Abstract
Poly(styrene 4-sulfonate), cellulose sulfate, polymethylenehydroquinone, and PRO 2000 are sulfated or sulfonated polymers (SPs) under development as topical microbicides. They are presumed to work through similar mechanisms of action, although to date there has been no extensive comparison of their anti-human immunodeficiency virus activities. To determine whether any of these candidate microbicides offers a potential advantage, their in vitro activities, mechanisms of action, stabilities in biological secretions, and toxicities were compared. All four compounds were found to be active against X4, R5, and dualtropic primary isolates and against X4 and R5 laboratory-adapted strains in CD4+ T cells, macrophages, and single-coreceptor cell lines. Our single-cycle experiments using pseudotyped virus suggest that all four SPs function at the binding and entry stages of the viral life cycle but differ in degree of postentry effect. Surface plasmon resonance analyses demonstrate that SPs bind to X4 and R5 monomeric glycoprotein 120 with similar high binding affinities. When mixed with cervicovaginal lavage fluid, SPs maintain inhibitory activity at concentrations achievable in formulations.
It is estimated that, in 2004, 39.4 million people were living with human immunodeficiency virus (HIV) worldwide, the highest number ever, and that HIV/AIDS was the leading cause of death in sub-Saharan Africa. In many parts of the developing world, the majority of new infections occur in young adults, especially in women through heterosexual contact. Women and girls make up 57% of all people infected with HIV in sub-Saharan Africa, while a staggering 76% of young people (ages 15 to 24) living with HIV are female (27). In the absence of an effective HIV vaccine, alternative approaches to prevention are imperative. There are a number of compounds being developed as topical microbicides that could be applied vaginally or rectally to prevent transmission of HIV and other sexually transmitted infections. Nonoxynol-9 (N-9), available in over-the-counter spermicidal and lubricant formulations, is a nonionic surfactant originally shown to inactivate enveloped viruses such as HIV (12). Despite its in vitro antiviral activity, N-9 can be damaging to tissues both in vivo and in vitro. A recent paper by Catalone et al. using a mouse model of cervicovaginal toxicity and inflammation showed that 1% N-9 intravaginal inoculation of Swiss Webster mice resulted in acute disruption of cervical epithelial cells (3). Another study utilizing an in vitro model demonstrated that a KY-N-9 formulation was 20 to 50 times more toxic than other candidate microbicides, including PRO 2000 and cellulose acetate phthalate (CAP) (6). Clinical studies evaluating the efficacy of N-9 as a topical microbicide have also produced disturbing results. In a controlled study of 1,292 HIV-negative female sex workers in Cameroon, N-9 failed to reduce the rate of new HIV, gonorrhea, or chlamydia infection (24). In another randomized, placebo-controlled, triple-blinded, phase II/III trial with COL-1492, an N-9 vaginal gel, the risk of HIV type 1 (HIV-1) infection for N-9 users who reported a mean of 3.5 applications per working day was almost twice that for placebo users (28). Furthermore, repeated applications of N-9 have been shown to disrupt the rectal mucosa of the pig-tailed macaque (23).
The experience with N-9 highlights the need for full evaluation of both the safety and efficacy of compounds to be used as topical microbicides. A number of potential candidate microbicides have been shown to inhibit HIV and other sexually transmitted infections in vitro. Several candidate compounds have already progressed to various stages of clinical trials. Among the candidate agents are sulfated or sulfonated negatively charged polymers that presumably work through similar mechanisms of action. These include poly(styrene 4-sulfonate) (T-PSS), cellulose sulfate (CS), polymethylenehydroquinone sulfonate (PMHS), and PRO 2000, a naphthalenesulfonic acid polymer. Another negatively charged polymer in development is carrageenan (Carraguard), which is derived from seaweed. PRO 2000, CS, and carrageenan have been formulated and are in phase II/III clinical trials (5, 8, 20, 29). While CS, T-PSS, PMHS, and PRO 2000 have been shown to have similar profiles against herpes simplex virus (4), to date there has been little comparison of the sulfated or sulfonated polymers (SPs) to determine their relative efficacies and mechanisms of action against HIV-1 as well as their anti-HIV activities in the presence of cervicovaginal lavage (CVL) fluid. Such a comparison may assist in the evaluation of presently planned clinical trials and in the selection of compounds for future development.
MATERIALS AND METHODS
Primary cells and cell lines.
Primary macrophage cultures were prepared from Ficoll-Hypaque purified peripheral blood mononuclear cells and purified by adherence after 14 days in culture in RPMI medium containing 20% fetal calf serum. They were cultured in 96-well plates at a concentration of 1.5 × 105 cells/ml (200 μl/well). CD4+ T cells were prepared from peripheral blood mononuclear cells by magnetic cell sorting using a CD4 T-cell isolation kit (Miltenyi Biotec, Auburn, CA) and activated with 5 μg/ml of phytohemagglutinin (PHA) for 48 h. After activation, they were cultured in 96-well plates at a concentration of 1 × 105 cells/ml (200 μl/well) in RPMI medium supplemented with 50 U/ml of interleukin-2. The U87MG astrocytoma-derived cell lines engineered to express CD4 and single HIV-1 coreceptors (U87CD4CCR5 or U87CD4CXCR4) were obtained through the AIDS Research and Reference Reagent Program (ARRRP), Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID), NIH (2). The cells were grown in Dulbecco's minimal essential medium supplemented with 15% fetal bovine serum, 1 μg/ml puromycin, 300 μg/ml G418, glutamine, penicillin, and streptomycin.
Viral strains and glycoproteins.
Laboratory-adapted HIV-1 strains included the R5 isolate HIV-1BaL and the X4 isolate HIV-1IIIB (Advanced Biotechnologies, Inc., Columbia, MD). Primary isolates included the X4 isolates 92UG021 and 92HT599, dualtropic R5/X4 isolate 92RW009 (obtained from ARRRP, UNAIDS Network for HIV Isolation and Characterization, and the Division of AIDS, NIAID) (15), and the R5 isolate RB23 generated by using a primary viral envelope cloned directly from renal epithelial cells (donated by B. Zerhouni-Layachi, Department of Medicine, Mount Sinai School of Medicine) (19). For the single-cycle experiments, replication-defective pseudotyped viruses were prepared. Briefly, the pseudotyped recombinant viruses were produced in 293T cells using a three-plasmid cotransfection system. The packaging construct pCMVΔR8.2 was previously described (26). The HIV-1 reporter construct pNL4-3.Luc.R-E (obtained from ARRRP) contains the firefly luciferase gene inserted into the pNL4-3 nef gene. Two frameshift mutations (in 5′ Env and Vpr at amino acid 26) render this clone replication defective. Pseudotyped replication-defective viruses were generated by cotransfection of the packaging and reporter constructs with the R5 envelope-expressing construct pJR-FL (donated by D. Littman, Skirball Institute, New York University, New York, NY) (10). Transfections were performed in 10-cm2 tissue culture dishes by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Viral supernatants were harvested 48 h posttransfection and were treated with 29 U/ml DNase I (Invitrogen, Carlsbad, CA) for 1 h at 37°C to remove any residual plasmid DNA.
Recombinant monomeric envelope glycoprotein 120 (gp120) from an X4 (MN) and an R5 (YU2) virus produced in the baculovirus expression system was obtained from ImmunoDiagnostics, Inc. (Woburn, MA).
Reagents.
T-PSS, CS, PMHS, and SAMMA, a nonsulfonated polyanion under development as a topical microbicide (11), were provided by Topical Prevention for Conception and Disease (TOPCAD, Chicago, IL). PRO 2000 was obtained from Indevus Pharmaceuticals, Inc. (Lexington, MA). The structures and peak molecular masses of these compounds have been previously published (4). Zidovudine (AZT) was purchased from Sigma (St. Louis, Mo.), and TAK-779 was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH (1).
Infectivity assays.
Macrophages and PHA-stimulated CD4+ T cells were preincubated with either microbicide compound (concentration, 1 μg/ml to 1 mg/ml) or control medium for 1 h at 37°C, followed by a 2-h infection with HIV at 37°C. Laboratory-adapted strains were used at a multiplicity of infection of 0.1 to 0.25, and primary isolates were used at a final concentration of 34 to 117 ng p24/ml. Similarly, U87CD4CCR5 or U87CD4CXCR4 cells were preincubated with either compound or control medium for 1 h at 37°C, followed by a 2-h infection with HIV-1BaL or HIV-1IIIB. Following infection, cells were washed twice with phosphate-buffered saline (PBS) to remove unbound virus and compound and incubated in fresh culture medium with no further addition of compound. In parallel experiments, compound and cells were washed with PBS following the 1-h preincubation prior to addition of HIV, so that the compounds were present before but not during the infection. Viral replication was monitored by use of an HIV-1 p24 (gag) protein enzyme-linked immunosorbent assay (ELISA) (DuPont, Wilmington, DE).
Time course assays.
For the single-cycle-infection time course assay, U87CD4CCR5 cells were cultured in 96-well plates at a concentration of 1 × 105 cells/ml (200 μl/well) and allowed to grow until confluent. They were preincubated with either compound or control medium for 1 h at 37°C (t = −1), followed by a 2-h infection with the replication-defective virus (JR-FL). Cells were washed twice with PBS to remove unbound virus, and compound was added either immediately (t = +2) or at 6 h (t = +8) after infection. The reverse transcriptase inhibitor AZT and the small-molecule CCR5 inhibitor TAK-779 were used as controls (7). Cells were lysed in luciferase lysis buffer after 48 h, and a luciferase assay was performed using the Promega luciferase assay system (Madison, WI). Values were normalized according to total protein concentrations.
SPR assays.
Surface plasmon resonance (SPR) analyses were performed on a BIAcore 2000 system with CM5 sensor chips (Biacore, Inc., Piscataway, NJ) (16, 17). HIV-1 recombinant gp120 (rgp120) MN and rgp120 YU2 were diluted in 10 mM sodium acetate buffer at pH 4.5 to a final concentration of 100 μg/ml and immobilized on the surface of the chip using the amine coupling method. The carboxyl groups on the sensor surfaces were activated with a mixture of 0.2 M N-ethyl-N′-(3-diethylamino-propyl) carbodiimide (EDC) and 0.05 M N-hydroxysuccinimide (NHS), and the protein was coupled under the continuous flow of HBS-EP buffer (10 mM HEPES with 0.15 M NaCl, 3 mM EDTA, and 0.005% surfactant P20, pH 7.4). A flow cell containing an unmodified dextran surface treated with EDC and NHS and not exposed to the coupling protein served as the control flow cell surface for each chip. Nonreaction residual reactive groups were blocked with 1.0 M ethanolamine hydrochloride at pH 8.5 in both control and test flow cell surfaces. Immobilization levels of 5,000 resonance units (RU) for rgp120 MN and 6,000 RU for rgp120 YU2 were achieved individually in channel 2 and channel 3, and channel 1 worked as a control.
For kinetic binding determinations, individual compounds were diluted in HBS-EP buffer and injected over the test and control flow cell surfaces at 20 μl/min, including 3 min of association followed by 17 min of dissociation and 1 min of regeneration for all samples. Each experiment was repeated at least three times. Data transformation and overlay plots were prepared with BIA evaluation version 3.1 software. Binding signals were normalized for nonspecific binding by subtracting the signal of the control. The affinities of the binding interactions between MN and YU2 to each candidate microbicide were calculated using the 1:1 Langmuir binding model in the BIA evaluation software.
CVL assay.
Following Institutional Review Board approval at Mount Sinai School of Medicine, CVL fluid samples were obtained from healthy volunteers with low identifiable risk factors for HIV by washing with 10 ml of sterile normal saline directed at the cervical os and posterior vaginal wall. The CVL was immediately transported on ice to the laboratory and centrifuged at 2,000 to 2,500 rpm for 15 min at 4°C. A cocktail of penicillin (500 U/ml), streptomycin (50 μg/ml), and amphotericin (0.5 μg/ml) was added to the supernatants, which were aliquoted and stored at −80°C. CVL or saline was mixed (50% by volume) with compound to achieve final concentrations of 1, 10, and 100 μg/ml, and the mixtures were incubated for 1 h at 37°C. U87CD4CCR5 cells were treated with CVL or saline for 1 h, followed by a 2-h infection with the replication-defective virus pseudotyped with JR-FL envelope. The virus and CVL were removed after 2 h, and the cells were washed, cultured in fresh medium, and lysed at 48 h. Luciferase activity was estimated using the Promega luciferase assay system (Madison, WI).
Toxicity assays.
The toxicities of all compounds were assessed on primary macrophages. The cells were exposed to 0, 100 μg/ml, or 1 mg/ml of microbicide for 2 h daily for 5 consecutive days or to 1 mg/ml for 48 continuous hours. Cell viability was assayed using a tetrazolium compound assay (CellTiter96; Promega, Madison, WI).
Statistical analysis.
The effects of microbicides in all experiments were analyzed using the Student t test, and results are included in the figures where applicable. Statistical significance was defined as a P value of <0.05. Curves for 50% inhibitory concentration (IC50) were generated using the SigmaPlot version 8.0 software.
RESULTS
Spectrum of anti-HIV activity.
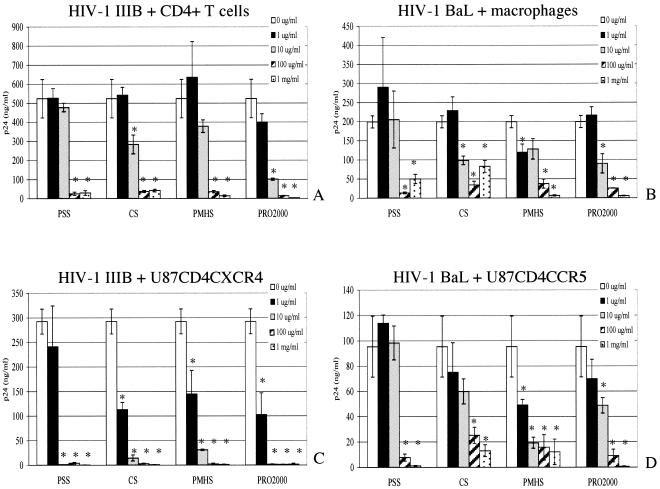
To determine the spectrum of activity of each microbicide, primary human CD4+ T cells and primary human macrophages were infected with the X4 (HIV-1IIIB) and the R5 (HIV-1BaL) laboratory-adapted strains, respectively. T-PSS, CS, PMHS, and PRO 2000 inhibited HIV-1IIIB and HIV-1BaL in primary T cells and macrophages with IC50s ranging from 2.75 to 12 μg/ml (Fig. 1A and B, respectively, and Table 1). HIV-1IIIB and HIV-1BaL were also used to infect the U87CD4CXCR4 and U87CD4CCR5 single-coreceptor cell lines. These cell lines were chosen on the basis of the fact that the parent line, U87CD4, has no background expression of X4 or R5 coreceptors and is not permissive to HIV-1. All four compounds inhibited infection in both cell lines; however, the dose-response curves differed between the X4 and R5 viruses (Fig. 1C and D, respectively). For all of the drugs tested, higher concentrations were needed to achieve the same level of inhibition of HIV-1BaL as of HIV-1IIIB (Table 1). For all drugs tested, there was a sharp drop in the X4 viral replication in CXCR4-expressing cells between 1 and 10 μg/ml (Fig. 1C and D). The IC50s against HIV-1IIIB in U87CD4CXCR4 cells were consistently lower than the IC50s in primary CD4+ T cells, which might reflect differences in receptor and coreceptor densities for these target cells. The IC50s calculated from our experiments were comparable to previously published IC50s for T-PSS against HIV-1IIIB (30).
FIG. 1.
Activities of SPs against laboratory-adapted strains of HIV. HIV-1IIIB was used in a 2-h infection in either primary CD4+ T cells (A) or U87CD4CXCR4 cells (C) that had been preincubated with the indicated concentrations of microbicide (in μg/ml or mg/ml) for 1 h. HIV-1BaL was used to infect either primary macrophages (B) or U87CD4CCR5 cells (D). Viral replication was monitored by determining supernatant viral p24 (gag) protein by ELISA after 48 h (for U87 cell lines) or after 7 days (for primary cells). Results represent the average p24 concentrations from triplicate wells (error bars indicate standard deviations) and are representative of at least two independent experiments. Asterisks indicate statistical significance (P < 0.05) as calculated by the Student t test.
TABLE 1.
Effects of candidate SPs on infection with laboratory-adapted HIV strainsa
| Microbicide | IC50 (μg/ml) for:
|
|||
|---|---|---|---|---|
| HIV-1IIIB
|
HIV-1BaL
|
|||
| CD4+ T cells | U87CD4CXCR4 | Macrophages | U87CD4CCR5 | |
| T-PSS | 11.6 | 1.1 | 12 | 32 |
| CS | 10 | 0.65 | 8.8 | 18.67 |
| PMHS | 10.6 | 0.98 | 9.8 | 7.4 |
| PRO 2000 | 2.75 | 0.85 | 9.5 | 7.8 |
HIV-1IIIB was used in a 2-h infection in either primary CD4+ T cells or U87CD4CXCR4 cells that had been preincubated with the indicated concentrations of microbicide for 1 h. HIV-1BaL was used to infect either primary macrophages or U87CD4CCR5 cells. Viral replication was monitored by viral p24 (gag) protein ELISA after 48 hours (for U87 cell lines) or after 7 days (for primary cells). IC50s were determined using dose-response curves generated by SigmaPlot. Results are representative of at least two experiments performed in triplicate for each compound.
In parallel experiments where the U87 lines were preincubated with microbicides but the compounds were washed off prior to challenge with virus, there was a substantial loss of activity for all compounds, suggesting that the compounds interact preferentially with the virus and not the cell (data not shown).
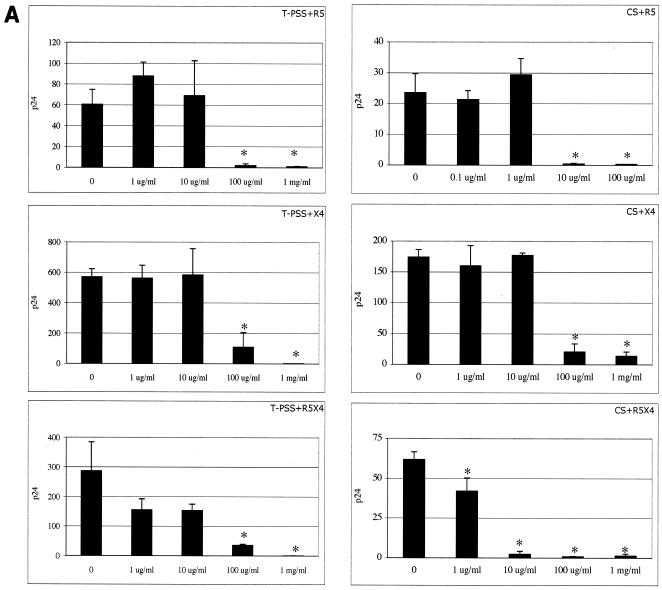
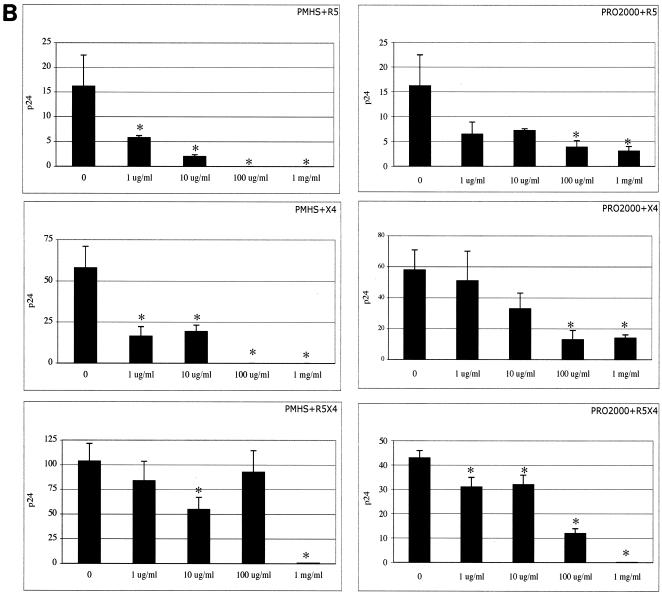
Primary isolates of HIV, including T-cell-tropic (R5), macrophage-tropic (X4), and dualtropic (X4/R5) isolates, were used to infect CD4+ T cells in the presence and absence of microbicides. T-PSS, CS, PMHS, and PRO 2000 inhibited the X4, dualtropic X4/R5, and R5 primary strains at comparable concentrations (Fig. 2A and B).
FIG. 2.
Activities of T-PSS and CS (A) and PMHS and PRO 2000 (B) against X4, R5, and R5/X4 primary isolates of HIV-1. Primary CD4+ T cells were preincubated with the indicated concentrations of SPs (in μg/ml or mg/ml) and infected with the X4 isolates UG021 and 9ZTH599, R5 isolate IN/93/905, or dualtropic R5/X4 isolate RW/92/09. Viral replication was monitored by p24 ELISA on culture supernatants after 7 or 10 days. Results represent the average p24 concentrations in ng/ml from triplicate wells, and error bars indicate standard deviations. Asterisks indicate statistical significance compared to untreated (P < 0.05) as calculated by the Student t test.
Mechanism of anti-HIV activity.
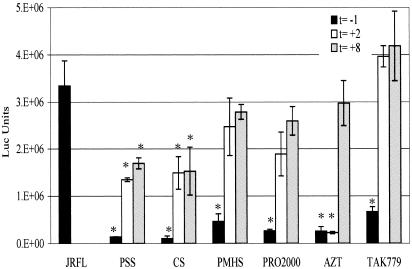
Single-cycle infections in U87CD4CCR5 cells were performed using replication-defective pseudotyped virus (HIV-1JR-FL). The times of addition of microbicides or controls (TAK-779 and AZT) were varied relative to challenge with HIV. T-PSS, CS, PMHS, and PRO 2000 were all active if added 1 h before and still present at the time of infection (t = −1 h), similar to results with the coreceptor inhibitor TAK-779 (Fig. 3). If added after the 2-h infection (t = +2 h), there was considerable loss of inhibitory activity. This result is consistent with these compounds all working predominantly at the points of attachment and entry. However, in contrast to TAK-779, whose activity is completely lost when added after the 2-h infection (t = +2 h), two of the sulfonated compounds maintained some residual activity even when added up to 8 h following initiation of infection. T-PSS inhibited HIV by 39.5% (P = 0.04) and CS inhibited HIV by 45.5% (P = 0.02) at 6 h after infection (t = +8 h). PMHS and PRO 2000 also inhibited HIV at 6 h after infection (t = +8 h), but the inhibitory activities were not statistically significant. The reverse transcriptase inhibitor, AZT, retained its activity when added after infection (t = +2 h), but its activity was essentially lost when added 6 h after infection (t = +8 h), consistent with the completion of reverse transcription by this point.
FIG. 3.
Anti-HIV activities of SPs, determined by single-cycle-infection assays. SPs, AZT, and TAK-779 were added at different times to U87CD4CCR5 cells and infected with the replication-defective virus JR-FL. Cells were preincubated with microbicide, AZT, TAK-779, or control medium for 1 h at 37°C (t = −1), followed by a 2-h infection. Cells were washed twice with PBS to remove virus and compound and cultured in fresh medium. Alternatively, the compounds were added either immediately after infection and wash (t = +2 h) or 6 h after infection and wash (t = +8 h). Cells were lysed in luciferase buffer after 48 h. Luciferase values were standardized with respect to total protein concentration. Results are mean luciferase (Luc) values from triplicate wells (error bars represent standard deviations) and are representative of two independent experiments. Asterisks indicate statistical significance compared to luciferase values in control cells infected with JR-FL in the absence of any microbicide and were calculated by the Student t test.
Since it appears that the compounds all work at least at the point of entry, interactions between the compounds and monomeric gp120 were investigated using SPR analysis. MN and YU2 gp120 were immobilized on the surface of the chip, and the affinities of the on and off interactions of the microbicides with the viral protein were calculated. The results are shown in Table 2. PRO 2000, T-PSS, and PMHS bound to gp120 MN and YU2 with similar high affinities. While the rank order of the compounds from highest to lowest affinity is significant for MN and YU2 gp120, it is unclear whether this difference is functionally important since all compounds had relatively high affinities for gp120. When the binding affinities of individual compounds for MN or YU2 were compared, only T-PSS and SAMMA showed differences that were statistically significant (P < 0.05; n = 4). Affinities observed here were comparable to the affinities observed for the herpes simplex virus glycoprotein B (4) and to the high affinity of the compound SAMMA (11). It was not possible to obtain conclusive results for CS due to its higher molecular mass.
TABLE 2.
Kinetic analysis of MN gp120 (X4) and YU2 gp120 (R5) binding to candidate topical microbicidesa
| Microbicide | Avg (±SD) for:
|
|||||
|---|---|---|---|---|---|---|
| MN gp120 (X4)
|
YU2 gp120 (R5)
|
|||||
| Ka | Kd | Kd/Ka | Ka | Kd | Kd/Ka | |
| T-PSS | 1.99 × 105 (±0.31 × 105) | 2.54 × 10−4 (±0.36 × 10−4) | 1.27 × 10−9 (±0.1 × 10−9) | 1.47 × 105 (±0.31 × 105) | 3.81 × 10−4 (±0.33 × 10−4) | 2.59 × 10−9 (±0.7 × 10−9) |
| PMHS | 9.59 × 103 (±3.2 × 103) | 1.06 × 10−4 (±0.39 × 10−4) | 1.11 × 10−8 (±1.31 × 10−8) | 1.52 × 104 (±0.35 × 104) | 1.39 × 10−4 (±0.46 × 10−4) | 9.19 × 10−9 (±5.9 × 10−9) |
| PRO 2000 | 537 (±187) | 2.85 × 10−5 (±0.31 × 10−5) | 5.27 × 10−8 (±2.4 × 10−8) | 716 (±122) | 2.93 × 10−5 (±0.36 × 10−6) | 4.09 × 10−8 (±0.1 × 10−8) |
| SAMMA | 1.7 × 104 (±0.43 × 104) | 2.21 × 10−4 (±0.37 × 10−4) | 1.3 × 10−7 (±0.1 × 10−7) | 1.95 × 104 (±0.31 × 104) | 1.17 × 10−3 (±0.59 × 10−3) | 6.5 × 10−8 (±2.6 × 10−8) |
Binding signals were corrected for nonspecific binding by subtracting the signal of the control flow cell. The affinities of the binding interactions between MN and YU2 to each candidate microbicide were calculated using the 1:1 Langmuir binding model in the BIA evalution software. Each binding experiment was repeated four times, and the average values ± standard deviations (SD) are shown. Kd, diffusion constant; Ka, association constant.
Cell viability.
All compounds were tested on primary macrophages for potentially toxic effects. We observed no toxicity in a setting of either repeated exposure (2 h for 5 days) or chronic exposure (48 continuous hours) to SPs (Table 3). The toxicities of these compounds have also been determined for human endocervical cells and were previously published (4).
TABLE 3.
Cell viability in primary macrophages exposed to SPsa
| Microbicide | Result after repeated exposure at:
|
Result after chronic exposure at:
|
||||
|---|---|---|---|---|---|---|
| 100 μg/ml
|
1 mg/ml
|
1 mg/ml
|
||||
| % Viability | P value | % Viability | P value | % Viability | P value | |
| PSS | 92 ± 7 | 0.799 | 86 ± 7 | 0.523 | 79 ± 12 | 0.130 |
| CS | 116 ± 4 | 0.197 | 100 ± 5 | 0.784 | 163 ± 32 | 0.015 |
| PMHS | 115 ± 3 | 0.228 | 87 ± 4 | 0.527 | 111 ± 27 | 0.677 |
| PRO 2000 | 120 ± 4 | 0.149 | 76 ± 7 | 0.227 | 106 ± 24 | 0.901 |
Human macrophages were exposed to 0, 100 μg/ml, or 1 mg/ml of microbicide compound for 2 hours daily for 5 consecutive days (repeated exposure) or to 1 mg/ml for 48 continuous hours (chronic exposure). Cell viability was assayed using a tetrazolium compound assay (CellTiter96; Promega, Madison, WI). Viability is expressed as a percentage of the value for cells not treated with microbicide. Results represent the means from triplicate wells ± standard deviations and are representative of two independent experiments.
Activity in the presence of CVL fluid.
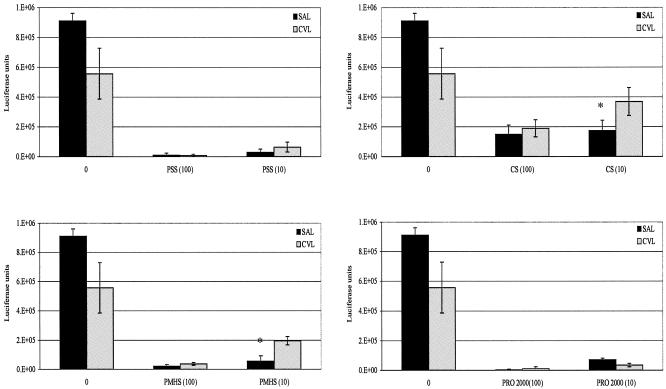
It is important to establish microbicide activity in the presence of CVL fluid. Some CVL fluids from HIV-negative donors had modest intrinsic inhibitory activities compared to saline alone. These values ranged from 38.7% to 72.7% and were statistically significant. T-PSS, PMHS, CS, and PRO 2000 were preincubated with CVL or saline, and their HIV inhibitory activities were evaluated. At equivalent drug doses, inhibitory activities of T-PSS and PRO 2000 were the same in CVL as in saline. With PMHS and CS, there was a small loss of inhibition at the lower dose of 10 μg/ml (Fig. 4). Similar results were obtained from three additional experiments using two other donor CVL fluids (data not shown).
FIG. 4.
CVL from healthy donors or saline (SAL) was mixed with 0, 10, or 100 μg/ml of microbicide and incubated for 1 h at 37°C. CVL or saline with or without microbicide was added to U87CD4CCR5 cells for 1 h at 37°C, followed by a 2-h infection with the replication-defective strain JR-FL. The cells were washed after 2 h, cultured in fresh medium, and lysed at 48 h. Results are mean luciferase values from triplicate wells, and error bars represent standard deviations. This is one of four experiments carried out using a total of three CVL fluids (one CVL fluid provided enough sample for two experiments), and results shown are representative of other experiments.
DISCUSSION
The goal of this study was to directly compare the efficacies and mechanisms of HIV inhibition of candidate sulfated or sulfonated polyanions. T-PSS, CS, PMHS, and PRO 2000 differ from other proposed microbicides in that they are neither detergents nor surfactants and they do not appear to have cytotoxic effects on human cervical epithelial cells (4).
Our data illustrate that T-PSS, CS, PMHS, and PRO 2000 inhibit primary isolates and laboratory-adapted strains of HIV-1. All compounds at 100 μg/ml inhibit X4, R5, and dual-tropic primary isolates of HIV in CD4+ cells. Their profiles of activity against two laboratory-adapted strains, HIV-1IIIB and HIV-1BaL, in primary CD4+ T cells and macrophages, respectively, revealed similar IC50s. In all assays using replication-competent virus, the compounds were present only during the first round of infection and not during subsequent rounds from released virus; therefore, these IC50s probably underrepresent the true inhibitory capacities of the compounds, as any residual virus would be amplified in the absence of compound. Only in engineered cell lines expressing a single coreceptor did these compounds inhibit infection of HIV-1IIIB in U87CD4CXCR4 cells at lower concentrations than they inhibited infection of HIV-1BaL in U87CD4CCR5 cells. IC50s were calculated using molecular weights because the compounds are being formulated on a weight basis, not a molar basis. Because the compounds are large polymers, formulation based on molarity would be difficult.
A number of reports have suggested that sulfated compounds, such as dextran sulfate, bind the V3 loop of X4 viruses more readily than they bind the V3 loop of R5 viruses (21). Consistent with this is the demonstration that dextran sulfate fails to inhibit R5 viruses in vitro (18). This differential activity has been attributed to a difference in the net charge of the V3 loop between R5 and X4 viruses. The more cationic X4 isolates are thought to interact better with the negatively charged sulfate groups. However, our results looking at inhibitory activity as well as gp120 binding affinity argue against the V3 loop charge being a major determinant of differential activity in these SPs.
Our data from the SPR analysis demonstrated that all compounds bind to gp120 with high affinity without significant differences between their affinities for X4 and R5 gp120. PRO 2000 was previously reported to inhibit binding of rgp120 to CD4 (25) and to block HIV-1 X4 and R5 virus infection of cervical explants (9) and partially protect macaques challenged with SHIV89.6PD. Together, our studies and these results support planned phase II/III trials with PRO 2000 gel.
In a similar fashion, dextrin sulfate derivatives inhibit both syncytium-inducing and non-syncytium-inducing primary viral isolates in lymphocytes and monocyte-derived macrophages (14). The cell surface binding abilities and inhibitory concentrations for the dextrin sulfate derivatives are however dependent on the positions of the negatively charged sulfate groups. In a study where T-PSS and CS were among a number of compounds compared to micronized CAP, both CS and T-PSS, similarly to CAP, were found to inactivate HIV-1IIIB and HIV-1BaL, while dextran sulfate was unable to inactivate HIV-1BaL (22). These studies are consistent with our results and suggest that several of the SPs inhibit both X4 and R5 viruses in vitro.
Our single-pass virus studies have also suggested that two of these compounds, CS and T-PSS, may have some effect when added to cells long after entry of the virus into the target cells has been completed. This could be due to some of the compound irreversibly binding to cell surface molecules and triggering downstream signaling events that ultimately inhibit steps in viral replication, or it could be due to some of the compound being internalized and binding to other targets within the cell.
The ability of these SPs to effectively block infection by R5 viruses is an extremely important point relative to the transmission of virus in vivo. HIV-1 isolates from newly infected individuals are predominantly M-tropic and utilize CCR5, while T-tropic isolates that use CXCR4 evolve later in the course of the disease. R5, X4, and dualtropic R5/X4 isolates can all infect PHA-activated cervical tissues (9). Coreceptor blocking experiments with cervical explant tissue have demonstrated that CCR5 and CXCR4 are the two predominant coreceptors involved in infection (13). In our experiments, SPs were effective at inhibiting R5, X4, and dualtropic primary isolates, as well as laboratory-adapted strains, in primary cells and engineered cell lines. Single-cycle-infection assays confirmed that these compounds exert their predominant effect on viral entry, with some postentry effect seen with T-PSS and CS.
Potential topical microbicides should also maintain activity in the biological milieu and remain nontoxic to human tissues. Specifically, they should not be inhibited in the cervicovaginal environment that includes an acid pH and rich protein content. CVL fluids from three independent uninfected donors in our study demonstrated intrinsic inhibitory activity against HIV-1. Importantly, SPs retained their anti-HIV activity in the presence of CVL and inhibited HIV infection at concentrations readily achievable in vivo. In a recently completed clinical study, the concentration of PRO 2000 in CVL obtained 1 h postapplication of a 0.5% formulation was >100 μg/ml (M. J. Keller et al., submitted for publication). Since CS is formulated as a 6% solution and T-PSS as a 2 or 5% solution, and based on the PRO 2000 data, it would be reasonable to assume that in vivo concentrations would exceed inhibitory concentrations. Additional studies examining the impact of seminal fluid both in vitro and in animal models are needed to fully evaluate the activities of SPs in biological systems.
While elucidation of early primary events in HIV-1 transmission at human mucosal surfaces is currently under intense investigation, several compounds have been considered for development of topical microbicides. Five of those, including CS and PRO 2000, are scheduled for large-scale trials. Our data suggest that the candidate microbicides T-PSS, CS, PMHS, and PRO 2000 have similar profiles against HIV in vitro. Prior to initiation of these costly clinical trials, studies focused on the more subtle effects of SPs on host cell defenses should be pursued. They might in fact reveal differing profiles that could guide selective instead of random advancement.
Acknowledgments
This work was supported by NICHD grants PO1HD041763 and PO1HD43733 and GCRC grant M01-RR-00071.
We thank Melissa Dzuzelewski for technical support and Bouchra Zerhouni-Layachi for providing us with the HIV-1 RB23 primary isolate.
REFERENCES
- 1.Baba, M., O. Nishimura, N. Kanzaki, M. Okamoto, H. Sawada, Y. Iizawa, M. Shiraishi, Y. Aramaki, K. Okonogi, Y. Ogawa, K. Meguro, and M. Fujino. 1999. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. USA 96:5698-5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjorndal, A., H. Deng, M. Jansson, J. R. Fiore, C. Colognesi, A. Karlsson, J. Albert, G. Scarlatti, D. R. Littman, and E. M. Fenyo. 1997. Coreceptor usage of primary human immunodeficiency virus type 1 isolates varies according to biological phenotype. J. Virol. 71:7478-7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catalone, B. J., T. M. Kish-Catalone, L. R. Budgeon, E. B. Neely, M. Ferguson, F. C. Krebs, M. K. Howett, M. Labib, R. Rando, and B. Wigdahl. 2004. Mouse model of cervicovaginal toxicity and inflammation for preclinical evaluation of topical vaginal microbicides. Antimicrob. Agents Chemother. 48:1837-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheshenko, N., M. J. Keller, V. MasCasullo, G. A. Jarvis, H. Cheng, M. John, J. H. Li, K. Hogarty, R. A. Anderson, D. P. Waller, L. J. Zaneveld, A. T. Profy, M. E. Klotman, and B. C. Herold. 2004. Candidate topical microbicides bind herpes simplex virus glycoprotein B and prevent viral entry and cell-to-cell spread. Antimicrob. Agents Chemother. 48:2025-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coggins, C., K. Blanchard, F. Alvarez, V. Brache, E. Weisberg, P. H. Kilmarx, M. Lacarra, R. Massai, D. J. Mishell, A. Salvatierra, P. Witwatwongwana, C. Elias, and C. Ellertson. 2000. Preliminary safety and acceptability of a carrageenan gel for possible use as a vaginal microbicide. Sex. Transm. Infect. 76:480-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dezzutti, C. S., V. N. James, A. Ramos, S. T. Sullivan, A. Siddig, T. J. Bush, L. A. Grohskopf, L. Paxton, S. Subbarao, and C. E. Hart. 2004. In vitro comparison of topical microbicides for prevention of human immunodeficiency virus type 1 transmission. Antimicrob. Agents Chemother. 48:3834-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dragic, T., A. Trkola, D. A. Thompson, E. G. Cormier, F. A. Kajumo, E. Maxwell, S. W. Lin, W. Ying, S. O. Smith, T. P. Sakmar, and J. P. Moore. 2000. A binding pocket for a small molecule inhibitor of HIV-1 entry within the transmembrane helices of CCR5. Proc. Natl. Acad. Sci. USA 97:5639-5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elias, C. J., C. Coggins, F. Alvarez, V. Brache, I. S. Fraser, M. Lacarra, P. Lahteenmaki, R. Massai, D. R. J. Mishell, D. M. Phillips, and A. M. Salvatierra. 1997. Colposcopic evaluation of a vaginal gel formulation of iota-carrageenan. Contraception 56:387-389. [DOI] [PubMed] [Google Scholar]
- 9.Greenhead, P., P. Hayes, P. S. Watts, K. G. Laing, G. E. Griffin, and R. J. Shattock. 2000. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J. Virol. 74:5577-5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He, J., S. Choe, R. Walker, P. Di Marzio, D. O. Morgan, and N. R. Landau. 1995. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J. Virol. 69:6705-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herold, B. C., I. Scordi-Bello, N. Cheshenko, D. Marcellino, M. Dzuzelewski, F. Francois, R. Morin, V. M. Casullo, R. A. Anderson, C. Chany II, D. P. Waller, L. J. D. Zaneveld, and M. E. Klotman. 2002. Mandelic acid condensation polymer: novel candidate microbicide for prevention of human immunodeficiency virus and herpes simplex virus entry. J. Virol. 76:11236-11244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hicks, D. R., L. S. Martin, J. P. Getchell, J. L. Heath, D. P. Francis, J. S. McDougal, J. W. Curran, and B. Voeller. 1985. Inactivation of HTLV-III/LAV-infected cultures of normal human lymphocytes by nonoxynol-9 in vitro. Lancet ii:1422-1423. [DOI] [PubMed] [Google Scholar]
- 13.Hu, Q., I. Frank, V. Williams, J. J. Santos, P. Watts, G. E. Griffin, J. P. Moore, M. Pope, and R. J. Shattock. 2004. Blockade of attachment and fusion receptors inhibits HIV-1 infection of human cervical tissue. J. Exp. Med. 199:1065-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Javan, C. M., N. J. Gooderham, R. J. Edwards, D. S. Davies, and S. Shaunak. 1997. Anti-HIV type 1 activity of sulfated derivatives of dextrin against primary viral isolates of HIV type 1 in lymphocytes and monocyte-derived macrophages. AIDS Res. Hum. Retrovir. 13:875-880. [DOI] [PubMed] [Google Scholar]
- 15.Korber, B. T., S. Osmanov, J. Esparza, and G. Myers. 1994. The World Health Organization global programme on AIDS proposal for standardization of HIV sequence nomenclature. WHO network for HIV isolation and characterization. AIDS Res. Hum. Retrovir. 10:1355-1358. [DOI] [PubMed] [Google Scholar]
- 16.Leng, L., C. N. Metz, Y. Fang, J. Xu, S. Donnelly, J. Baugh, T. Delohery, Y. Chen, R. A. Mitchell, and R. Bucala. 2003. MIF signal transduction initiated by binding to CD74. J. Exp. Med. 197:1467-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu, S., G. Xiao, Y. Chen, Y. He, J. Niu, C. R. Escalante, H. Xiong, J. Farmar, A. K. Debnath, P. Tien, and S. Jiang. 2004. Interaction between heptad repeat 1 and 2 regions in spike protein of SARS-associated coronavirus: implications for virus fusogenic mechanism and identification of fusion inhibitors. Lancet 363:938-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lynch, G., L. Low, S. Li, A. Sloane, S. Adams, C. Parish, B. Kemp, and A. L. Cunningham. 1994. Sulfated polyanions prevent HIV infection of lymphocytes by disruption of the CD4-gp120 interaction, but do not inhibit monocyte infection. J. Leukoc. Biol. 56:266-272. [DOI] [PubMed] [Google Scholar]
- 19.Marras, D., L. A. Bruggeman, F. Gao, N. Tanji, M. M. Mansukhani, A. Cara, M. D. Ross, G. L. Gusella, G. Benson, V. D. D'Agati, B. H. Hahn, M. E. Klotman, and P. E. Klotman. 2002. Replication and compartmentalization of HIV-1 in kidney epithelium of patients with HIV-associated nephropathy. Nat. Med. 8:522-526. [DOI] [PubMed] [Google Scholar]
- 20.Mauck, C., D. H. Weiner, S. Ballagh, M. Creinin, D. F. Archer, J. Schwartz, H. Pymar, J. J. Lai, and M. Callahan. 2001. Single and multiple exposure tolerance study of cellulose sulfate gel: a phase I safety and colposcopy study. Contraception 64:383-391. [DOI] [PubMed] [Google Scholar]
- 21.Moulard, M., H. Lortat-Jacob, I. Mondor, G. Roca, R. Wyatt, J. Sodroski, L. Zhao, W. Olson, P. D. Kwong, and Q. J. Sattentau. 2000. Selective interactions of polyanions with basic surfaces on human immunodeficiency virus type 1 gp120. J. Virol. 74:1948-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neurath, A. R., N. Strick, S. Jiang, Y. Y. Li, and A. K. Debnath. 2002. Anti-HIV-1 activity of cellulose acetate phthalate: synergy with soluble CD4 and induction of “dead-end” gp41 six-helix bundles. BMC Infect. Dis. 2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patton, D. L., Y. T. Cosgrove Sweeney, L. K. Rabe, and S. L. Hillier. 2002. Rectal applications of nonoxynol-9 cause tissue disruption in a monkey model. Sex. Transm. Dis. 29:581-587. [DOI] [PubMed] [Google Scholar]
- 24.Roddy, R. E., L. Zekeng, K. A. Ryan, U. Tamoufe, S. S. Weir, and E. L. Wong. 1998. A controlled trial of nonoxynol 9 film to reduce male-to-female transmission of sexually transmitted diseases. N. Engl. J. Med. 339:504-510. [DOI] [PubMed] [Google Scholar]
- 25.Rusconi, S., M. Moonis, D. P. Merrill, P. V. Pallai, E. A. Neidhardt, S. K. Sing, K. J. Willi, M. S. Osburne, A. T. Profy, J. C. Jenson, and M. S. Hirsch. 1996. Naphthalene sulfonate polymers with CD4-blocking and anti-human immunodeficiency virus type 1 activities. Antimicrob. Agents Chemother. 40:234-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szilagyi, J. F., and C. Cunningham. 1991. Identification and characterization of a novel non-infectious herpes simplex virus-related particle. J. Gen. Virol. 72:661-668. [DOI] [PubMed] [Google Scholar]
- 27.UNAIDS. 2004. AIDS epidemic update—December 2004. United Nations, Geneva, Switzerland.
- 28.Van Damme, L., G. Ramjee, M. Alary, B. Vuylsteke, V. Chandeying, H. Rees, P. Sirivongrangson, L. Mukenge-Tshibaka, V. Ettiegne-Traore, C. Uaheowitchai, S. S. Karim, B. Masse, J. Perriens, and M. Laga. 2002. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet 360:971-977. [DOI] [PubMed] [Google Scholar]
- 29.Van Damme, L., A. Wright, K. Depraetere, I. Rosenstein, V. Vandersmissen, L. Poulter, M. McKinlay, E. Van Dyck, J. Weber, A. Profy, M. Laga, and V. Kitchen. 2000. A phase I study of a novel potential intravaginal microbicide, PRO 2000, in healthy sexually inactive women. Sex. Transm. Infect. 76:126-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaneveld, L. J., D. P. Waller, R. A. Anderson, C. Chany II, W. F. Rencher, K. Feathergill, X. H. Diao, G. F. Doncel, B. Herold, and M. Cooper. 2002. Efficacy and safety of a new vaginal contraceptive antimicrobial formulation containing high molecular weight poly(sodium 4-styrenesulfonate). Biol. Reprod. 66:886-894. [DOI] [PubMed] [Google Scholar]