Abstract
The effects of simvastatin treatment on Chlamydia pneumoniae lung infection, inflammation, and serum lipids in mouse model were studied. Simvastatin decreased viable chlamydial counts and increased inflammatory cell infiltrates in the lung tissue, suggesting that simvastatin treatment had both antichlamydial and immunomodulatory effects during an acute C. pneumoniae infection.
.Numerous studies have addressed the possible role of infectious agents in the pathogenesis of atherosclerosis, and Chlamydia pneumoniae, a human respiratory pathogen, displays the strongest association (7). Atherosclerosis is a combination of chronic inflammation and a cholesterol overload of endothelial macrophages (32). Statins are widely used as cholesterol-lowering drugs, and recently, growing interest has also been focused on their immunomodulatory actions (11, 18, 33a, 37). Clinical studies have shown that statins decrease cardiac events in persons with average cholesterol levels and slightly elevated C-reactive protein levels, and most importantly, they reduce elevated inflammatory markers, suggesting other protective mechanisms (26, 30, 31, 33). Several animal model and in vitro studies have also reported anti-inflammatory action (20, 34, 38). Recently, in humans and in two mouse models, statins have been shown to be beneficial during bacteremia and sepsis (1, 2, 22, 25).
Statins have been shown to affect C. pneumoniae infection in vitro: cerivastatin slightly decreases the infection rate in human macrophages and the infection rate of vascular smooth muscle cells through C. pneumoniae-infected monocytes (8, 19). Our aim was to study how lipophilic simvastatin affects C. pneumoniae infection in a mouse model and whether a high-fat diet modulates the outcome.
The Animal Care and Use Committee of National Public Health Institute, Helsinki, Finland, approved all procedures. Eight- to nine-week-old female NIH/S mice fed a regular chow diet (n = 138) (Altromin) or a high-fat diet (n = 137) (21% total fat, 0.2% cholesterol, and 19.5% casein; Harlan Teklad) were given simvastatin (L-644; Merck & Co., Inc.) in daily intraperitoneal injections (100 μl in 1% dimethyl sulfoxide) for 24 days (days −3 to 21 postinfection [p.i.]). At day 0, the mice were intranasally inoculated with C. pneumoniae Kajaani 7 (5.3 × 105 inclusion-forming units in 40 μl of saccharose-phosphate-glutamate [SPG] solution) (12). Samples (n = 6 mice) were collected after a minimum 4-h fast. (Fig. 1). The right lung was mechanically homogenized in 2 ml of SPG solution, and the supernatant was cultured in HL cells. For inclusion detection, the Pathfinder Chlamydia Confirmation System was used (Kallestad Diagnostics). DNA was extracted using the QIAamp tissue kit (QIAGEN GmbH). C. pneumoniae LightCycler real-time quantitative PCR (Roche) was performed using 16S rRNA-specific primers and a hybridization probe (13, 29). C. pneumoniae immunoglobulin G antibodies (serum dilution, 1:100) were measured by enzyme immunoassay (AniLabsystems). Inflammatory changes in the lungs were determined by histology from hematoxylin and eosin-stained longitudinal cross sections and graded as no changes (histology score = 0), minimal (score = 1), slight (score = 2), moderate (score = 3), marked (score = 4), or severe (score = 5), depending on the number of mononuclear cells and the area affected. In the milder forms, the inflammatory cell infiltrates were limited to focal areas or occurred in small scattered foci, but in the severe cases, large tissue areas were affected. Serum amyloid A concentrations were measured by enzyme immunoassay (BioSource International), and lipids were measured with fully enzymatic methods (Roche Diagnostics and Wako Chemicals GmbH). For statistical analysis, the nonparametric Mann-Whitney U test was used.
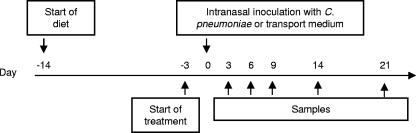
FIG. 1.
Experimental design for the two separate experiments using different diets. Feeding with the high-fat diet was initiated 2 weeks, and treatment with simvastatin (0.5 mg/kg of body weight) or 1% dimethyl sulfoxide 3 days, prior to the C. pneumoniae challenge/SPG inoculation. Samples were taken until day 20 p.i.
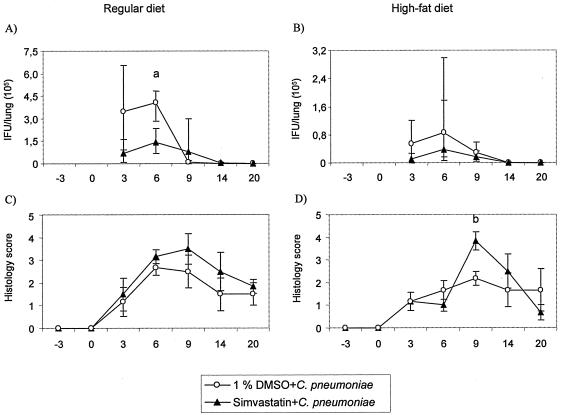
Following simvastatin treatment, inclusion-forming-unit counts in the lungs at the early stages of infection (days 3 and 6 p.i., respectively) were reduced 65 to 80% from those for the vehicle-treated mice with a regular diet (Fig. 2A) and 55 to 82% for mice on a high-fat diet (Fig. 2B). Similar decreases in chlamydial genome numbers, 78 to 83% (in regular diet-fed mice, day 6 p.i.; P = 0.002) were also demonstrated by PCR.
FIG. 2.
Chlamydia culture and pulmonary histopathology findings of C. pneumoniae-infected NIH/S mice fed either a regular diet (A and C) or a high-fat diet (B and D). Mice were treated with simvastatin (0.5 mg/kg of body weight) or 1% dimethyl sulfoxide (DMSO). (A, B) Chlamydia culture results from lung tissue, median ± interquartile range (25th and 75th percentile). “a” indicates simvastatin-treated C. pneumoniae-infected regular-diet-fed mice compared to 1% DMSO-treated C. pneumoniae-infected regular-diet-fed mice; P = 0.026. (C, D) Pulmonary histopathology results, mean histology scores ± standard errors. “b” indicates simvastatin-treated C. pneumoniae-infected high-fat-diet-fed mice compared to 1% DMSO-treated C. pneumoniae-infected high-fat-diet-fed mice; P = 0.015. The nonparametric Mann-Whitney U test was used.
Simvastatin treatment slightly increased pulmonary inflammation at each time point in mice on a regular diet (histology score, 1.5 to 3.2 versus. 1.2 to 2.7, respectively) (Fig. 2C) and also on a high-fat diet (Fig. 2D). Serum immunoglobulin G antibody responses against C. pneumoniae and the serum amyloid A concentrations were not affected by simvastatin treatment (data not shown).
After 2 weeks on a high-fat diet, total cholesterol levels increased by 70.9% (day −3 p.i.; P = 0.002) and triglyceride levels by 37.5% (P = not significant). Simvastatin had no effects on serum lipid levels (Table 1).
TABLE 1.
Serum lipid levels at baseline (day −3 p.i.) and at the end point (day 20 p.i) of SPG inoculated mice treated with 0.5-mg/kg simvastatin
| Diet | Amt of total cholesterol, mmol/liter, median (IQRa)
|
Amt of triglycerides, mmol/liter, median (IQRa)
|
||
|---|---|---|---|---|
| Baseline | End point | Baseline | End point | |
| Regular diet | 3.3 (2.6-3.6) | 3.3 (3.1-3.6) | 1.9 (1.6-2.4) | 2.0 (1.9-2.1) |
| Fatty diet | 5.7 (5.4-6.6) | 6.4 (5.9-6.9) | 2.6 (2.4-3.0) | 2.3 (2.2-2.4) |
Interquartile range, 25th to 75th percentile.
The present study showed that simvastatin treatment affects the course of acute C. pneumoniae infection by decreasing chlamydial counts in the lungs and, surprisingly, by amplifying the pulmonary inflammatory response in infected mice. Interestingly, statins have displayed antimicrobial effects in recent studies: they reduce the intracellular growth of Salmonella enterica serovar Typhimurium both in vitro and in vivo and the intracellular replication of cytomegalovirus, human immunodeficiency virus, and C. pneumoniae in vitro (6, 9, 19, 27). Hydroxymethylglutaryl (HMG)-coenzyme A reductase is an important enzyme catalyzing the rate-limiting reaction of the mevalonate pathway, leading to biosynthesis of isoprenoids and cholesterol in eucaryotes. The enzyme is also found in some gram-positive bacteria; thus, statins, inhibitors of the HMG-coenzyme A reductase, might have a direct antibiotic effect (14). Chlamydia is a gram-negative bacterium and does not have HMG-coenzyme A reductase but has genes encoding nonmevalonate isoprenoid pathway enzymes (21). Further, Chlamydia and Salmonella do not have the capacity to synthesize cholesterol, but cholesterol is required for their intracellular multiplication and is also an essential component of, e.g., chlamydial particles (39). Thus, these bacteria are dependent on the availability of host cholesterol inside the cells where they replicate (5, 6). Chlamydia may use cholesterol derived either from the extracellular space via low-density lipoprotein uptake or from intracellular cholesterol stores (5). Lipophilic simvastatin has easy access to all cell types in which C. pneumoniae multiplies, and by decreasing host cell isoprenoid and cholesterol levels or by disturbing intracellular trafficking of cholesterol, statins may affect chlamydial intracellular multiplication (8, 15, 16). Statins may also interfere with the chlamydial cell entry, as during human immunodeficiency virus infection (9). Specific plasma membrane microdomains, caveolae or lipid rafts, are rich in cholesterol and are important for the entry of several Chlamydia species, including C. pneumoniae (36).
The second interesting finding was that simvastatin amplified pulmonary inflammatory response by increasing inflammatory cell infiltration into the lungs during acute C. pneumoniae infection. The anti-inflammatory effect of statins with noninfectious stimuli has been reported in several in vivo studies (10, 24, 28, 34, 35, 40). However, Kiener et al. have previously pointed to the possible proinflammatory effects of statins, and they also showed that lipophilic statins increase cellular influx to the peritoneal cavity after injection of thioglycolate (17). The present study is the first one in which the effect of a statin treatment on immunological parameters after an active infection caused by an intracellularly multiplying pathogen has been studied in vivo under controlled experimental conditions.
In mice, a cholesterol-lowering effect of statins has been achieved with high doses even in mice lacking low-density lipoprotein receptors (4). In the present study, with the simvastatin dose similar to the therapeutic dose for treating hypercholesterolemia in humans, we did not see any decrease in the total cholesterol or triglyceride levels independent of the diet. The response to a high-fat diet was displayed as elevated serum cholesterol and triglycerides. Cholesterol increased especially in the high-density lipoprotein (HDL) fraction (data not shown). Since mice preferentially have HDL in circulation, it might well be that simvastatin does not affect this pool of cholesterol. Human statin trials depict only minor effects on HDL levels (23).
In conclusion, the present study confirms the previous in vitro findings that statin treatment may have an antichlamydial effect in vivo, too (19). The present data further suggest a possible proinflammatory effect in the antichlamydial process in vivo. However, probably due to the low numbers of mice in different groups, statistically significant differences between treatment and control groups were reached only at a few time points. The short follow-up of the present study does not allow any speculation on the effects of statin treatment on chronic C. pneumoniae infection, which is considered important in the development of atherosclerosis.
Acknowledgments
The skillful technical assistance of Maija Holtinkoski, Terttu Korpela, Leena Kuisma, and Anu Ahlroos is acknowledged.
This work was supported by National Public Health Institute, Finland, and the Retro Life Assurance Company Ltd.
REFERENCES
- 1.Almog, Y., A. Shefer, V. Novack, N. Maimon, L. Barski, M. Eizinger, M. Friger, L. Zeller, and A. Danon. 2004. Prior statin therapy is associated with a decreased rate of severe sepsis. Circulation 110:880-885. [DOI] [PubMed] [Google Scholar]
- 2.Ando, H., T. Takamura, T. Ota, Y. Nagai, and K. Kobayashi. 2000. Cerivastatin improves survival of mice with lipopolysaccharide-induced sepsis. J. Pharmacol. Exp. Ther. 294:1043-1046. [PubMed] [Google Scholar]
- 3.Reference deleted.
- 4.Bisgaier, C. L., A. D. Essenburg, B. J. Auerbach, M. E. Pape, C. S. Sekerke, A. Gee, S. Wolle, and R. S. Newton. 1997. Attenuation of plasma low density lipoprotein cholesterol by select 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors in mice devoid of low density lipoprotein receptors. J. Lipid Res. 38:2502-2515. [PubMed] [Google Scholar]
- 5.Carabeo, R. A., D. J. Mead, and T. Hackstadt. 2003. Golgi-dependent transport of cholesterol to the Chlamydia trachomatis inclusion. Proc. Natl. Acad. Sci. USA 100:6771-6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catron, D. M., Y. Lange, J. Borensztajn, M. D. Sylvester, B. D. Jones, and K. Haldar. 2004. Salmonella enterica serovar Typhimurium requires nonsterol precursors of the cholesterol biosynthetic pathway for intracellular proliferation. Infect. Immun. 72:1036-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danesh, J., R. Collins, and R. Peto. 1997. Chronic infections and coronary heart disease: is there a link? Lancet 350:430-436. [DOI] [PubMed] [Google Scholar]
- 8.Dechend, R., J. Gieffers, R. Dietz, A. Joerres, J. Rupp, F. C. Luft, and M. Maass. 2003. Hydroxymethylglutaryl coenzyme A reductase inhibition reduces Chlamydia pneumoniae-induced cell interaction and activation. Circulation 108:261-265. [DOI] [PubMed] [Google Scholar]
- 9.del Real, G., S. Jimenez-Baranda, E. Mira, R. A. Lacalle, P. Lucas, C. Gomez-Mouton, M. Alegret, J. M. Pena, M. Rodriguez-Zapata, M. Alvarez-Mon, A. C. Martinez, and S. Manes. 2004. Statins inhibit HIV-1 infection by down-regulating Rho activity. J. Exp. Med. 200:541-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diomede, L., D. Albani, M. Sottocorno, M. B. Donati, M. Bianchi, P. Fruscella, and M. Salmona. 2001. In vivo anti-inflammatory effect of statins is mediated by nonsterol mevalonate products. Arterioscler. Thromb. Vasc. Biol. 21:1327-1332. [DOI] [PubMed] [Google Scholar]
- 11.Endo, A., Y. Tsujita, M. Kuroda, and K. Tanzawa. 1977. Inhibition of cholesterol synthesis in vitro and in vivo by ML-236A and ML-236B, competitive inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Eur. J. Biochem. 77:31-36. [DOI] [PubMed] [Google Scholar]
- 12.Erkkilä, L., M. E. Rottenberg, and K. Laitinen. 2000. Comparison of anesthetics for inoculation of mice with Chlamydia pneumoniae. Comp. Med. 50:46-48. [PubMed] [Google Scholar]
- 13.Gaydos, C. A., T. C. Quinn, and J. J. Eiden. 1992. Identification of Chlamydia pneumoniae by DNA amplification of the 16S rRNA gene. J. Clin. Microbiol. 30:796-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hedl, M., L. Tabernero, C. V. Stauffacher, and V. W. Rodwell. 2004. Class II 3-hydroxy-3-methylglutaryl coenzyme A reductases. J. Bacteriol. 186:1927-1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keidar, S., M. Aviram, I. Maor, J. Oiknine, and J. G. Brook. 1994. Pravastatin inhibits cellular cholesterol synthesis and increases low density lipoprotein receptor activity in macrophages: in vitro and in vivo studies. Br. J. Clin. Pharmacol. 38:513-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kempen, H. J., M. Vermeer, E. de Wit, and L. M. Havekes. 1991. Vastatins inhibit cholesterol ester accumulation in human monocyte-derived macrophages. Arterioscler. Thromb. 11:146-153. [DOI] [PubMed] [Google Scholar]
- 17.Kiener, P. A., P. M. Davis, J. L. Murray, S. Youssef, B. M. Rankin, and M. Kowala. 2001. Stimulation of inflammatory responses in vitro and in vivo by lipophilic HMG-CoA reductase inhibitors. Int. Immunopharmacol. 1:105-118. [DOI] [PubMed] [Google Scholar]
- 18.Kobashigawa, J. A., S. Katznelson, H. Laks, J. A. Johnson, L. Yeatman, W. Xiu Ming, D. Chia, P. I. Terasaki, A. Sabad, G. A. Cogert, K. Trosian, M. A. Hamilton, J. D. Moriguchi, N. Kawata, A. Hage, D. C. Drinkwater, and L. W. Stevenson. 1995. Effect of pravastatin on outcomes after cardiac transplantation. N. Engl. J. Med. 333:621-627. [DOI] [PubMed] [Google Scholar]
- 19.Kothe, H., K. Dalhoff, J. Rupp, A. Muller, J. Kreuzer, M. Maass, and H. A. Katus. 2000. Hydroxymethylglutaryl coenzyme A reductase inhibitors modify the inflammatory response of human macrophages and endothelial cells infected with Chlamydia pneumoniae. Circulation 101:1760-1763. [DOI] [PubMed] [Google Scholar]
- 20.Kwak, B., F. Mulhaupt, S. Myit, and F. Mach. 2000. Statins as a newly recognized type of immunomodulator. Nat. Med. 6:1399-1402. [DOI] [PubMed] [Google Scholar]
- 21.Lange, B. M., T. Rujan, W. Martin, and R. Croteau. 2000. Isoprenoid biosynthesis: the evolution of two ancient and distinct pathways across genomes. Proc. Natl. Acad. Sci. USA 97:13172-13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liappis, A. P., V. L. Kan, C. G. Rochester, and G. L. Simon. 2001. The effect of statins on mortality in patients with bacteremia. Clin. Infect. Dis. 33:1352-1357. [DOI] [PubMed] [Google Scholar]
- 23.Maron, D. J., S. Fazio, and M. F. Linton. 2000. Current perspectives on statins. Circulation 101:207-213. [DOI] [PubMed] [Google Scholar]
- 24.McKay, A., B. P. Leung, I. B. McInnes, N. C. Thomson, and F. Y. Liew. 2004. A novel anti-inflammatory role of simvastatin in a murine model of allergic asthma. J. Immunol. 172:2903-2908. [DOI] [PubMed] [Google Scholar]
- 25.Merx, M. W., E. A. Liehn, U. Janssens, R. Lutticken, J. Schrader, P. Hanrath, and C. Weber. 2004. HMG-CoA reductase inhibitor simvastatin profoundly improves survival in a murine model of sepsis. Circulation 109:2560-2565. [DOI] [PubMed] [Google Scholar]
- 26.Plenge, J. K., T. L. Hernandez, K. M. Weil, P. Poirier, G. K. Grunwald, S. M. Marcovina, and R. H. Eckel. 2002. Simvastatin lowers C-reactive protein within 14 days: an effect independent of low-density lipoprotein cholesterol reduction. Circulation 106:1447-1452. [DOI] [PubMed] [Google Scholar]
- 27.Potena, L., G. Frascaroli, F. Grigioni, T. Lazzarotto, G. Magnani, L. Tomasi, F. Coccolo, L. Gabrielli, C. Magelli, M. P. Landini, and A. Branzi. 2004. Hydroxymethyl-glutaryl coenzyme a reductase inhibition limits cytomegalovirus infection in human endothelial cells. Circulation 109:532-536. [DOI] [PubMed] [Google Scholar]
- 28.Pruefer, D., J. Makowski, M. Schnell, U. Buerke, M. Dahm, H. Oelert, U. Sibelius, U. Grandel, F. Grimminger, W. Seeger, J. Meyer, H. Darius, and M. Buerke. 2002. Simvastatin inhibits inflammatory properties of Staphylococcus aureus alpha-toxin. Circulation 106:2104-2110. [DOI] [PubMed] [Google Scholar]
- 29.Reischl, U., N. Lehn, U. Simnacher, R. Marre, and A. Essig. 2003. Rapid and standardized detection of Chlamydia pneumoniae using LightCycler real-time fluorescence PCR. Eur. J. Clin. Microbiol. Infect. Dis. 22:54-57. [DOI] [PubMed] [Google Scholar]
- 30.Ridker, P. M., N. Rifai, M. A. Pfeffer, F. Sacks, E. Braunwald, et al. 1999. Long-term effects of pravastatin on plasma concentration of C-reactive protein. Circulation 100:230-235. [DOI] [PubMed] [Google Scholar]
- 31.Ridker, P. M., N. Rifai, M. A. Pfeffer, F. M. Sacks, L. A. Moye, S. Goldman, G. C. Flaker, E. Braunwald, et al. 1998. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Circulation 98:839-844. [DOI] [PubMed] [Google Scholar]
- 32.Ross, R. 1999. Atherosclerosis—-an inflammatory disease. N. Engl. J. Med. 340:115-126. [DOI] [PubMed] [Google Scholar]
- 33.Sacks, F. M., M. A. Pfeffer, L. A. Moye, J. L. Rouleau, J. D. Rutherford, T. G. Cole, L. Brown, J. W. Warnica, J. M. Arnold, C. C. Wun, B. R. Davis, E. Braunwald, et al. 1996. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N. Engl. J. Med. 335:1001-1009. [DOI] [PubMed] [Google Scholar]
- 33a.Scandinavian Simvastatin Survival Study Group. 1994. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 344:1383-1389. [PubMed] [Google Scholar]
- 34.Sparrow, C. P., C. A. Burton, M. Hernandez, S. Mundt, H. Hassing, S. Patel, R. Rosa, A. Hermanowski-Vosatka, P. R. Wang, D. Zhang, L. Peterson, P. A. Detmers, Y. S. Chao, and S. D. Wright. 2001. Simvastatin has anti-inflammatory and antiatherosclerotic activities independent of plasma cholesterol lowering. Arterioscler. Thromb. Vasc. Biol. 21:115-121. [DOI] [PubMed] [Google Scholar]
- 35.Stanislaus, R., K. Pahan, A. K. Singh, and I. Singh. 1999. Amelioration of experimental allergic encephalomyelitis in Lewis rats by lovastatin. Neurosci. Lett. 269:71-74. [DOI] [PubMed] [Google Scholar]
- 36.Stuart, E. S., W. C. Webley, and L. C. Norkin. 2003. Lipid rafts, caveolae, caveolin-1, and entry by Chlamydiae into host cells. Exp. Cell Res. 287:67-78. [DOI] [PubMed] [Google Scholar]
- 37.Vaughan, C. J., M. B. Murphy, and B. M. Buckley. 1996. Statins do more than just lower cholesterol. Lancet 348:1079-1082. [DOI] [PubMed] [Google Scholar]
- 38.Weitz-Schmidt, G. 2002. Statins as anti-inflammatory agents. Trends Pharmacol. Sci. 23:482-486. [DOI] [PubMed] [Google Scholar]
- 39.Wylie, J. L., G. M. Hatch, and G. McClarty. 1997. Host cell phospholipids are trafficked to and then modified by Chlamydia trachomatis. J. Bacteriol. 179:7233-7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeh, Y. F., and S. L. Huang. 2004. Enhancing effect of dietary cholesterol and inhibitory effect of pravastatin on allergic pulmonary inflammation. J. Biomed. Sci. 11:599-606. [DOI] [PubMed] [Google Scholar]