). We purport that any assessments of potential clinical breakpoints for echinocandins based on susceptibility testing in the future will likely need to carefully incorporate the susceptibility patterns of Candida strains which have demonstrated acquired resistance to these agents.
In summary, our data provide additional confirmatory evidence regarding the in vitro activity of caspofungin against a variety of clinical stains of Candida species. The lack of a clear relationship between MICs for caspofungin and clinical outcomes for patients with esophageal candidiasis or invasive candidiasis prevents us from assigning an interpretative MIC breakpoint for caspofungin based solely on the results of these studies. Ongoing surveillance of the caspofungin susceptibility pattern of strains from current clinical trials and from marketed use may indeed identify a sufficient sample of isolates with reduced susceptibility to caspofungin, thereby ultimately assisting in the assessment of the clinical breakpoints for caspofungin. Until that time, caution should be routinely exercised regarding the use and interpretation of susceptibility testing for caspofungin in the clinical care of patients with Candida infections.
Acknowledgments
Merck Research Laboratories provided support.
Statement on potential conflicts of interest: all authors are employees of Merck Research Laboratories, Merck & Company, Inc.
REFERENCES
- 1.Abruzzo, G. K., A. M. Flattery, C. J. Gill, L. Kong, J. G. Smith, V. B. Pikounis, J. M. Balkovec, A. F. Bouffard, J. F. Dropinski, H. Rosen, H. Kropp, and K. Bartizal. 1997. Evaluation of the echinocandin antifungal MK-0991 (L-743,872): efficacies in mouse models of disseminated aspergillosis, candidiasis, and cryptococcosis. Antimicrob. Agents Chemother. 41:2333-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abruzzo, G. K., C. J. Gill, A. M. Flattery, L. Kong, C. Leighton, J. G. Smith, V. B. Pikounis, K. Bartizal, and H. Rosen. 2000. Efficacy of the echinocandin caspofungin against disseminated aspergillosis and candidiasis in cyclophosphamide-induced immunosuppressed mice. Antimicrob. Agents Chemother. 44:2310-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arathoon, E. G., E. Gotuzzo, L. M. Noriega, R. S. Berman, M. J. DiNubile, and C. A. Sable. 2002. Randomized, double-blind, multicenter study of caspofungin versus amphotericin B for treatment of oropharyngeal and esophageal candidiases. Antimicrob. Agents Chemother. 46:451-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barchiesi, F., A. M. Schimizzi, A. W. Fothergill, G. Scalise, and M. G. Rinaldi. 1999. In vitro activity of the new echinocandin antifungal, MK-0991, against common and uncommon clinical isolates of Candida species. Eur. J. Clin. Microbiol. Infect. Dis. 18:302-304. [DOI] [PubMed] [Google Scholar]
- 5.Bartizal, K., C. J. Gill, G. K. Abruzzo, A. M. Flattery, L. Kong, P. M. Scott, J. G. Smith, C. E. Leighton, A. Bouffard, J. F. Dropinski, and J. Balkovec. 1997. In vitro preclinical evaluation studies with the echinocandin antifungal MK-0991 (L-743,872). Antimicrob. Agents Chemother. 41:2326-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reference deleted.
- 7.Douglas, C. M., J. A. D'Ippolito, G. J. Shei, M. Meinz, J. Onishi, J. A. Marrinan, and W. Li. 1997. Identification of the FKS1 gene of Candida albicans as the essential target of 1,3-β-d-glucan synthase inhibitors. Antimicrob. Agents Chemother. 41:2471-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graybill, J. R., L. K. Najvar, M. F. Luther, and A. W. Fothergill. 1997. Treatment of murine disseminated candidiasis with L-743,872. Antimicrob. Agents Chemother. 41:1775-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graybill, J. R., R. Bocanegra, M. Luther, A. Fothergill, and M. J. Rinaldi. 1997. Treatment of murine Candida krusei or Candida glabrata infection with L-743,872. Antimicrob. Agents Chemother. 41:1937-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenwood, D. 1981. In vitro veritas? Antimicrobial susceptibility tests and their clinical relevance. J. Infect. Dis. 144:380-385. [DOI] [PubMed] [Google Scholar]
- 11.Hernandez, S., J. L. López-Ribot, L. K. Najvar, D. I. McCarthy, R. Bocanegra, and J. R. Graybill. 2004. Caspofungin resistance in Candida albicans: correlating clinical outcome with laboratory susceptibility testing of three isogenic isolates serially obtained from a patient with progressive Candida esophagitis. Antimicrob. Agents Chemother. 48:1382-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kartsonis, N. A., A. Saah, J. Lipka, A. Taylor, and C. A. Sable. 2004. Second-line therapy with caspofungin for mucosal or invasive candidiasis: results from the caspofungin compassionate-use study. J. Antimicrob. Chemother. 53:878-881. [DOI] [PubMed] [Google Scholar]
- 13.Kurtz, M. B., G. K. Abruzzo, A. M. Flattery, J. A. Marrinan, W. Li., J. A. Milligan, K. Nollstadt, and C. M. Douglas. 1995. Isolation and characterization of echinocandin resistant mutants of Candida albicans: genetic, biochemical and virulence studies. Yeast 11:S561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez-Suarez, J. V., and J. L. Rodriguez-Tudela. 1996. In vitro activities of semisynthetic pneumocandin L-733,560 against fluconazole-resistant and -susceptible Candida albicans isolates. Antimicrob. Agents Chemother. 40:1277-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mora-Duarte, J., R. Betts, M. C. Rotstein, A. L. Colombo, J. Thompson-Moya, J. Smietana, and R. Lupinacci. 2002. Comparison of caspofungin and amphotericin B for invasive candidiasis. N. Engl. J. Med. 347:2020-2029. [DOI] [PubMed] [Google Scholar]
- 16.NCCLS. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard, 2nd ed. Document M27-A2. NCCLS, Wayne, Pa.
- 17.Nelson, P. W., M. Lozano-Chiu, and J. H. Rex. 1997. In vitro growth-inhibitory activity of pneumocandins L-733,560 and L-743,872 against putatively amphotericin B- and fluconazole-resistant Candida isolates: influence of assay conditions. J. Med. Vet. Mycol. 35:285-287. [PubMed] [Google Scholar]
- 18.Odds, F., M. Motyl, R. Andrade, J. Bille, E. Canton, M. Cuenca-Estrella, A. Davidson, C. Durussel, D. Ellis, E. Foraker, A. W. Fothergill, M. A. Ghannoum, R. A. Giacobbe, M. Gobernado, R. Handke, M. Laverdiere, W. Lee-Yang, W. G. Merz, L. Ostrosky-Zeichner, J. Peman, S. Perea, J. R. Perfect, M. A. Pfaller, L. Proia, J. H. Rex, M. G. Rinaldi, J. L. Rodriguez-Tudela, W. A. Schell, C. Shields, D. A. Sutton, P. E. Verweij, and D. W. Warnock. 2004. Interlaboratory comparison of results of susceptibility testing with caspofungin against Candida and Aspergillus species. J. Clin. Microbiol. 42:3475-3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park, S., R. Kelly, J. N. Kahn, J. Robles, M.-J. Hsu, E. Register, W. Li, V. Vyas, H. Fan, G. Abruzzo, A. Flattery, C. Gill, G. Chrebet, S. A. Parent, M. Kurtz, H. Teppler, C. M. Douglas, and D. S. Perlin. 2005. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob. Agents Chemother. 8:3264-3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfaller, M. A., D. J. Diekema, S. A. Messer, R. J. Hollis, and R. N. Jones. 2003. In vitro activities of caspofungin compared with those of fluconazole and itraconazole against 3,959 clinical isolates of Candida spp., including 157 fluconazole-resistant isolates. Antimicrob. Agents Chemother. 47:1068-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfaller, M. A., S. A. Messer, L. Boyken, S. Tendolkar, R. J. Hollis, and D. J. Diekema. 2004. Geographic variation in the susceptibilities of invasive isolates of Candida glabrata to seven systemically active antifungal agents: a global assessment from the ARTEMIS antifungal surveillance program conducted in 2001 and 2002. J. Clin. Microbiol. 42:3142-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfaller, M. A., S. A. Messer, L. Boyken, C. Rice, S. Tendolkar, R. J. Hollis, and D. J. Diekema. 2004. Further standardization of broth microdilution methodology for in vitro susceptibility testing of caspofungin against Candida species by use of an international collection of more than 3,000 clinical isolates. J. Clin. Microbiol. 42:3117-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rex, J. H., J. E. Bennett, A. M. Sugar, P. G. Pappas, C. M. van der Horst, J. E. Edwards, and R. G. Washburn. 1994. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. N. Engl. J. Med. 331:1325-1330. [DOI] [PubMed] [Google Scholar]
- 24.Rex, J. H., M. A. Pfaller, A. L. Barry, P. W. Nelson, and C. D. Webb. 1995. Antifungal susceptibility testing of isolates from a randomized, multicenter trial of fluconazole versus amphotericin B as treatment of nonneutropenic patients with candidemia. Antimicrob. Agents Chemother. 39:40-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rex, J. H., M. A. Pfaller, M. G. Rinaldi, A. Polak, and J. N. Galgiani. 1993. Antifungal susceptibility testing. Clin. Microbiol. Rev. 6:367-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rex, J. H., P. G. Pappas, A. W. Karchmer, J. Sobel, J. E. Edwards, S. Hadley, C. Brass, J. A. Vazquez, S. W. Chapman, H. W. Horowitz, M. Zervos, D. McKinsey, J. Lee, T. Babinchak, R. W. Bradsher, J. D. Cleary, D. M. Cohen, L. Danziger, M. Goldman, J. Goodman, E. Hilton, N. E. Hyslop, D. H. Kett, J. Lutz, R. H. Rubin, W. M. Scheld, M. Schuster, B. Simmons, D. K. Stein, R. G. Washburn, L. Mautner, T. C. Chu, H. Panzer, R. B. Rosenstein, J. Booth, and the National Institute of Allergy and Infectious Diseases Mycoses Study Group. 2003. A randomized and blinded multicenter trial of high-dose fluconazole plus placebo versus fluconazole plus amphotericin B as therapy for candidemia and its consequences in nonneutropenic subjects. Clin. Infect. Dis. 36:1221-1228. [DOI] [PubMed] [Google Scholar]
- 27.Sanders, W. E., Jr., and C. C. Sanders. 1981. In vitro antimicrobial susceptibility tests accurately predict therapeutic responsiveness in infected patients?, p. 325-340. In V. Lorian (ed.), Significance of medical microbiology in the care of patients, 2nd ed. The Williams & Wilkins Co., Baltimore, Md.
- 28.Straton, C. W. 1991. In vitro testing: correlations between bacterial susceptibility, body fluid levels, and effectiveness of antibacterial therapy, p. 849-879. In V. Lorian (ed.), Antibiotics in laboratory medicine, 3rd ed. The Williams & Wilkins Co., Baltimore, Md.
- 29.Vazquez, J. A., M. Lynch, D. Boikov, and J. D. Sobel. 1997. In vitro activity of a new pneumocandin antifungal, L-743,872, against azole-susceptible and -resistant Candida species. Antimicrob. Agents Chemother. 41:1612-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villanueva, A., E. G. Arathoon, E. Gotuzzo, R. S. Berman, M. J. DiNubile, and C. A. Sable. 2001. A randomized double-blind study of caspofungin versus amphotericin for the treatment of candidal esophagitis. Clin. Infect. Dis. 33:1529-1535. [DOI] [PubMed] [Google Scholar]
- 31.Villanueva, A., E. Gotuzzo, E. G. Arathoon, L. M. Noriega, N. A. Kartsonis, R. J. Lupinacci, and J. M. Smietana. 2002. A randomized double-blind study of caspofungin versus fluconazole for the treatment of esophageal candidiasis. Am. J. Med. 113:294-299. [DOI] [PubMed] [Google Scholar]
- 32.Walsh, T. J., H. Teppler, G. R. Donowitz, J. A. Maertens, L. R. Baden, A. Dmoszynska, O. A. Cornely, M. R. Bourque, R. J. Lupinacci, C. A. Sable, and B. E. dePauw. 2004. Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. N. Engl. J. Med. 351:1391-1402. [DOI] [PubMed] [Google Scholar]