Abstract
We have determined the antifungal susceptibilities of 34 clinical isolates of the dimorphic fungus Sporothrix schenckii to 11 drugs using a microdilution method. In general, the type of growth phase (mycelial or yeast) and the temperature of incubation (30 or 35°C) exerted a significant influence on the MICs.
Sporothrix schenckii is a thermally dimorphic fungus and the most common agent of subcutaneous mycosis in Latin America (2, 12). Pulmonary and disseminated sporotrichosis can also occur when S. schenckii conidia are inhaled (16). Itraconazole (ITC) is generally used for the treatment of lymphocutaneous infection (3, 13, 21), while amphotericin B (AMB) is indicated for severe infections or when ITC therapy fails (9). Although these drugs are generally effective, the long duration of therapy and the strong toxicity of AMB make it necessary to explore new alternatives for the treatment of severe infections.
The infective form of sporotrichosis is the yeast phase (Y). However, the reference methods for in vitro antifungal susceptibility testing propose conditions only for the testing of its mycelial phase (M) (19). Testing of Y requires the use of other conditions. Although the optimal temperature for the in vitro growth of S. schenckii ranges from 25°C to 30°C (7), the reference method recommends a temperature of incubation of 35°C. The few studies that have determined the in vitro antifungal susceptibility of S. schenckii have used as the inoculum a mixture of hyphae and conidia obtained from its M (8, 14). We have evaluated the in vitro activities of 11 antifungal agents against clinical isolates of S. schenckii in order to determine if the form of growth and temperature of incubation can influence the MICs.
A total of 34 isolates were tested (18 were from patients with disseminated sporotrichosis, 15 were from patients with the lymphocutaneous or cutaneous form of infection, and 1 was from domestic dust). Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258 were used as control strains.
The following 11 antifungal agents were tested by a microdilution method (18, 19): albaconazole (ABC), AMB, eberconazole (EBC), flucytosine (5FC), fluconazole (FLC), ITC, ketoconazole (KTC), micafungin (MFG), ravuconazole (RVC), terbinafine (TRB), and voriconazole (VRC).
To convert S. schenckii into Y, the procedure was performed as described by Ghosh et al. (7). Mycelial cultures grown on potato dextrose agar (PDA) were subcultured on brain heart infusion agar supplemented with 5% defibrinated sheep blood at 37°C for approximately 6 to 9 days. Several successive passages were done to achieve Y. The suspensions were adjusted hemacytometrically, diluted 1:50, and further diluted 1:20 in RPMI to obtain double the final inoculum, which ranged from 1.0 × 105 to 5.0 × 105 CFU/ml. This inoculum was similar to that used by Nakai et al. (17). The size of the inoculum was verified by determination of quantitative colony counts on PDA. The microplates were incubated at 35°C for 6 days. For AMB and MFG the MICs were defined as the lowest concentration that showed complete inhibition of growth, and for the rest of the drugs the MICs were defined as the lowest concentration that showed 80% inhibition of growth.
The M inoculum was obtained as recommended by the CLSI (formerly the NCCLS) (19). Two sets of microplates were incubated at 35°C and 30°C, respectively, and were read after 6 days of incubation. For AMB, ABC, EBC, ITC, MFG, RVC, TRB, and VRC, the MICs were defined as the lowest concentration that showed complete inhibition of growth. For 5FC, FLC, and KTC the MICs were defined as the lowest concentration that showed 80% growth inhibition compared with that for the growth control. The minimum fungicidal concentrations (MFCs), defined as the lowest drug concentration that produced either no growth or less than three colonies, were determined as described previously (22) and were read at 6 days of incubation.
The MICs obtained with the two forms of growth could be clearly read after 6 days of incubation, although it was possible to develop Y from only 24 of the 34 isolates tested.
The MICs obtained with M at 30°C were significantly higher than those obtained with the same phase at 35°C for ABC, AMB, TRB, and VRC. The only exception was ITC, which showed a lower MIC at 30°C than at 35°C.
The MICs obtained with M at 35°C were higher than those obtained with Y at the same temperature for EBC, ITC, TRB, and VRC. Significant differences were also observed when the MICs for M at 30°C and those for Y at 35°C were compared. The MICs were significantly lower for Y at 35°C than for M at 30°C for ABC, AMB, EBC, TRB, and VRC. The only exception was RVC, which showed a lower MIC for M at 30°C than for Y at 35°C (Table 1).
TABLE 1.
MICs of 11 antifungal agents against the two phases of growth of Sporothrix schenckii
| Growth phase/temp (°C) (no. of isolates tested) and parameter | MIC (μg/ml)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ABC | AMB | EBC | ITC | 5FC | FLC | MFG | KTC | RVC | TRB | VRC | |
| M/35 (34) | |||||||||||
| Range | 0.125-16 | 1-4 | 0.06-4 | 0.25->16 | 16->64 | >64 | >128 | 0.03-16 | 1-16 | 0.125-4 | 0.5->16 |
| GM | 2.95a | 1.73a | 0.80 | 4.08a | >64 | >64 | >128 | 0.81 | 3.07 | 0.80a | 9.81a |
| M/30 (34) | |||||||||||
| Range | 0.5-16 | 1->16 | 0.06->16 | 0.125->16 | 64->64 | >64 | 16->128 | 0.25-2 | 0.125-8 | 1-4 | 1->16 |
| GM | 7.37b | 2.71b | 0.96b | 1.47 | >64 | >64 | >128 | 0.64 | 2.45b | 2.55b | >16b |
| Y/35 (24) | |||||||||||
| Range | 0.06-8 | 1-4 | <0.03-2 | 0.25->16 | 16->64 | 16->64 | >128 | 0.125-4 | 0.25-8 | 0.06-1 | 2->16 |
| GM | 2.37 | 1.63 | 0.28c | 1.06c | >64 | >64 | >128 | 0.65 | 5.66 | 0.48c | 8.24c |
Statistically significant with respect to the geometric mean MIC in the mycelial growth phase at 30°C (P < 0.05; Student's t test, paired).
Statistically significant with respect to the geometric mean MIC in the yeast growth phase at 35°C (P < 0.05; Student's t test, paired).
Statistically significant with respect to the geometric mean MIC in the mycelial growth phase at 35°C (P < 0.05; Student's t test, paired).
All the azoles with the exception of EBC showed geometric mean (GM) MFCs of >16 μg/ml against both growth forms. For the azoles, the differences between the MFCs and the MICs were notable (3 or more dilutions) for the two forms of growth. This seems to indicate that the azoles tested have fungistatic activity against this fungus. In the case of AMB and TRB, the differences between the MFCs and the MICs were less than 2 dilutions. No statistically significant differences were found between the GM MFCs for Y at 35°C and M at 35°C for any antifungal agent (Table 2).
TABLE 2.
MFCs of eight antifungal agents against the two phases of growth of Sporothrix schenckii
| Growth phase/temp (°C) (no. of isolates tested) and parameter | MFC (μg/ml)a
|
|||||||
|---|---|---|---|---|---|---|---|---|
| ABC | AMB | EBC | ITC | KTC | RVC | TRB | VRC | |
| M/35 (34) | ||||||||
| Range | 0.125->16 | 1->16 | 0.125-16 | 4->16 | 1->16 | 4->16 | 0.125-16 | 4->16 |
| GM | >16 | 3.38 | 12.53 | >16 | >16 | >16 | 2.21 | >16 |
| Y/35 (24) | ||||||||
| Range | 0.125->16 | 1->16 | 0.03-16 | 1->16 | 2->16 | 16->16 | 0.25->16 | 4->16 |
| GM | >16 | 2.74 | 9.37 | >16 | >16 | >16 | 2.31 | >16 |
MFCs for 5FC, FLC, and MFG were not determined (MICs, >64 μg/ml) (P < 0.05, Student's t test, paired).
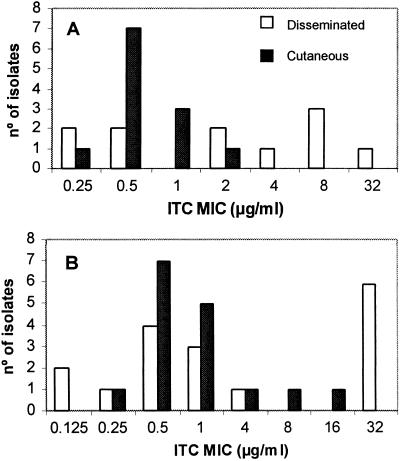
One of the most interesting findings of the present study was that the isolates from disseminated infections were less susceptible to ITC than those from localized infections. This is relevant because, as mentioned above, ITC is a drug recommended for the treatment of sporotrichosis (3, 13, 21). These differences were statistically significant when Y was tested. In contrast, with M, there were no differences in the activities of ITC against the two forms of sporotrichosis (Fig. 1). These data correlate with the high frequency of therapeutic failure in the cases of disseminated sporotrichosis observed in our Brazilian institution (Fundação Oswaldo Cruz [FIOCRUZ]). This suggests that a possibly different genetic species of S. schenckii, hardly distinguishable morphologically, was responsible for the different clinical manifestations, as happens with other pathogenic fungi (Trichosporon, Malassezia, etc.). This is an interesting topic for further study. FLC is considered the second-line treatment for sporotrichosis (4). In spite of the poor activity that this drug has shown in this and other studies (6, 17), its clinical efficacy has been estimated to be 71% for cases of lymphocutaneous infection (10). KTC has shown poor efficacy against different forms of sporotrichosis (13). However, our in vitro results agree with those obtained by Shadomy et al. (20), who also found that this drug has very good activity in vitro. In our study, in agreement with Kohler et al. (11), who used a macrodilution method, TRB has also shown good activity, mainly when Y was tested. Although a few reports on the clinical efficacy of TRB exist (1, 15), the data are still too scarce to recommend its use as a possible alternative treatment. Our data seem to indicate that one of the most promising drugs for the treatment of mold infections, VRC, is not a good candidate for sporotrichosis management. McGinnis et al. (14) and Johnson et al. (8) also reported high MICs for VRC when a mixture of hyphae and conidia was used as the inoculum. EBC is a topical antifungal agent that has been shown to have excellent activity both in vitro and in vivo against yeasts and dermatophytes (5). In this study, this drug was very active against the two forms of growth of S. schenckii. In conclusion, our data demonstrate that the growth form and temperature of incubation considerably influence the antifungal susceptibility of S. schenckii. Further in vivo studies are required to prove which growth phase and temperature of incubation are the most predictive of the clinical outcome.
FIG. 1.
Distribution of the ITC MICs, tested at 35°C, against the yeast (A) and the mycelial (B) phases of S. schenckii from patients with cutaneous and disseminated sporotrichosis. Geometric means for panel A, disseminated, 2.13 μg/ml; cutaneous, 0.63 μg/ml (P = 0.035, independent samples t test); geometric means for panel B, disseminated, 2.26 μg/ml; cutaneous, 1 μg/ml (P = 0.402, Mann-Whitney U test). P values refer to differences between the MICs for isolates from disseminated and cutaneous cases. Statistical significance was assessed at a P value of <0.05.
Acknowledgments
This work was partially supported by Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro-FAPERJ, Rio de Janeiro, Brazil, and by a grant from Fondo de Investigaciones Sanitarias from the Ministerio de Sanidad y Consumo of Spain (PI 020114).
REFERENCES
- 1.Chapman, S. W., P. Pappas, C. Kauffman, E. B. Smith, R. Dietze, N. Tiraboschi-Foss, A. Restrepo, A. B. Bustamante, C. Opper, S. Emady-Azar, and R. Bakshi. 2004. Comparative evaluation of the efficacy and safety of two doses of terbinafine (500 and 1000 mg day−1) in the treatment of cutaneous or lymphocutaneous sporotrichosis. Mycoses 47:62-68. [DOI] [PubMed] [Google Scholar]
- 2.Conti, I. A. 1989. Epidemiology of sporotrichosis in Latin America. Mycopathologia 108:113-116. [DOI] [PubMed] [Google Scholar]
- 3.Conti, I. A., E. Civila, and E. Gezuele. 1992. Treatment of human cutaneous sporotrichosis with itraconazole. Mycoses 35:153-156. [DOI] [PubMed] [Google Scholar]
- 4.Diaz, M., R. Negroni, F. Montero-Gei, L. G. Castro, S. A. Sampaio, D. Borelli, A. Restrepo, L. Franco, J. L. Bran, and E. G. Arathoon. 1992. A pan-American 5-year study of fluconazole therapy for deep mycoses in the immunocompetent host. Clin. Infect. Dis. 14(Suppl. 1):68-76. [DOI] [PubMed] [Google Scholar]
- 5.Fernández-Torres, B., I. Inza, and J. Guarro. 2003. In vitro activities of the new antifungal drug eberconazole and three other topical agents against 200 strains of dermatophytes. J. Clin. Microbiol. 41:5209-5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghosh, A., A. Chakrabarti, B. M. Hemanshettar, and P. K. Maity. 2001. In vitro susceptibility pattern of Sporothrix schenckii strains isolated from three centers in India. Indian J. Med. Res. 113:214-220. [PubMed] [Google Scholar]
- 7.Ghosh, A., P. K. Maity, B. M. Hemashettar, V. K. Sharma, and A. Chakrabarti. 2002. Physiological characters of Sporothrix schenckii isolates. Mycoses 45:449-454. [PubMed] [Google Scholar]
- 8.Johnson, E. M., A. Szekely, and D. W. Warnock. 1998. In-vitro activity of voriconazole, itraconazole and amphotericin B against filamentous fungi. J. Antimicrob. Chemother. 42:741-745. [DOI] [PubMed] [Google Scholar]
- 9.Kauffman, C. A., R. Hajjeh, and S. W. Chapman. 2000. Practice guidelines for the management of patients with sporotrichosis. Clin. Infect. Dis. 30:684-687. [DOI] [PubMed] [Google Scholar]
- 10.Kauffman, C. A., P. G. Pappas, D. S. McKinsey, R. A. Greenfield, J. R. Perfect, G. A. Cloud, C. J. Thomas, and W. E. Dismukes. 1996. Treatment of lymphocutaneous and visceral sporotrichosis with fluconazole. Clin. Infect. Dis. 22:46-50. [DOI] [PubMed] [Google Scholar]
- 11.Kohler, L. M., P. C. Fialho, R. C. Hahn, and J. Soares. 2004. In vitro susceptibilities of isolates of Sporothrix schenckii to itraconazole and terbinafine. J. Clin. Microbiol. 42:4319-4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopes, J. O., S. H. Alves, C. R. Mari, L. M. Brum, J. B. Westphalen, M. J. Altermann, and F. B. Prates. 1999. Epidemiologia da esporotricose na região central do Rio Grande do Sul. Rev. Soc. Bras. Med. Trop. 32:541-545. [DOI] [PubMed] [Google Scholar]
- 13.Lortholary, O., D. W. Denning, and B. Dupont. 1999. Endemic mycoses: a treatment update. J. Antimicrob. Chemother. 43:321-331. [DOI] [PubMed] [Google Scholar]
- 14.McGinnis, M. R., N. Nordoff, R. K. Li. L. Pasarell, and D. W. Warnock. 2001. Sporothrix schenckii sensitivity to voriconazole, itraconazole and amphotericin B. Med. Mycol. 39:369-371. [DOI] [PubMed] [Google Scholar]
- 15.Morishita, N., K. Yamazaki, J. Ninomiya, T. Hamaguchi, Y. Sei, and I. Takiuchi. 2001. A case of lymphocutaneous sporotrichosis. Jpn. J. Med. Mycol. 42:149-154. [DOI] [PubMed] [Google Scholar]
- 16.Morris-Jones, R. 2002. Sporotrichosis. Clin. Exp. Dermatol. 27:427-431. [DOI] [PubMed] [Google Scholar]
- 17.Nakai, T., J. Uno, F. Ikeda, S. Tawara, K. Nishimura, and M. Miyaji. 2003. In vitro antifungal activity of micafungina (FK463) against dimorphic fungi: comparison of yeast-like and mycelial forms. Antimicrob. Agents Chemother. 47:1376-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 19.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard M38-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 20.Shadomy, S., S. C. White, M. P. Yu, and W. E. Dismukes. 1985. Treatment of systemic mycoses with ketoconazole: in vitro susceptibilities of clinical isolates of systemic and pathogenic fungi to ketoconazole. J. Infect. Dis. 152:1249-1256. [DOI] [PubMed] [Google Scholar]
- 21.Sharkey-Mathis, P. K., C. A. Kauffman, J. R. Graybill, D. A. Stevens, T. S. Hostetler, G. Cloud, and W. E. Dismukes. 1993. Treatment of sporotrichosis with itraconazole. Am. J. Med. 95:279-285. [DOI] [PubMed] [Google Scholar]
- 22.Trilles, L., B. Fernández-Torres, M. dos Santos Lazéra, B. Wanke, and J. Guarro. 2004. In vitro antifungal susceptibility of Cryptococcus gattii. J. Clin. Microbiol. 42:4815-4817. [DOI] [PMC free article] [PubMed] [Google Scholar]