Abstract
Sulfadoxine-pyrimethamine (SP) has been widely used in recent years to treat acute uncomplicated Plasmodium falciparum malaria. Risk factors for SP therapeutic failure include young age, subtherapeutic SP concentrations, and resistance-conferring genetic mutations in parasite target enzymes. A substantial proportion of patients are able to clear genetically highly resistant P. falciparum genotypes. To determine whether blood SP concentrations independently affect the patient's ability to clear resistant genotypes, we compared SP pharmacokinetics of cases of adequate clinical and parasitological response (ACPR) with cases of treatment failure (TF). When patients with ACPR and TF were compared, mean values were similar for the day 3 blood pyrimethamine (205 ng/ml versus 172 ng/ml; P = 0.25) and estimated maximum sulfadoxine (79 ± 6.52 versus 69 ± 6.27 μg/ml; P = 0.60) concentrations, for sulfadoxine terminal-phase elimination half-lives (7.15 versus 6.41 days; P = 0.42), and for the extents of sulfadoxine absorption (areas under the concentration-time curve of 932 ± 100 versus 888 ± 78.9 μg day ml−1; P = 0.72). Among patients infected with the quintuple resistant parasites, day 3 blood pyrimethamine concentrations were higher in those who cleared the infection than in those who did not (305 ± 35.4 versus 228 ± 21.7 ng/ml; P = 0.037). Within this subgroup, this finding remained significant after adjusting for endogenous folate levels, age, site, and resistance-conferring mutations (odds ratio: 1.011 [1.003 to 1.024]; P = 0.018). However, as a subgroup analysis, our biologically plausible observation that higher blood pyrimethamine concentrations enhance the ability of patients to clear resistant P. falciparum should be interpreted with caution and needs further validation.
The combination of sulfadoxine (SDX) and pyrimethamine (PYR) (sulfadoxine-pyrimethamine [SP]) has been widely used in the treatment of acute uncomplicated malaria in countries faced with chloroquine-resistant Plasmodium falciparum (15). Pyrimethamine is a synthetic diaminopyridine that inhibits the dihydrofolate reductase (DHFR) of plasmodia and thereby blocks the biosynthesis of purines and pyrimidines, which are essential for DNA synthesis and cell multiplication. This leads to a failure of nuclear division and subsequent cell death. Sulfadoxine is a long-acting sulfonamide, and it inhibits dihydropteroate synthase (DHPS), an enzyme that utilizes para-aminobenzoic acid in the synthesis of dihydropteroic acid. This enzyme is also a component of the folate metabolic pathway and is upstream of DHFR, the enzyme targeted by pyrimethamine. The combination of pyrimethamine and sulfadoxine thus offers a two-step synergistic blockade of plasmodial division.
Mutations at defined codons in the DHFR gene have been associated with in vivo SP therapeutic failure in various epidemiological studies (5, 14). It is established that the step-wise accumulation of the mutations S108N, C59R, N51I, and I164L in the P. falciparum DHFR gene results in high-level pyrimethamine resistance, as reviewed by Hyde (4). Similarly, most sulfadoxine-resistant parasites that have been assayed in vitro carry the DHPS mutations A437G and K540E (14). According to recent observations (5) in Africa, the best marker of SP resistance in the field is the presence of quintuple mutant P. falciparum parasites. The quintuple mutants are parasites carrying three mutations in DHFR (S108N plus C59R plus N51I) and two mutations in DHPS (A437G plus K540E) genes.
There are many potential causes of SP treatment failure, including these resistance-conferring mutations in the parasite and high host folate levels (13). Interindividual variation in host pharmacokinetics (PK) is another potential cause. The rate of absorption of the drug, its distribution in the various body compartments, tissue and plasma protein binding, and the rate of metabolism and excretion of the drugs all influence drug concentrations at the active sites (11), hence the importance of determining the effect of interindividual pharmacokinetic variability on therapeutic efficacy.
Information about the pharmacokinetics of these drugs in children with malaria is very limited. When orally administered, SP is well absorbed. Its components display peak plasma levels within 2 to 8 h and are excreted mainly by the kidneys (12). The elimination half-life ranges from 4.8 to 10.6 days for sulfadoxine and from 3.3 to 4.8 days for pyrimethamine (6, 12, 16, 19). To evaluate pharmacological determinants of SP therapeutic failure, it is necessary to measure the drug levels after therapy to determine the duration of parasiticidal SP concentrations. The minimum effective concentrations required in vivo are, however, not clearly established. These concentrations are usually predicted from in vitro observations, but the relevance of in vitro-to-in vivo efficacy is uncertain.
We studied the SP concentration-time profiles for Malawian children with uncomplicated malaria yielding P. falciparum isolates with well-characterized genotypic resistance profiles. We aimed to test the hypothesis that subtherapeutic levels of SP at specified posttreatment time points are associated with SP treatment failure and to investigate whether differences in posttreatment blood SP concentrations affect the outcomes for patients harboring quintuple mutants.
MATERIALS AND METHODS
Study area and population.
We studied patients presenting to the Dedza and Mangochi district hospitals, Malawi, during a dry low-malaria-transmission season (August to October 2000 and June to September 2000, respectively). Patients were eligible for the study if they were between 6 months and 12 years of age, had axillary temperatures of ≥37.5°C, had microscopically confirmed single P. falciparum infection, and had no complications of malaria (complications include the inability to drink or breastfeed, the inability to sit or stand, and severe anemia, cerebral malaria, or a recent history of convulsions). Informed consent was obtained from parents or guardians before enrollment into the study. The ethics committee of the University of Malawi College of Medicine approved the study protocol.
Treatment, follow-up of children, and sample collection.
Children received supervised treatment with a standard SP dose (one-fourth of a tablet per 5 kg body weight for ages ≤12 to a maximum of three tablets; one tablet contained 25 mg pyrimethamine plus 500 mg sulfadoxine) on the enrollment day (day 0). Each subject was followed again on days 3, 7, 14, 21, and 28. Finger-prick blood samples (100 μl placed on 3MM Whatman filter paper and air dried) were collected before treatment and at each of the follow-up days and stored in desiccated resealable plastic bags at room temperature for parasite genetic and drug analysis and for measurement of pretreatment folate concentrations. Posttreatment blood samples were collected at any time during working hours, between 8 a.m. and 5 p.m.
Definition of treatment outcomes.
Therapeutic efficacy was defined according to the World Health Organization protocols (20). Definitions were as below.
Treatment success.
Adequate clinical and parasitological response (ACPR) consisted of the absence of parasitemia on day 28 irrespective of axillary temperature without previously meeting any of the criteria for early treatment failure (ETF), late clinical failure (LCF), or late parasitological failure (LPF).
Treatment failure.
ETF consisted of the development of danger signs or severe malaria on day 1, 2, or 3 in the presence of parasitemia; a day 2 parasitemia count higher than the day 0 count irrespective of axillary temperature; parasitemia on day 3 with axillary temperature of >37.5°C; or day 3 parasitemia more than 25% of the count on day 0. Late treatment failure (LTF) consisted of the following: (i) LCF, consisting of the development of danger signs or severe malaria after day 3 in the presence of parasitemia without meeting any criteria of early treatment failure or the presence of parasitemia and axillary temperature of ≥37.5°C on any day from day 4 to day 28 without previously meeting any of the criteria of early treatment failure; and (ii) LPF, consisting of the presence of parasitemia on any day from day 4 to 28 and axillary temperature of <37°C without meeting any criteria for early treatment failure or late clinical failure.
For the purpose of analysis in this study we combined LPF and LCF cases to make the LTF category, which we compared with the ACPR group.
Drug analysis.
Whole-blood concentrations of pyrimethamine and sulfadoxine were recovered from filter paper blood spots and measured by a slight modification of a reversed-phase high-performance liquid chromatography procedure previously described for serum samples (19). The blood for the PK work was collected in a 100-μl capillary tube, transferred onto filter paper, dried at room temperature, and then stored at 4°C until extraction.
Genetic analysis.
A previously described (9) nested PCR with mutation-specific restriction enzyme digestion was used to detect resistance-associated mutations at Plasmodium falciparum DHFR and DHPS codons 51, 59, 108, and 164 and 437 and 540, respectively. The prevalence of the quintuple mutant genotype (encoding S108N plus C59R plus N51I plus A437G plus K540E) was determined.
Pharmacokinetics and statistical analysis.
Mean values of C0 (drug concentration at time zero) were obtained by back extrapolation of the log-linear portion of the concentration-time profile. As SP blood concentrations peak within 2 to 3 h of administration (12), days 3, 7, 14, 21, and 28 were considered data points in the terminal phase. Data points day 3, day 7, day 14, and day 21 were used to calculate the terminal rate constant (λz) by log-linear regression. The drug elimination half-life was estimated as ln2/λz. The area under the concentration-time curve from time zero to infinity (AUC0-∞) was estimated by use of the linear trapezoidal rule.
Noncompartmental analysis with WinNonlin V4.0.1 (Pharsight Corporation, Mountain View, California) was used to obtain PK parameters. We used STATA 7.0 (Stata Corporation, Texas) and the two-sample Mann-Whitney rank-sum test with a two-tailed level of significance (significance set at a P value of <0.05) to compare PK parameters and other characteristics between groups. Multivariate logistic regression analysis was used to measure the independent relationship between variables and outcomes. The Bonferroni method (significance set at a P value of <0.007) was used in the multivariate analysis to determine risk factors for failure to clear infections with quintuple mutants in 74 cases. The Bonferroni adjustment is a statistical correction for multiple comparisons. It effectively raises the standard of proof needed when testing a wide range of hypotheses simultaneously. If we are testing n outcomes instead of a single outcome, we divide our alpha level by n as opposed to testing at the conventional 0.05 alpha level. Parameters included in the multivariate analysis were chosen based on published literature that has shown that they are important for treatment outcomes. Backward elimination was then used to build the logistic regression model.
RESULTS
Of 217 enrolled subjects, 191 completed the study and were eligible for analysis. Therapeutic efficacy could be determined in 164 of the 191 subjects who completed the study. Eighty-one (49%) children had ACPR outcomes, and there were no cases of ETF. Fifteen (9.1%) and 68 (41%) children had LCF and LPF therapeutic outcomes, respectively. Blood sulfadoxine and pyrimethamine concentration measurements were determined for 115 and 104 children, respectively. Pharmacokinetic parameters were compared for the 115 and 104 children for whom day 3 drug levels, SP genotypic resistance profiles, blood folate levels, and complete baseline demographic data were available, and the baseline clinical and laboratory characteristics of these subjects are summarized in Table 1. Of the 217 enrolled subjects, those excluded from the present analysis included 4 who withdrew their consent, 21 who were lost to follow-up, and 1 who violated the study protocol. Twenty had incomplete DHFR/DHPS genotype results, 34 had no baseline hemoglobin recorded, and drug concentrations could not be determined for 24 patients. There were no differences in baseline geometric mean parasitemias (8,282 versus 6,546 parasites/μl; P = 0.68), ages (2.75 versus 2.50 years; P = 0.70), and gender ratios (44% versus 46% female; P = 1.0) between the subjects reported in Table 1 and those who were excluded.
TABLE 1.
Clinical and laboratory findings on the day of enrollmenta
| Parameter | ACPR (n = 49) | LTF (n = 66) | P value |
|---|---|---|---|
| Dose of sulfadoxine (oral, mg) | 382 ± 326 (250-2,501) | 362 ± 268 (250-2,501) | 0.81 |
| Sex (% female) | 53 | 41 | 0.14 |
| Age (yr) | 2.58 ± 2.42 (0.500-10.0) | 2.48 ± 1.74 (0.500-10.0) | 0.28 |
| Body weight (kg) | 11.0 ± 4.36 (6.00-26.0) | 11.2 ± 3.70 (6.00-29.0) | 0.89 |
| Parasitemia (parasites/μl) | 7,900 (6,325-9,868) | 8,497 (7,108-10,159) | 0.63 |
| Hemoglobin (g/liter) | 7.78 ± 1.84 (3.20-11.9) | 7.80 ± 1.55 (5.30-12.0) | 0.78 |
| Blood folate (ng/ml) | 31 ± 1.6 (8.0-58) | 34 ± 1.3 (12-56) | 0.12 |
| Quintuple mutant prevalence (%) | 61.2 | 68.2 | 0.30 |
Parasite densities are reported as geometric means. The rest of the values are means±SEM with ranges in parentheses.
Sulfadoxine.
The lower limit of quantitation for SDX from whole blood spotted on filter paper was less than 6 μg/ml. Blood sulfadoxine concentration-time profiles for ACPR and LTF cases were similar. For each time point, those with ACPR had blood sulfadoxine concentrations that were similar to those for LTF cases (at day 3, 77.1 μg/ml and 61.3 μg/ml [P = 0.061]; at day 7, 40.9 μg/ml and 35.6 μg/ml [P = 0.31]; at day 14, 25.1 μg/ml and 20.9 μg/ml [P = 0.34]; and at day 21, 14.5 μg/ml and 14.6 μg/ml [P = 0.80], all for ACPR and LTF, respectively). Day three sulfadoxine concentrations varied widely between individuals (range, 0.0935 to 230 μg/ml).
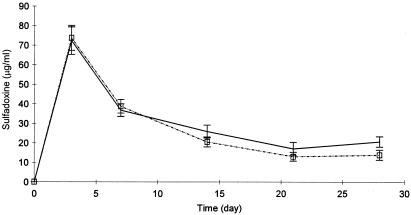
The extents of exposure to sulfadoxine (reported as AUC0-∞) were similar in subjects with ACPR and in those with LTF (932 versus 888 μg day ml−1; P = 0.72), and sulfadoxine elimination half-lives were similar for the two groups (7.15 versus 6.41 days, respectively; P = 0.42). Although there was a significant difference in gender ratios between the two outcome groups, there were no significant differences in sulfadoxine blood concentrations between males and females at any time after SP administration, as shown in Fig. 1. Derived sulfadoxine pharmacokinetic parameters, half-lives, and AUC0-∞s were, as reported in Table 2, similar for ACPR and LTF cases.
FIG. 1.
Blood sulfadoxine concentrations (mean ± standard error of the mean [SEM]) in males (dotted line) and females (solid line) following single oral doses of pyrimethamine-sulfadoxine (one-fourth of a tablet per 5 kg body weight for ages ≤12 to a maximum of three tablets; one tablet contained 25 mg pyrimethamine plus 500 mg sulfadoxine).
TABLE 2.
Pharmacokinetic parameters following the administration of pyrimethamine-sulfadoxine in patients with acute uncomplicated P. falciparum malariaa
| Parameter | ACPR (n = 49) | LTF (n = 66) | P value |
|---|---|---|---|
| Day 3 blood SDX (μg/ml) | 77.1 ± 48.1 (0.23-230) | 61.3 ± 42.3 (0.0935-213) | 0.061 |
| Day 3 blood PYR (ng/ml) | 267 ± 165 (245-668) | 216 ± 130 (17.6-513) | 0.083 |
| SDX elimination half-life (days) | 7.15 | 6.41 | 0.42 |
| AUC0-∞ (μg day ml−1) ± SE | 932 ± 100 | 888 ± 78.9 | 0.72 |
| C0 (SDX, μg/ml) ± SE | 79 ± 6.52 | 69 ± 6.27 | 0.60 |
Values are means±SEM, with ranges in parentheses. See text for dosages. AUC0-∞ is for sulfadoxine.
Pyrimethamine.
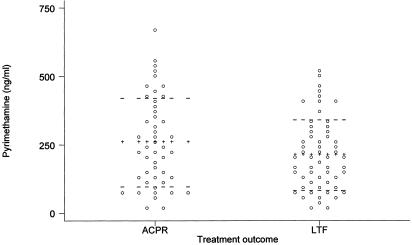
The lower limit of quantitation for pyrimethamine from 100 μl of whole blood spots was 31 ng/ml. With pyrimethamine concentrations after day 7 below the limit of quantitation, we failed to do a full PK study for this drug and we therefore measured day 3 pyrimethamine concentrations. The geometric mean concentrations of pyrimethamine on day 3 were similar for patients with ACPR and those with LTF (205 ng/ml [95% confidence interval {CI}, 162 to 210] versus 172 ng/ml; P = 0.25), but there was wide interindividual variation in measured day 3 pyrimethamine concentrations (Fig. 2).
FIG. 2.
Day 3 blood PYR levels dot plotted by treatment outcome. LTF, late treatment failure (n = 60); ACPR, sensitive treatment outcome or adequate clinical and parasitological response (n = 44). The lines of pluses mark the mean PYR level for each outcome group, and the horizontal dashed lines are means plus or minus standard deviations.
Drug levels and ability to clear the resistant quintuple mutant.
We found that SP strongly selected for resistant parasite genotypes as evidenced by the observation that there was a greater proportion of resistant genotypes after treatment than in pretreatment patient samples (92% versus 67% [P = 0.002], respectively, for DHFR triple mutant P. falciparum and 90% versus 61% [P < 0.001], respectively, for the quintuple mutant).
Among children infected with the highly SP-resistant quintuple mutation (n = 74), 31 (42%) cleared their infection. The characteristics of and comparisons between children who cleared and those who did not clear this resistant P. falciparum type are reported in Table 3. Mean ages, baseline parasite densities, weights, baseline hemoglobin levels, and gender ratios were similar for these two groups. However, we found that children who cleared infection with the SP-resistant quintuple mutant had higher blood pyrimethamine concentrations than those who failed to clear the infection with the quintuple mutant (P = 0.037).
TABLE 3.
Univariate comparison of the characteristics of children who cleared and those who failed to clear the PYR-SDX-resistant P. falciparum quintuple-mutant infectiona
| Parameter | Cleared quintuple mutant (n = 31) | Failed to clear quintuple mutant (n = 43) | P valueb |
|---|---|---|---|
| Gender ratio (male:female) | 14:17 | 27:16 | 0.16 |
| Age (yr) | 2.54 ± 0.63 | 2.11 ± 0.24 | 0.49 |
| Weight (kg) | 10.6 ± 0.96 | 10.1 ± 0.50 | 0.25 |
| Parasitemia (parasites/μl) | 8,920 ± 1,339 | 8,217 ± 1,534 | 0.32 |
| Baseline hemoglobin (g/liter) | 8.0 ± 0.46 | 7.7 ± 0.27 | 0.60 |
| Baseline blood folate (ng/ml) | 29.5 ± 1.87 | 34.2 ± 1.57 | 0.056 |
| Day 3 SDX (μg/ml) | 69.8 ± 8.43 | 65.3 ± 7.01 | 0.72 |
| Day 3 PYR (ng/ml) | 305 ± 35.4 | 228 ± 21.7 | 0.037* |
Parasite densities are reported as geometric means. The rest of the values are means ± SEM.
The P value with an asterisk is significant at <0.05.
Multivariate analysis.
A multivariate logistic regression analysis was done incorporating pyrimethamine and sulfadoxine day 3 concentrations, ages, gender ratios, pretreatment geometric mean parasite densities, pretreatment hemoglobin levels, and DHFR/DHPS genotypes. We found that low blood concentrations of PYR (odds ratio [OR]: 1.011 [1.003 to 1.024]; P = 0.018) and high blood concentrations of folate (OR: 1.5 [1.08 to 1.98]; P = 0.013) were independent risk factors for SP treatment failure. Pretreatment presence of the quintuple mutant and study site were the most significant risk factors for an LTF treatment outcome, based on the 28-day follow-up. Complete data on folate and molecular markers will be reported elsewhere. Following the Bonferroni adjustment, neither high folate (P > 0.007) nor decreased sulfadoxine-pyrimethamine (P > 0.007) blood concentrations were risk factors for failure to clear resistant malaria parasites.
DISCUSSION
This study did not find evidence of differences in posttreatment blood sulfadoxine-pyrimethamine concentrations between cases with ACPR and LTF treatment outcomes. The clinically relevant therapeutic range for sulfadoxine-pyrimethamine is not well established, but a previous study found that nonimmune patients (travelers) with RII (patients still parasitemic at day 7 after treatment) SP-resistant P. falciparum infections from Tanzania in 1986, who attained maximum sulfadoxine and pyrimethamine concentrations below the range of 62 to 115 μg/ml and below 49.7 ng/ml, respectively, developed secondary parasitemia (10). Clearly, in the present study, most subjects (on day 3 only), irrespective of treatment outcome, sustained SDX and PYR concentrations (mean of 73.2 μg/ml [range, 51 to 89 μg/ml] and mean of 290 ng/ml [95% CI, 182 to 398], respectively) that seemed to be the same or below those that were associated with failure in the 1986 Tanzanian study. In contrast, Chulay et al. found that sulfadoxine and pyrimethamine concentrations of ≥60 μg/ml and ≥15 ng/ml, respectively, were effective against K39, a resistant strain of P. falciparum in vitro (2). Blood sulfadoxine-pyrimethamine levels attained in the present study were above the concentrations found by Chulay et al. Thus, values for parasite-growth-inhibitory concentrations for resistant or sensitive P. falciparum isolates obtained in vitro may not always be an accurate reference for assessing the drug's efficacy in vivo.
In the univariate analysis, we found that among patients infected with the quintuple mutants, day 3 blood pyrimethamine concentrations were higher in those who cleared the infection than in those who did not. This difference could not be detected, however, in multivariate analysis (with Bonferroni adjustment), probably because there were numerous explanatory variables between the two groups (those that cleared quintuple mutants and those that failed to clear quintuple mutants) with only less than half (n = 74) of the enrolled subjects analyzed. It is therefore possible that a greater sample size for the analysis would have been required to find that low blood pyrimethamine concentration is indeed a risk factor for failure to clear the quintuple mutants. The pharmacologic activity of drugs is a function of the unbound drug concentration. Since sulfadoxine and pyrimethamine are highly protein bound (88% and 93%, respectively [8, 17]) and since our drug assays looked only at the total drug, we might not have been able to notice the difference in plasma concentrations between the groups compared.
In a separate multivariate analysis that included all patients that had LTF and ACPR outcomes (n = 115), whether they had quintuple-mutant infections or not, it was found that low day 3 blood concentration of PYR (OR, 1.011 [1.003 to 1.024]; P = 0.018) was a risk factor for late treatment failure. The magnitude of the odds ratio from this analysis and the borderline significance of the results from the univariate analysis of the subgroup infected with quintuple mutants suggested that our observation that higher blood pyrimethamine concentrations enhance the ability of patients to clear resistant P. falciparum must be interpreted with caution and needs further validation.
There was substantial interindividual variation in sulfadoxine-pyrimethamine disposition. The cause of these differences is unknown, but the interindividual variations may reflect the effect of disease severity and/or differences in the time of day at which posttreatment day 3 blood samples were collected. Blood samples were collected at any time during working hours on day 3 between 8 a.m. and 5 p.m. The observed ranges of day 3 sulfadoxine and pyrimethamine concentrations (59 to 75 μg/ml and 208 to 264 ng/ml, respectively) in this study were, nevertheless, similar to the ranges (sulfadoxine range: 51 to 89 μg/ml [mean, 73.2 μg/ml]; pyrimethamine range: 252 to 484 ng/ml [mean, 368 ng/ml]) found by Hellgren et al. in Tanzania (3) and Bustos et al. (1) in the Philippines. In contrast to our results and those of Hellgren et al., Bustos et al. found a mean sulfadoxine day 3 concentration of 184 ± 40 μg/ml (1). In our study population, back-extrapolated sulfadoxine C0 was found to fall within the previously observed range of the highest blood concentration levels of a drug after administration, 51 to 169 μg/ml, following oral administration of the standard SP dose (1, 16, 19). The apparent elimination half-life of sulfadoxine agreed with previous reports of a range of 4 to 11 days (1, 3, 19).
Sulfadoxine-pyrimethamine acts through a two-step synergistic blockade of plasmodial division. A decline in the concentration of one of the component drugs of this combination to a concentration below that required for effective synergy would result in the loss of the antiplasmodial synergistic action. We found a trend towards lower sulfadoxine levels in patients with treatment failure than in those with ACPR (P = 0.061). We observed that after day 3 posttreatment (Fig. 1), sulfadoxine concentrations decreased rapidly (in both ACPR and LTF) to concentration levels that are below those required to kill resistant P. falciparum isolates in vivo (i.e., below the range of 62 to 115 μg/ml). This is of concern, since therapeutic concentrations need to be maintained for three life cycles to eradicate P. falciparum (18). Additional studies are needed to determine whether after day 3 the pyrimethamine concentration remained above that required for effective synergistic action against resistant parasites. However, we postulate that the rapid decline of sulfadoxine levels below the concentrations required for synergy immediately after day three should result in an overall loss of the required effective synergy between pyrimethamine and sulfadoxine as a combination. Such a situation would result in differential pyrimethamine susceptibility among parasites that would otherwise be susceptible to the combination, resulting in the selection of the low-grade resistant parasites observed in this study.
For this study population, it has been shown that predicted SP therapeutic levels were sustained, at least until day 3 after treatment, in subjects who cleared quintuple-mutant infections as well as in those who could not clear them and in ACPR and LTF groups in general. However, therapeutic levels are very poorly defined by the small numbers of patients with uncomplicated malaria for whom published data on the association of pharmacokinetics and treatment response (pharmacodynamics) are available (1, 3, 19). This is further complicated by the rightward shift in the dose response curve as resistance spreads as well as by the effects of other factors, including humoral immunity (7, 18) and blood folate levels (13), on therapeutic response. We therefore suggest that larger pharmacokinetic-pharmacodynamic studies are needed to elucidate factors that may account for interindividual variation in the observed SP therapeutic responses.
Acknowledgments
Funding for the study reported in this paper was provided in part by the WHO/MIM/TDR Task Force on Malaria Research Capability Strengthening in Africa grant (principal investigator: Allan Macheso, grant number 980041), Christopher Plowe's NIH grant (R01AI44824), and the Department of Pharmacology at the University of Cape Town.
We thank the children of Dedza and Mangochi, G. Gamadzi (laboratory technician, Lilongwe Central Hospital, Malawi), and the clinical staff who supported this study in various ways.
REFERENCES
- 1.Bustos, D. G., J. E. Lazaro, F. Gay, A. Pottier, C. J. Laracas, B. Traore, and B. Diquet. 2002. Pharmacokinetics of sequential and simultaneous treatment with the combination chloroquine and sulfadoxine-pyrimethamine in acute uncomplicated Plasmodium falciparum malaria in the Philippines. Trop. Med. Int. Health 7:584-591. [DOI] [PubMed] [Google Scholar]
- 2.Chulay, J. D., W. M. Watkins, and D. G. Sixsmith. 1984. Synergistic antimalarial activity of pyrimethamine and sulfadoxine against Plasmodium falciparum in vitro. Am. J. Trop. Med. Hyg. 33:325-330. [DOI] [PubMed] [Google Scholar]
- 3.Hellgren, U., C. M. Kihamia, Y. Bergqvist, M. Lebbad, Z. Premji, and L. Rombo. 1990. Standard and reduced doses of sulfadoxine-pyrimethamine for treatment of Plasmodium falciparum in Tanzania, with determination of drug concentrations and susceptibility in vitro. Trans. R. Soc. Trop. Med. Hyg. 84:469-473. [DOI] [PubMed] [Google Scholar]
- 4.Hyde, J. E. 1990. The dihydrofolate reductase-thymidylate synthetase gene in the drug resistance of malaria parasites. Pharmacol. Ther. 48:45-59. [DOI] [PubMed] [Google Scholar]
- 5.Kublin, J. G., F. K. Dzinjalamala, D. D. Kamwendo, E. M. Malkin, J. F. Cortese, L. M. Martino, R. A. G. Mukadam, S. J. Rogerson, A. G. Lescano, M. E. Molyneux, P. A. Winstanley, P. Chimpeni, T. E. Taylor, and C. V. Plowe. 2002. Molecular markers for failure of sulfadoxine-pyrimethamine and chlorproguanil-dapsone treatment of Plasmodium falciparum malaria. J. Infect. Dis. 185:380-388. [DOI] [PubMed] [Google Scholar]
- 6.Mansor, S. M., V. Navaratnam, M. Mohamad, S. Hussein, A. Kumar, A. Jamaludin, and W. H. Wernsdorfer. 1989. Single dose kinetic study of the triple combination mefloquine/sulphadoxine/pyrimethamine (Fansimef) in healthy male volunteers. Br. J. Clin. Pharmacol. 27:381-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayxay, M., K. Chotivanich, S. Pukrittayakamee, P. Newton, S. Looareesuwan, and N. J. White. 2001. Contribution of humoral immunity to the therapeutic response in falciparum malaria. Am. J. Trop. Med. Hyg. 65:918-923. [DOI] [PubMed] [Google Scholar]
- 8.Panisko, D. M., and J. S. Keystone. 1990. Treatment of malaria—1990. Drugs 39:160-189. [DOI] [PubMed] [Google Scholar]
- 9.Plowe, C. V., J. F. Cortese, A. Djimde, O. C. Nwanyanu, W. M. Watkins, P. A. Winstanley, J. G. Estrada-Franco, R. E. Mollinedo, J. C. Avila, J. L. Cespedes, D. Carter, and O. K. Doumbo. 1997. Mutations in Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase and epidemiologic patterns of pyrimethamine-sulfadoxine use and resistance. J. Infect. Dis. 176:1590-1596. [DOI] [PubMed] [Google Scholar]
- 10.Schapira, A., I. C. Bygbjerg, S. Jepsen, H. Flachs, and M. W. Bentzon. 1986. The susceptibility of Plasmodium falciparum to sulfadoxine and pyrimethamine: correlation of in vivo and in vitro results. Am. J. Trop. Med. Hyg. 35:239-245. [DOI] [PubMed] [Google Scholar]
- 11.Turner, P., and A. Richens. 1978. Clinical pharmacology, 3rd ed. Churchill Livingstone, Edinburgh, United Kingdom.
- 12.USP DI. 1995. Drug information for the health care professional, 15th ed., vol. 1. The United States Pharmacopeial Convention, Inc., Rockville, Md.
- 13.van Hensbroek, M. B., S. Morris-Jones, S. Meisner, S. Jaffar, L. Bayo, R. Dackour, C. Phillips, and B. M. Greenwood. 1995. Iron, but not folic acid, combined with effective antimalarial therapy promotes haematological recovery in African children after acute falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 89:672-676. [DOI] [PubMed] [Google Scholar]
- 14.Wang, P., M. Read, P. F. Sims, and J. E. Hyde. 1997. Sulfadoxine resistance in the human malaria parasite Plasmodium falciparum is determined by mutations in dihydropteroate synthetase and an additional factor associated with folate utilization. Mol. Microbiol. 23:979-986. [DOI] [PubMed] [Google Scholar]
- 15.Watkins, W. M., E. K. Mberu, P. A. Winstanley, and C. V. Plowe. 1997. The efficacy of antimalarial combinations in Africa: a predictive model based on pharmacokinetic and pharmacodynamic analysis. Parasitol. Today 13:459-464. [DOI] [PubMed] [Google Scholar]
- 16.Weidekamm, E., H. Plozza-Nottebrock, I. Forgo, and U. C. Dubach. 1982. Plasma concentrations in pyrimethamine and sulfadoxine and evaluation of pharmacokinetic data by computerized curve fitting. Bull. W. H. O. 60:115-122. [PMC free article] [PubMed] [Google Scholar]
- 17.Weidekamm, E., D. E. Schwartz, U. C. Dubach, and B. Weber. 1987. Single-dose investigation of possible interactions between the components of the antimalarial combination Fansimef. Chemotherapy 33:259-265. [DOI] [PubMed] [Google Scholar]
- 18.White, N. J. 2003. Malaria, p. 1244. In G. C. Cook and A. Zumla (ed.), Manson's tropical diseases, 21st ed. Elsevier Science Limited, Edinburgh, United Kingdom.
- 19.Winstanley, P. A., W. M. Watkins, C. R. J. C. Newton, C. Nevill, E. Mberu, P. A. Warns, C. M. Waruiru, I. N. Mwangi, D. A. Warrell, and K. Marsh. 1992. The disposition of oral and intramuscular pyrimethamine/sulfadoxine in Kenyan children with high parasitaemia but clinically non-severe falciparum malaria. Br. J. Clin. Pharmacol. 33:143-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. 2002. Monitoring antimalarial resistance. Report of a W.H.O. consultation. W.H.O./CDS/CSR/EPH/2002.17. Geneva, Switzerland.