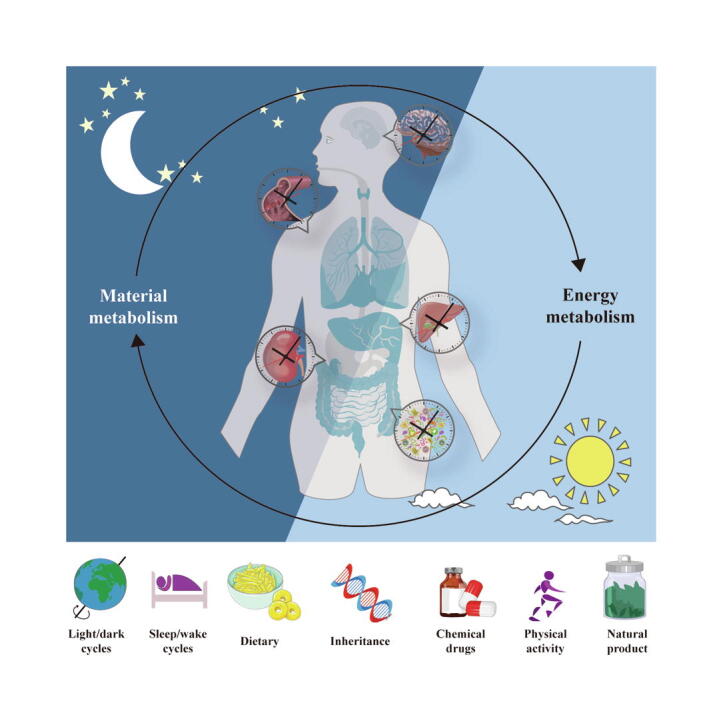
Graphical abstract
Keywords: Circadian rhythm, Natural products, Metabolic system, Cardiovascular system, Nervous system
Highlights
-
•
Impaired circadian disorders have been observed in many diseases.
-
•
Natural products improve metabolic system disease though circadian rhythm.
-
•
Natural products improve cardiovascular system disease though circadian rhythm.
-
•
Natural products improve nervous system disease though circadian rhythm.
-
•
Circadian rhythm is a new therapeutic target for these diseases.
Abstract
Background
The treatment of metabolic system, cardiovascular system, and nervous system diseases remains to be explored. In the internal environment of organisms, the metabolism of substances such as carbohydrates, lipids and proteins (including biohormones and enzymes) exhibit a certain circadian rhythm to maintain the energy supply and material cycle needed for the normal activities of organisms. As a key factor for the health of organisms, the circadian rhythm can be disrupted by pathological conditions, and this disruption accelerates the progression of diseases and results in a vicious cycle. The current treatments targeting the circadian rhythm for the treatment of metabolic system, cardiovascular system, and nervous system diseases have certain limitations, and the identification of safer and more effective circadian rhythm regulators is needed.
Aim of the review
To systematically assess the possibility of using the biological clock as a natural product target for disease intervention, this work reviews a range of evidence on the potential effectiveness of natural products targeting the circadian rhythm to protect against diseases of the metabolic system, cardiovascular system, and nervous system. This manuscript focuses on how natural products restore normal function by affecting the amplitude of the expression of circadian factors, sleep/wake cycles and the structure of the gut microbiota.
Key scientific concepts of the review
This work proposes that the circadian rhythm, which is regulated by the amplitude of the expression of circadian rhythm-related factors and the sleep/wake cycle, is crucial for diseases of the metabolic system, cardiovascular system and nervous system and is a new target for slowing the progression of diseases through the use of natural products. This manuscript provides a reference for the molecular modeling of natural products that target the circadian rhythm and provides a new perspective for the time-targeted action of drugs.
Introduction
The vast majority of organisms on earth exhibit physiological activity rhythms corresponding to the light/dark cycle of the outside world, which are referred to as circadian rhythms [1]. Specifically, under the influence of the natural light/dark cycle, the physiological activities of an organism follow a stable 24-hour cycle to maintain homeostasis of the internal environment and the normal physiological functions of the body. For example, the physiological cycle of blood pressure during the day includes a sharp increase in the morning and a slow decrease at night [2], [3], [4], [5], [6], [7]. This circadian pattern of activities is also influenced by other factors, such as the timing of meals, the sleep/wake cycle and pathological states [8], [9]. Notably, the circadian rhythm plays a key role in almost all chronic diseases, including metabolic-associated fatty liver disease (MAFLD), Alzheimer’s disease (AD) and atherosclerosis [10], [11], [12]. For example, working at night is a risk factor for metabolic syndrome and hypertension, but fasting patterns can alter the rhythms of digestion of exogenous substances and reverse the metabolic syndrome process [9], [13], [14], [15]. In addition, disease progression in brain injuries, including AD, anxiety, and depression, overlaps with fundamental regulatory mechanisms of circadian rhythms, including the sleep/wake cycle, protein homeostasis, and immune and inflammatory responses [16], [17], [18], [19], [20]. Prolonged insomnia can promote the progression of depression, and certain chemical drugs can regulate the circadian rhythm via melatonin, relieving anxiety and depression [21]. In summary, circadian rhythm disturbance can lead to the occurrence of disease, and the development of disease is often accompanied by exacerbation of circadian rhythm disturbance, which forms a vicious cycle. Targeting factors that regulate the circadian rhythm and blocking the vicious cycle of disease and circadian disorders may be a new approach for the treatment of some complex chronic diseases.
According to chronomedicine and chronobiology, there are two main approaches for regulating circadian rhythms: behavioral therapy and drug therapy [22]. The former refers to patients changing their sleep or dietary patterns on their own, whereas the latter involves the use of chemical drugs such as sleeping pills or anti-anxiety and depression drugs. However, in today’s fast-paced world, patients need to have elevated levels of self-control to adapt to a strict daily routine and dietary structure, which is difficult for most patients to achieve [23]. Most patients who receive a drug treatment use sleeping pills such as dexamethasone or antidepressants such as agomelatine, but unfortunately, the long-term use of sleeping pills such as dexamethasone increases the risk of dependence. Agomelatine has hepatotoxicity, and the use of these drugs has an age limit [24], [25]. Therefore, the identification of safer and more effective therapeutic drugs is urgently needed.
Natural products, including polysaccharides, volatile oils, amino acids, alkaloids, flavonoids and other natural compounds with many biological activities, have become more effective sources of drugs [26]. Studies have demonstrated that natural products can play a complementary therapeutic role as good dietary supplements in the treatment of various metabolic, cardiovascular and central nervous system disorders [27], [28], [29]. Many natural products, such as cocaine and caffeine, which can prolong the circadian rhythm cycle in mice, have shown potential to regulate circadian rhythms [30], [31]. Myricetin and nobiletin can change the circadian rhythm amplitude [32], [33]. Studying the health benefits of these natural products that target circadian rhythms has become a solid idea for the treatment of certain chronic diseases.
This review aims to elaborate on the research on circadian rhythm as a new target in the treatment of metabolic system, cardiovascular system, and nervous system diseases, to summarize the research progress on natural products affecting the circadian rhythm and to provide a reasonable and valuable reference basis for subsequent research on natural products that target the circadian rhythm and their underlying molecular mechanisms.
Circadian rhythm system
Central clock and peripheral clocks
The circadian rhythm system of mammals is composed of a central clock and peripheral clocks [34]. The central clock is located in the superior optic chiasm nucleus (SCN), which is responsible for sensing external light/dark cycle signals and regulating peripheral clocks. In brief, intrinsically photosensitive retinal ganglion cells (ipRGCs) receive light and transmit information directly to the SCN. The central clock in the SCN governs the central release of circadian hormones and signals that travel throughout the body and entrain the peripheral clocks to a consistent phase [35]. Almost all peripheral clocks, such as those in the heart, adipose tissue and pancreas, have melatonin receptors [36], [37], [38], [39]. Upon receiving a “signal” from the central clock, the peripheral clocks undergo rhythmic actions, including fat storage and insulin secretion [40]. For example, ipRGCs receive dark cycle signals and transmit the information directly to the SCN; the SCN then sends a signal to urge the pineal gland to secrete melatonin [41]. After melatonin enters the peripheral clocks throughout the body, the breathing and heart rates become slower, nerve activity and other activities are inhibited, the repair of DNA damage and other problems are accelerated, and the “night life” of the human body begins [42], [43], [44], [45]. As mentioned above, the SCN coordinates a wide range of physiological and metabolic processes. In turn, the rhythm of the central clock is reinforced and enhanced by relying on and integrating feedback signals from the peripheral clocks. For example, leptin and adiponectin secreted by adipose tissue regulate the systemic energy status by altering the activity of the intracellular cyclic adenosine 5‘-monophosphate (AMP)-activated protein kinase (AMPK) pathway in the hypothalamus, which further transmits metabolic signaling projections to the SCN [46], [47], [48], [49], [50]. Similarly, the homeostasis of the liver, as an important peripheral clock, is closely related to the circadian rhythms of glucose and lipid metabolism and energy consumption, whereas in nonalcoholic fatty liver disease, the circadian rhythm of liver lipids is unbalanced, further leading to an imbalance in the rhythm of inflammatory factors, which often causes shock to the whole body [51], [52], [53]. In addition, local microenvironments such as the temperature, the gut microbiota, and dietary rhythms are involved in select peripheral clock fluctuations, providing tissue specificity to peripheral clocks [54], [55], [56]. This tissue specificity leads to a phase difference between the peripheral and central clocks and further strengthens the feedback signals [57], [58].
Taken together, these findings suggest that the SCN relies on signals such as hormones to synchronize the peripheral clock and also receives and integrates feedback signals from the peripheral clock to form an interconnected large oscillator that drives circadian rhythm physiology.
Transcriptional-translational feedback loops
It is widely accepted that mammalian circadian rhythms in the central clock and peripheral clocks are driven by a complex set of transcriptional-translational feedback loops (TTFLs). This complex feedback loop involves a series of rhythm regulators.
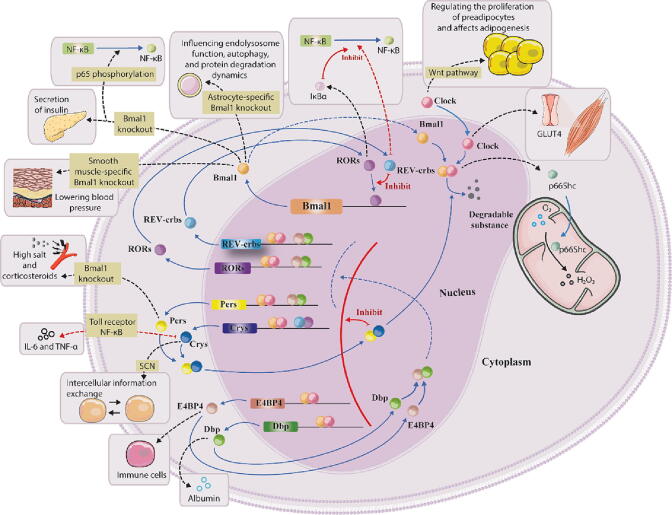
First, the core circadian factors circadian locomotor output cycles kaput (CLOCK) and brain and muscle Arnt-like 1 (BMAL1) are generated in the nucleus and then interact to form the heterodimer CLOCK-BMAL1. CLOCK-BMAL1 increases the expression of period circadian proteins (Pers; Per1, Per2 and Per3) and cryptochromes (Crys; Cry1 and Cry2) in the cytoplasm, and Pers and Crys are then transported to the nucleus through heterodimerization. These molecules act as transcription inhibitors to inhibit CLOCK-BMAL1-mediated transcription and thus form a negative feedback loop (first feedback loop). The CLOCK-BMAL1 heterodimer positively regulates the transcription of nuclear hormone receptors (Rev-erbs; Rev-erbα and Rev-erbβ). As transcriptional repressors, Rev-erbs enter the nucleus and competitively bind to the BMAL1 promoter element with retinoic acid-related orphan receptors (RORs; RORα, RORβ and RORγ) (which promote the transcription of BMAL1), inhibiting the transcription of BMAL1 and forming another negative feedback loop (second feedback loop) [59], [60]. In addition, the positive regulation of the CLOCK-BMAL1 heterodimer regulates the synthesis of D-box-binding protein (Dbp) and E4 promoter-binding factor 4 (E4BP4) proteins, whereas the Dbp and E4BP4 proteins alter the expression of Rev-erbs, RORs, and Pers, thereby negatively regulating the concentration of CLOCK-BMAL1 in cells (third feedback loop). Briefly, an increase in certain rhythmic factors, such as Crys, Pers, and Rev-erbs, in the nucleus can reduce the concentration of CLOCK-BMAL1, thereby reducing the level of transcriptional activation induced by CLOCK-BMAL1 and resulting in decreases in the synthesis of Crys, Pers, and Rev-erbs in the cell. These effects lead to the release of CLOCK-BMAL1, the gradual activation of new transcription, and the beginning of a new cycle of the circadian rhythm (Fig. 1). Notably, these factors act in the set of TTFLs, and a significant number of these factors also switch to other pathways to regulate cellular energy consumption, hormone synthesis, and other life activities. This “switching” function is often accompanied by the consumption of circadian factors, and the circadian factor levels are restored by the TTFLs. Undoubtedly, this process of “switching” brings virtually all of the cell’s vital activities into these TTFLs, forming a relatively stable oscillator. Surprisingly, this complex regulation occurs in every cell, and cells are connected through hormonal or neural signals, influence each other, and oscillate in a relatively stable manner.
Fig. 1.
Transcriptional-translational feedback loops. Explanatory notes: In cells, the main circadian rhythm factors CLOCK and BMAL1 promote the transcription of Rev-erbs, RORs, Pers, Crys, E4B4, and Dbp by forming the heterodimer CLOCK-BMAL1 and are negatively regulated by these transcription factors, forming transcriptional-translational feedback loops (TTFLs). In addition to the TTFLs, CLOCK can regulate glucose receptor expression and lipid cell proliferation, and BMAL1 is associated with cellular autophagy and intracellular oxidative stress, among other processes. Rev-erbs and RORs can participate in inflammatory responses, Pers is associated with vascular sensitivity, Crys can participate in cellular communication, and E4BP4 and Dbp can affect the body’s immune system. These factors outside the loops bring all important cellular activities into the TTFLs. The black and red dotted lines represent the diversion of circadian factors; the blue solid and blue dotted lines represent physiological processes such as transport and translation; and the red solid and red dotted lines represent inhibition. Abbreviations: BMAL1, brain and muscle Arnt-like 1; CLOCK, circadian locomotor output cycles kaput; Crys, cryptochromes; Dbp, D-box-binding protein; E4BP4, E4 promoter-binding factor 4; IκBα, inhibitor kappa B alpha; NF-κB, nuclear factor kappa-B; Pers, period circadian proteins; Rev-erbs, nuclear hormone receptors; RORs, retinoic acid-related orphan receptors; TTFLs, transcriptional-translational feedback loops; SCN, superior optic chiasm nucleus. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Circadian rhythm-regulating factors
The functional oscillations of the set of TTFLs depend on the function of circadian rhythm factors at the molecular level. CLOCK and BMAL1 are the most extensively studied factors and are known as “master clocks” or “circadian pacemakers” [61]. CLOCK plays an important role in regulating the circadian rhythm of mitochondrial oxidation and cell viability [62], [63]. The mitochondrial adaptor protein p66Shc was previously shown to promote hydrogen peroxide formation [64]. The p66Shc gene is expressed in a circadian manner at both the mRNA and protein levels, presumably because its promoter contains the CLOCK-BMAL1 recognition element. Additionally, CLOCK proteins promote the proliferation of preadipocytes by regulating the Wnt pathway, further inhibiting the initiation of the adipose differentiation program and suppressing cellular adipogenesis [65]. BMAL1 plays a crucial role in biological function rhythms, such as the rhythm of blood pressure fluctuation and that of glucose transport [66], [67]. The literature reports that BMAL1 knockdown in astrocytes can dysregulate autophagy and endolysosomal function in astrocytes [68]. BMAL1 knockdown in smooth muscle impairs the circadian rhythm of blood pressure and lowers blood pressure, and systemic BMAL1 knockdown accelerates senescent death in mice [66], [69]. BMAL1 plays a role in almost all cells, and CLOCK and BMAL1 often form heterodimers to play a role in rhythm regulation.
Crys play a role in the exchange of information between cells through signaling molecules such as hormones [70]. Some research has shown that Crys are the core inhibitors of many nuclear hormone receptors, which control metabolism and physiological regulation mediated by nuclear hormone receptors and thereby regulate cell growth and differentiation [71]. For example, Crys regulate the blood glucose levels by modulating glucocorticoid receptors and energy metabolism [72], [73]. Cy1 also regulates many inflammatory responses [74], [75]. Pers are important rhythm factors that regulate the cell cycle and promote DNA damage repair [76], [77]. In addition, Per1 can improve the blood pressure levels by regulating vascular cell sensitivity [78]. The feedback regulation of Pers and Crys affects rhythm cycles through the formation of heterodimers.
Rev-erbs are involved in many physiological processes and play important roles in anti-inflammatory and immune regulation. For example, Rev-erbα can interact with the promoter region of the nuclear factor kappa-B (NF-κB) signal transduction transcript, and Rev-erbα deletion leads to the activation of proinflammatory NF-κB [79]. In addition, Rev-erbα can regulate lipid metabolism by targeting the expression of interleukin 3 regulatory factor (NFIL3) [80]. RORs are typically associated with the circadian rhythms of inflammatory factors. For example, RORα has been shown to bind to promoter regions, increase the transcription of inhibitor kappa B alpha (IκBα), and inhibit the NF-κB pathway, thus reducing the inflammatory response [81]. Rev-erbs and RORs usually regulate inflammation together.
Dbp and E4BP4 are important output signals of circadian rhythms that rhythmically activate the transcription of various genes [82]. For example, Dbp can promote the differentiation of osteoblasts in rats [83]. E4BP4 is critical for the development of immune cells and plays an important role in osteoblast function, heart failure, ovulation, and cell metabolism [84], [85], [86].
In addition, other regulatory factors (such as those that regulate the cell cycle and sleep/wake cycle) have been identified. The abovementioned regulatory factors interact with each other to form a complex circadian rhythm regulation system.
Chronotherapy based on the time rhythm
Notably, chronotherapy based on the time rhythm has been widely used in most current treatments, which include but are not limited to temporal patterns of drug action, surgical time determination, and chronotropic nutrition [87], [88], [89], [90]. Drug action has a temporal pattern, i.e., the pharmacokinetics and pharmacodynamics (PK/PD) of many drugs are circadian; therefore, the efficacy and safety of a drug vary with the time of day. For example, temporal and theophylline exhibit different pharmacokinetics in the morning and evening [91], [92]. These changes are the result of several time-dependent modifications of physiological and molecular processes that affect drug absorption and distribution. The knowledge that drug action has a temporal pattern of relevance translates to a large extent into successful time-based therapies, the most obvious example being the use of an extended-release formulation of the L-type calcium channel blocker verapamil that can be matched to the blood pressure levels to produce therapeutically effective plasma levels for oral dosing in the early morning after bedtime [93]. Chronotropic nutrition implies that nutrients or mealtimes can positively or negatively affect the health of the circadian clock system (and vice versa). Consumption of the same nutrient at different meal times can yield different effects. For example, the consumption of fish oil improves the plasma and liver triglyceride (TG) and cholesterol concentrations; however, its consumption with breakfast results in better lipid-lowering activity than its consumption with dinner [94]. In contrast, some studies have shown that the timing of eating is critical for maintaining the synchronization of the circadian rhythms of the central clock and peripheral clocks that influence energy metabolism [95], [96]. For example, British adolescents who have an early main meal and the highest energy intake at night have a greater body mass index and girth [97]. In addition, several natural products are critical to the regulation of the circadian rhythm. EGCG can inhibit the self-renewal ability of cancer stem cells through the Wnt/beta-catenin pathway by targeting CLOCK [98]. Curcumin can regulate the rhythm of Per2, promote apoptosis, and effectively inhibit the growth of thyroid cancer cells [99]. Withania somnifera extract alleviates the age-induced changes in the circadian factors BMAL1 and Per1 with horizontal and diurnal rhythms, and lycorine affects the length of the mammalian cell cycle, which may provide a perspective for further exploration of the mechanism of aging [100], [101], [102]. Catecholamines are important in regulating the circadian rhythm of the mouse SCN, and quercetin can alter the sleep/wake cycle and thus exerts a potential protective effect against light/dark disturbances [103], [104].
All of these findings point to the importance of circadian rhythms. Thus, limiting the immutability of lifestyle habits, focusing on circadian rhythm research, and developing circadian regulators rather than artificially altering behavioral rhythms may constitute a better treatment strategy.
Protective effects of natural products through effects on the circadian rhythm
Protective effects of natural products against metabolic system diseases through effects on the circadian rhythm
Natural products that treat dysglycemia by affecting the circadian rhythm
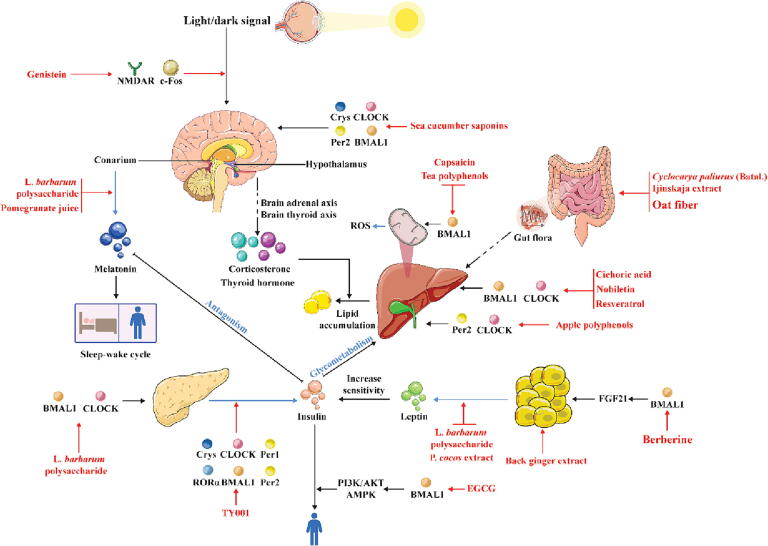
The circadian rhythm plays an influential role in regulating the balance of glucose metabolism in the animal body [105]. In the body, glucose homeostasis is determined by multiple factors, such as insulin, which is the glucose transport protein carrier responsible for the storage and decomposition of liver glycogen and promotes the conversion of glucose in the blood, and glucocorticoids, which reduce the transport of glucose from the blood circulation to tissues [106], [107], [108], [109], [110]. The central circadian factor BMAL1 regulates the expression rhythms of glucose transporter carriers through transcriptional, translational and feedback regulation [67]. In BMAL1-knockout mice, the secretion of insulin is also impaired [111], [112], [113], [114], [115]. Crys can interact with the glucocorticoid receptor [73]. Similar studies have also shown that Cry1 can block the activity of AMP in the liver and regulate the expression of hepatic gluconeogenesis-related genes, thus reducing glucose synthesis in the liver [72]. It is clear that the stable cycling of circadian rhythm factors plays an important role in the maintenance of glucose metabolic homeostasis in organisms. Targeting circadian factors and further regulating the corresponding hormones or transporters to restore the metabolic rhythm of glucose may be attributed to the efficacy of certain natural products (Fig. 2).
Fig. 2.
Protective effects of natural products against metabolic system diseases through effects on the circadian rhythm. The red solid line represents the targeting effect of natural products, the blue solid line represents the secretion process, and the black solid and black virtual solid lines represent transport and action processes, respectively. Abbreviations: AKT, protein kinase B; AMPK, intracellular cyclic adenosine 5′-monophosphate-activated protein kinase; BMAL1, brain and muscle Arnt-like 1; CLOCK, circadian locomotor output cycles kaput; Cry, cryptochrome; FGF21, fibroblast growth factor 21; Per, period circadian protein; RORα, retinoic acid-related orphan receptor α; ROS, reactive oxygen species. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Genistein, an isoflavone compound found in rare natural plants, can target mitochondria-dependent pathways to eliminate free radicals and reduce oxidative stress damage [116]. Zhou et al. showed that genistein regulates glucose metabolism by regulating the central circadian clock [117]. The light-induced protein c-Fos is considered a functional marker of the light-activated signal transduction pathway in the central circadian clock, whereas the glutamate receptor n-methyl-d-aspartic acid receptor (NMDAR) is a key protein that transmits light signals to the central circadian clock [118], [119], [120]. Genistein can increase the expression of the light-inducing protein c-Fos and the glutamate receptor NMDAR, affecting the ability of the central circadian clock to receive light signals from the outside world and thus regulate the circadian rhythm of the organism [117].
Lycium barbarum L. (L. barbarum), a nutritious and healthy vegetable that is both a medicinal and food source and a valuable traditional Chinese medicine, exerts antiviral and antitumor effects and regulates immune function [121], [122], [123], [124], [125]. On the one hand, L. barbarum polysaccharide directly affects the expression of CLOCK and BMAL1 in islet cells, thereby lessening damage to islets and thus alleviating blood sugar disorders [126]. On the other hand, L. barbarum polysaccharide treatment also increases the melatonin levels and decreases the leptin levels in diabetic rats. Increased melatonin levels and decreased leptin levels improve insulin sensitivity and hyperglycemia [127], [128], [129]. In summary, L. barbarum polysaccharide alleviates glucose metabolism disorders by regulating circadian rhythms and related mechanisms.
The tilapia collagen peptide mixture TY001 can be used as a supplement for various essential amino acids and vitamins to enhance the immune system [130], [131]. TY001 maintains the amplitude of the rhythm feedback factors BMAL1, CLOCK, Cry1, Cry2, Per1 and Per2 in the dark, restoring the shift in the Cry1 and RORα peaks [132]. Moreover, the rhythmic secretion of insulin and proinflammatory cytokines in the serum is reversed, which may explain the link between the circadian rhythm and diabetes [132]. Unfortunately, the exact mechanism by which TY001 affects circadian rhythm factors is unclear.
Pomegranate is a mature fruit of Punica granatum L. that is rich in vitamins, organic acids, sugar, protein, fat, calcium and other beneficial ingredients for the human body [133], [134], [135]. Drinking pomegranate juice for 1 h significantly decreases the melatonin level of all individuals and significantly increases their insulin levels [136]. There is “antagonism” between melatonin and insulin; that is, a decrease in the melatonin concentration promotes the secretion of insulin [137], [138]. In addition, pomegranate juice can affect the cell cycle, but the specific mechanism of action requires further investigation.
Natural products that treat dyslipidemia by affecting the circadian rhythm
Significant circadian disturbances, including external sleep/week cycle abnormalities and internal imbalances in rhythm factors, are often observed in obese patients [139]. Studies have shown that biological enzymes, transporter proteins and hormones involved in lipid metabolism, such as adrenaline, insulin, leptin and adiponectin, are associated with the regulation of circadian factors [140], [141]. For example, obese mice exhibit decreased CLOCK expression and reduced leptin sensitivity [142]. In BMAL1-knockout mice, the accumulation of TGs in the liver is blocked [111], [112], [113], [114], [115]. In addition, the circadian factor Rev-erbα directly regulates the expression of NFIL3, which controls the circadian rhythm of lipid metabolism and regulates the absorption and output of lipids in gut epithelial cells [80]. In conclusion, controlling the circadian rhythm can feasibly regulate lipid metabolism and prevent lipid abnormalities (Fig. 2).
Epigallocatechin gallate (EGCG) is the most potent active component of tea polyphenols and has antibacterial, antioxidant, anti-inflammatory, and antitumor effects [143], [144], [145], [146]. These cell experimental findings indicate that Bmal1 plays an important role in the remission of insulin resistance caused by EGCG. EGCG improves insulin resistance by increasing the level of tyrosine-phosphorylated insulin receptor substrate-1, promoting transcription of the gene encoding glucose transporter protein 2, and activating phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) and the AMPK pathway through a BMAL1-dependent mechanism [147]. Moreover, cotreatment with EGCG significantly reduces the excessive release of reactive oxygen species (ROS) stimulated by glucosamine and prevents depletion of the mitochondrial membrane potential in HepG2 cells [147]. Another study suggested that the specific molecular mechanism may be that EGCG induces the functional oscillation of the circadian clock by controlling the sirt1-pgc1α ring in liver and adipose tissue, which leads to a decrease in fatty acid synthesis, an increase in β-oxidation in the liver and energy consumption in brown adipose tissue to reverse fat storage [148]. Although further investigation is needed to elucidate the molecular mechanism by which EGCG regulates the biological clock, these experiments offer a viable research direction for exploring the relationship between the circadian clock factor Bmal1 and insulin resistance.
Capsaicin, an active ingredient of chili peppers, has anti-inflammatory, analgesic, anesthetic and weight loss properties [149], [150], [151], [152]. Research has shown that capsaicin improves the oleic acid-induced accumulation of lipids and ROS imbalance in relation to BMAL1 in HepG2 cells [153]. The specific mechanism may involve the binding of capsaicin to the transient receptor potential vanilloid subfamily member 1 receptor on the cell membrane, leading to the influx of calcium ions, altering the membrane potential and energy expenditure of the mitochondria in the cell, and thereby causing the rhythmic oscillation of BMAL1 transcription in the cell [153]. This finding further highlights the importance of the rhythm of the circadian clock transcription factor BMAL1 on the normal rhythm of cellular lipid metabolism; however, the mechanism of action of capsaicin in vivo needs further investigation.
Chicoric acid, an important immune active ingredient, inhibits hepatocyte lipid accumulation and improves cellular morphological changes and hepatic lipid levels, and these processes rely on BMAL1 or CLOCK [154]. Although the mechanism of action of chicoric acid needs to be further verified, this finding still provides a research direction for reducing lipid accumulation by regulating the expression of BMAL1 or CLOCK.
Berberine, an alkaloid isolated from Coptis chinensis Franch., has positive biological effects, such as antibacterial activity, the ability to regulate blood lipids and potential for diabetes treatment [155]. In C3H10T1/2 brown fat cells, coptisine can dose-dependently upregulate fibroblast growth factor 21 (FGF21) gene expression and production. In addition, the upregulation of FGF21 expression in C3H10T1/2 brown fat cells is prevented by the silencing of Bmal1, suggesting that Bmal1 plays a linking role in the mechanism through which berberine regulates FGF21 [156]. FGF21, an important metabolic regulator of peripheral glucose tolerance and lipid homeostasis, activates receptors through the peroxisome proliferator γ pathway and upregulates the expression and secretion of adiponectin; these effects ultimately lower the blood sugar levels and improve insulin sensitivity and lipid abnormalities [157], [158]. However, the specific regulatory mechanism involved has not been fully elucidated, and further studies are needed to reveal the detailed mechanism by which berberine regulates the circadian rhythm and lipid metabolism.
Nobiletin is a flavonoid that exerts important anti-inflammatory, antitumor and neuroprotective effects [159], [160], [161]. The levels of key enzymes for intracellular adipogenesis, the mitochondrial respiratory complex and ROS show obvious diurnal fluctuations, and adipose tissue lipolysis is also correlated with the circadian rhythm. In primary mouse hepatocytes induced by palmitic acid, nobiletin can alleviate the remodeling of relatively shallow circadian rhythm oscillations, including the amplitudes of CLOCK and BMAL1, and thus ameliorate lipid abnormalities [162].
Resveratrol, a flavonoid polyphenolic compound with anti-inflammatory and antioxidant activities, prevents obesity and hepatic lipid metabolism disorders [163], [164], [165], [166]. Resveratrol does not directly influence the interaction of negative feedback factors on the CLOCK-BMAL1 complex but can negatively regulate transcriptional activation mediated by the CLOCK-BMAL1 complex by affecting the activity of Sirt1 in the nucleus [167]. Li et al. reported that resveratrol controls the free fatty acid-induced imbalance of intracellular lipid metabolism in a BMAL1-dependent manner in HepG2 cells [168]. This finding indicates that resveratrol may be used for the treatment of lipid metabolism disorders via regulation of the circadian rhythm.
Piperine, the main stimulatory component of black pepper, has multiple activities, including antioxidant, immunomodulatory, and antitumor properties [169], [170], [171]. Piperine influences factors related to hepatic lipid metabolism through the circadian factors BMAL1 and CLOCK, the redox status, and mitochondrial function. Piperine can enter the nucleus and affect the activity of the CLOCK-BMAL1 complex, activating the sterol regulatory element-binding protein-1c/peroxisome proliferator-activated receptor γ (SREBP-1c/PPARγ) and AMPK/AKT-mammalian target of rapamycin pathways [172]. Although some studies have shown that the regulatory mechanism of piperine on circadian rhythm factors depends on Trpv1, the specific molecular mechanism still needs to be further studied [173].
Grape seed procyanidin extract is a bioflavonoid with a unique molecular structure [174]. Studies have shown that grape seed procyanidin extract can act as a hepatic clock gene regulator to ameliorate liver-related metabolic diseases by regulating mitochondrial dynamics and microRNAs, and this effect is accompanied by the restoration of liver circadian mechanisms [175], [176]. Notably, grape seed procyanidin extract can restore the rhythm of liver clock factors (BMAL1, Per2, Cry1, and RoRα), and nocturnal treatment yields a more pronounced effect [176]. These findings also suggest the importance of the timing of drug administration in the regulation of circadian rhythms.
Apples are mature fruits of Malus pumila Mill., which is known as the king of fruits, and have the effects of generating saliva, moistening the lungs and alleviating the pain of muscles and bones [177], [178]. Apple polyphenols can affect transcription by affecting Per2 activity. Zeitgeber time 0 (ZT0)-ZT12 feeding changes the diurnal rhythm oscillation of TGs, whereas apple polyphenol extract treatment tends to reverse these changes. The underlying mechanism may be that apple polyphenol extract treatment reduces the amplitude of CLOCK, increases the amplitude of Per2, and ultimately significantly alters the transcriptional rhythms of genes involved in lipid metabolism and genes involved in bile acid metabolism [179]. Notably, peroxisome proliferator activated receptor γ coactivator (PGC-1)-1α and silent information regulator factor 2-related enzyme 1 (SIRT1), which act as links between lipid metabolism and the circadian clock, can influence the expression of BMAL1 and Per2 by regulating the redox status and energy expenditure [180].
Tea produced from the leaves of Camellia sinensis (L.) O. Kuntze, a traditional Chinese drink, has many physiological activities, such as antioxidant, lipid-lowering, and hypoglycemic activities [181], [182]. The circadian factor BMAL1 controls the number of mitochondria by regulating the mitotic fusion of mitochondria and alleviating mitochondrial dysfunction and thus regulates lipid metabolism by mediating the mitochondrial dynamics in HepG2 cells [183]. Tea polyphenols ameliorate the hydrogen peroxide-induced diurnal oscillations and restore phase shifts in the transcription and protein expression of circadian factors, which may be related to the transmission of light signals to the circadian rhythm [184].
Sea cucumber saponins are the major secondary metabolites in sea cucumber and can bind to sterol molecules on biofilms to form complexes [185]. The results of a previous study showed that sea cucumber saponin treatment at ZT16-ZT20 affects the expression of circadian factors (CLOCK, BMAL1, Cry1, Cry2 and Per2) in the brain. Similarly, during sleep (ZT20-ZT4), the trends of the expression of circadian factors (CLOCK, BMAL1, Cry1, Cry2 and Per2) and corticosterone also change [186]. Cortisol is thought to signal that the central clock, the SCN, synchronizes certain peripheral clocks [187]. Sea cucumber saponins may induce brain oscillations through humoral signals (corticosterone/thyroid hormone), and these oscillations alter PPARα, SREBP-1c, carnitine palmitoyl transferase (CPT) and fatty acid synthase (FASN), which are related to lipid metabolism, to improve glycolipid metabolism in rodents.
Oat is the seed of Avena sativa L., a natural grain rich in natural nutrients that contains dietary fibers such as cellulose, hemicellulose, pectin, and lignin polymers [188]. These dietary fibers cannot be directly absorbed by the human body but affect the gut microbiota. After oat fiber treatment, the species diversity index and total short-chain fatty acids, such as propionic acid, acetic acid and butyric acid, in the intestine of mice show a rhythm similar to that of normal mice. These small molecules enter the bloodstream, circulate to the liver, maintain the trough of CLOCK and BMAL1, restore the peak of Per1 and Cry1, and change the activity rhythm of liver cells, thus reducing insulin resistance and the serum total cholesterol (TC) and TG levels [189]. The study showed that the gut microbiota can affect hepatic circadian factor expression via the gut-liver axis.
Cyclocarya paliurus (Batal.) Ijinskaja, an ancient grass species, exerts anti-inflammatory and analgesic effects, enhances human immunity and has antioxidant and antiaging effects [190], [191]. Its aqueous extract contains a gut prebiotic that can improve persistent dark-induced gut microbiota disorders. These improvements in the gut microbiota increase the expression of BMAL1 and CLOCK in the liver, decrease the expression of Crys and Pers through the gut-liver axis, and change the activity rhythm of the liver, thus decreasing the serum TC, TG, and low-density lipoprotein (LDL) cholesterol indices [192]. This finding proves that metabolites such as short-chain fatty acids, as dialog molecules, connect the circadian rhythm of the gut microbiota with the circadian rhythm of liver lipid metabolism.
Poria cocos (Schw.) Wolf (P. cocos), the dry sclerotium of Polyporaceae fungi, plays a role in weight loss and fat reduction [193]. High-fat diet (HFD)-fed mice often exhibit special eating patterns. P. cocos extract dose-dependently reduces the amplitude of lipoprotein lipase and sterol regulatory element-binding transcription factors in the lipid synthesis pathway and restores the rhythm of the secretion of adiponectin, leptin and other related hormones [194]. Treatment with P. cocos extract balances the circadian rhythms of food intake and energy metabolism in obese mice, which results in the typical fat distribution and normal glucose and lipid metabolism.
Black ginger, the rhizome of Kaempferia parviflora, has anticancer, anti-inflammatory and antioxidant activities [195], [196], [197]. Researchers have shown that the in vitro administration of black ginger extract to NIH3T3 cells increases the amplitude of the circadian rhythm and prolongs the circadian rhythm, which results in phase delay [198]. Obese mice treated with black ginger show improved circadian exercise activity, a decreased body weight and reduced serum TG levels [199]. The mechanism by which black ginger improves lipid accumulation may depend on enhancing the expression of clock genes in adipocytes and inducing the expression of genes related to lipolysis.
Protective effects of natural products against cardiovascular system diseases through effects on the circadian rhythm
Natural products that treat hypertension through effects on the circadian rhythm
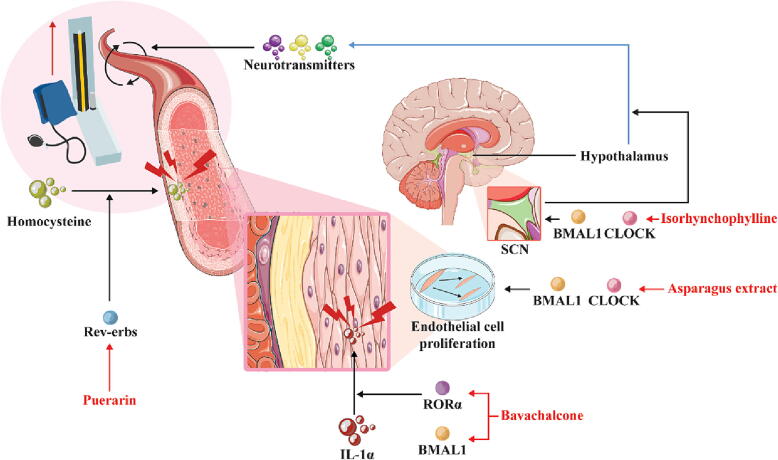
Blood pressure also has a certain physiological circadian rhythm [200]. A healthy blood pressure rhythm in the human body tends to manifest as a low levels at nighttime and high levels at daytime, i.e., low blood pressure levels at night and rapidly increases in the early morning before and after awakening, which may be related to changes in hormone levels [200]. Previous studies have shown that the levels of hormones such as renin and aldosterone can affect vascular function and thereby regulate blood pressure [201], [202]. In the adrenal glands of spontaneously hypertensive rats, the expression phase of the circadian factor Cry1 is advanced by approximately 4 h, and the amplitudes of Pers are decreased; these changes are consistent with changes in the rhythm of the secretion of plasma renin and aldosterone [203]. Another study found that BMAL1-knockout mice maintain a sustained hypotensive state and that the rhythm of their blood pressure activity is completely lost [66]. Moreover, the vascular cells of Per1-knockout mice show increased sensitivity to risk factors such as high salt and corticosteroid concentrations, leading to abnormal hypertension [78]. The circadian rhythm plays an undeniable role in blood pressure regulation, which may be a key factor in slowing the progression of hypertension (Fig. 3).
Fig. 3.
Protective effects of natural products against cardiovascular system diseases through effects on the circadian rhythm. The red solid line represents the targeting effect of natural products, the blue solid line represents the secretion process, and the black solid and black virtual solid lines represent transport and action processes, respectively. Abbreviations: BMAL1, brain and muscle Arnt-like 1; CLOCK, circadian locomotor output cycles kaput; Rev-erbs, nuclear hormone receptors; RORα, retinoic acid-related orphan receptor α; ROS, reactive oxygen species; SCN, superior optic chiasm nucleus. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Puerarin is an isoflavone that has sedative and vascular-protective effects [204], [205]. The metabolites resulting from high blood levels of homocysteine affect smooth muscle cells, leading to vascular dysfunction, which is a risk factor for hypertension [206], [207], [208], [209]. Puerarin targets a key circadian nuclear receptor, Rev-erbα, and Rev-erbα antagonism reduces hyperhomocysteinemia and promotes ammonia clearance [210], [211]. In addition, the administration of puerarin at ZT10 yields a more potent pharmacological effect, which is consistent with the circadian expression of Rev-erbα [211], [212]. This finding further encourages the establishment of a time-based therapy with drugs that target circadian rhythm-related factors, such as puerarin, to optimize their efficacy.
Isorhynchophylline is derived from Uncaria rhynchophylla (Miq.) Miq. ex Havil and exerts anti-inflammatory, antihypertensive and neuroprotective effects [213], [214], [215]. Recent studies have found that the antihypertensive effect of isorhynchophylline on hypertension occurs not only through regulation of the rhythm of serum neurotransmitters but also by acting on CLOCK and BMAL1 of the central circadian clock [216]. Moreover, metabolomics have confirmed that the rhythmic changes in serum neurotransmitters are consistent with the changes in hypothalamic rhythm-related genes [217].
Natural products that treat atherosclerosis through effects on the circadian rhythm
Lipid metabolism and the inflammatory processes of atherosclerosis exhibit their own rhythms of activity [139], [218]. Impaired expression of circadian rhythm factors can promote atherosclerosis [219]. For example, patients with atherosclerosis exhibit reduced expression of the plasma circadian transcription factor BMAL1, increased vascular inflammation, and elevated oxidative stress [220], [221]. The specific mechanism underlying these effects may be that the downregulation of BMAL1 expression promotes p65 phosphorylation, which further enhances NF-κB signaling and elevates oxidative stress and inflammatory responses in human aortic endothelial cells [10]. Interestingly, this process is reversed by overexpression of the circadian factor Cry1, which downregulates Toll receptor expression, inhibits NF-κB activity, and further reduces the levels of proinflammatory factors such as IL-6 and TNF-α [74], [75]. In addition, BMAL1 may be involved in atherosclerosis through other pathways, such as affecting the energy supply and endothelial function by influencing the PI3K/AKT pathway [222], [223]. In conclusion, the process of atherosclerosis is closely related to the circadian rhythm, and improving the circadian rhythm may be a new target in the treatment of atherosclerosis (Fig. 4).
Fig. 4.
Protective effects of natural products against nervous system diseases through effects on the circadian rhythm. The red solid line represents the targeting effect of natural products, the blue solid line represents the secretion process, and the black solid and black virtual solid lines represent transport and action processes, respectively. Abbreviations: Crys, cryptochromes; Pers, period circadian proteins; SCN, superior optic chiasm nucleus. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Bavachalcone, a natural component, increases the RORα transcript and protein levels and enhances the circadian amplitude of BMAL1 transcription in a dose-dependent manner [224]. Changes in RORα lead to the inhibition of aging and the expression of the inflammatory factor IL-1α in endothelial cells to protect the cardiovascular system [225], [226]. These findings provide new evidence showing that natural products provide vascular protection through circadian rhythms.
Asparagus extract significantly reverses the destruction of nuclear enriched abundant transcript 1, CLOCK and BMAL1 induced by trimethylamine oxide (TMAO) or acrolein and promotes endothelial cell proliferation, thereby reducing the risk of atherosclerosis [227], [228].
Protective effects of natural products against nervous system diseases through effects on the circadian rhythm
Natural products that treat Alzheimer’s disease through effects on the circadian rhythm
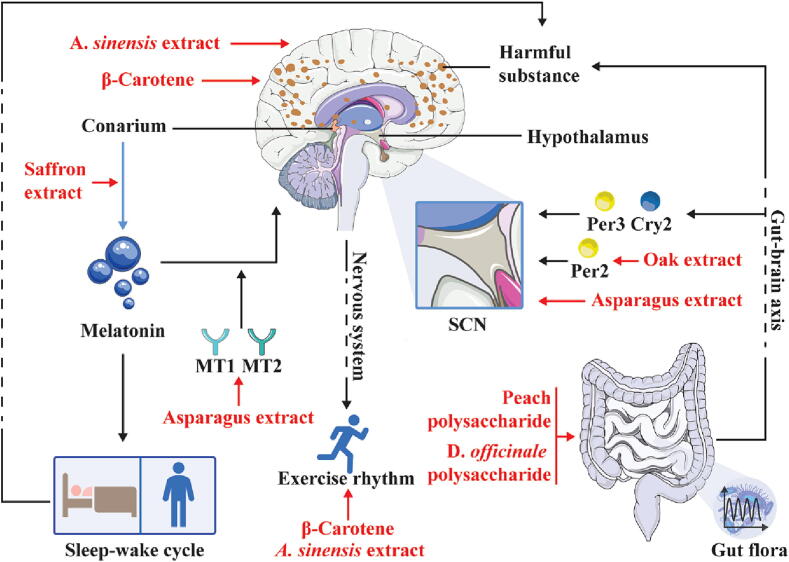
AD is characterized by impaired cognitive function and may stem from neuroinflammation and pathogenic protein aggregates, such as amyloid-beta (Aβ) and tau proteins in neurons [229]. Many studies have shown that the AD process and sleep/wake cycle disorders, such as biological clock disorders, are closely related each other [230], [231], [232], and this relationship may be related to the fact that a lack of sleep induces increases in the amyloid-beta and tau protein levels and their aggregation [233], [234]. In addition, at the circadian molecular level, AD patients exhibit abnormal expression of the circadian rhythm factors BMAL1, CLOCK, and Cry1 [235], [236], [11]. Restoration of the sleep and circadian rhythms in AD patients may represent a viable strategy for early intervention to delay the disease process (Fig. 3).
Asparagus (Asparagus officinalis L.) has been proven to have many physiological benefits, such as antioxidant and antitumor effects and the ability to lower the blood lipid and glucose levels [237], [238], [239]. Studies have shown that asparagus extract can significantly reduce the pathological progression of cognitive impairment, and this process induced by asparagus extract significantly reduces Aβ accumulation in the brain, significantly increases the number of neurons in the SCN and normalizes the expression of melatonin receptor 1 (MT1) and MT2 [240]. Unfortunately, the regulatory effect of asparagus on circadian factors in AD patients may require further investigation.
Peach, a mature fruit of Prunus persica (L.) Batsch, is a delicious fruit that is rich in sugars, mainly glucose, fructose, sucrose and xylose [241]. Peach polysaccharide administration improves the structural disorder of the gut microbiota in mice induced by persistent darkness, which indicates that peach polysaccharide affects the circadian rhythm factors Cry2 and Per3 in the hypothalamus through the gut–brain axis. In addition, peach polysaccharide improves the circadian rhythm of astrocytes and oligodendrocytes in the hypothalamus and increases the amplitude of the rhythm of the expression of neuroprotective genes, such as basic proteins [242].
Dendrobium officinale Kimura & Migo (D. officinale), a nourishing traditional Chinese medicine, enhances immunity, has antiaging effects, and lowers the blood sugar levels [243], [244], [245]. D. officinale polysaccharide treatment reshapes the gut microbiota disorders caused by circadian rhythm disorders and improves gut barrier dysfunction, and these effects reduce the invasion of endotoxins into the blood and brain [246]. The resulting decrease in the endotoxin levels weakens the levels of metabolites associated with improved cognitive function. D. officinale polysaccharide provides neuroprotection against circadian rhythm disorders and related cognitive dysfunction.
Natural products that treat depression through effects on the circadian rhythm
The available evidence suggests an interaction between depression and environmental disruptions to the circadian clock (e.g., shift work) [247], [248], [249]. In addition, anxiety behavior exhibits a clear circadian pattern regulated by the sleep/wake cycle and circadian rhythm oscillators, and these behaviors are elevated in mice lacking the cycle clock gene [250]. Patients with depression typically exhibit abnormal circadian rhythms, including emotional instability, daytime fatigue, and sleep deprivation [251]. Moreover, patients with endogenous depression exhibit an obvious rhythm of “severe in the morning and mild at night”, and this rhythm may be associated with abnormal expression of the circadian factor Per3 [252], [253]. Correcting these rhythmic disorders may aid the development of new treatment strategies for depression (Fig. 4).
β-Carotene, one of the most common vitamin A supplements, plays a protective role in cell development and reproductive function [254]. β-Carotene reverses the adverse reactions, including the amplitude and translocation of behavior and adverse factors (such as glutathione), in rats under stress conditions [255]. Unfortunately, this study did not involve specific molecules related to the circadian rhythm, and further experimental exploration is needed to determine the detailed pharmacological mechanism involved.
Oak (Quercus spp.) extracts have been shown to exhibit antioxidant activities [256]. Urolithin A and ellagic acid, which are metabolites of oak extracts, delay the temporary phase of the Per2 rhythm in SCN expansions [257]. In contrast, oak extract reverses the torpor of mice and increased their daily play frequency [257]. These data suggest that oak extract may act as a novel ex vivo circadian rhythm regulator to enhance biological activity, and this enhanced activity may be a positive factor for depression.
Saffron is the stigma of Crocus sativus L., a valuable traditional Chinese medicine with potential medicinal value in the prevention of cardiovascular, cerebrovascular, liver and kidney diseases [258], [259], [260], [261]. Saffron supplementation may improve the sleep quality and enhance the mood upon waking by modulating the circadian rhythm of melatonin secretion [262]. These findings may provide an important theoretical basis for the use of natural products to target the circadian rhythm and specifically alleviate the “early awakening” and “severe in the morning and mild at night” symptoms of patients with depression.
Agarwood, whose Latin name is Aquilaria sinensis (A. sinensis), is a plant with anti-inflammatory activities [263]. An experimental study of the circadian rhythm showed that the treatment of zebrafish with 100 ppm agarwood extract for 8 weeks effectively reversed the circadian rhythm of abnormal movement and reduced the anxiety levels [264]. The study provided evidence revealing the relationship between the circadian rhythm and anxiety, and the findings suggest that agarwood may be an effective therapeutic drug.
Conclusions and future outlook
The circadian rhythm is the regular cycle of physiological functions established by organisms to adapt to light/dark changes in the natural environment, and these functions include bacterial luminescence, plant photosynthesis and animal foraging [2], [4], [265]. Studies have found that the stability of metabolic homeostasis, energy expenditure, cell proliferation, and neural regulation in organisms depends on the circadian rhythm of the organism itself [266], [267], [268], [269], [270], [271]. Therefore, targeting the circadian rhythm may have a more comprehensive positive effect on the recovery of biological functions. Here, we demonstrate the remarkable capacity of natural products to modulate circadian rhythms, which play a crucial role in metabolic system, cardiovascular system, and nervous system diseases. Thus, the potential of natural products to modulate the regulation of circadian rhythms enhances their likelihood of exerting a favorable impact on the restoration of biological functions.
To date, studies of the circadian rhythm and the biological health status have achieved some progress [272]. The changes in circadian rhythm factors, biological hormones, and circadian rhythms of specific gut microbiota induced by natural product interventions play a crucial role in regulating the physiological health status, suggesting potential prospects for treating diseases of the metabolic system, cardiovascular system, and nervous system. The unique and complex structure of natural products indicates that their actions have a wide range of targets (Table 1 and Table 2). Using natural products as adjuvant therapeutic agents for the multitargeted systemic regulation of the human circadian rhythm may produce better results in the treatment of patients with a variety of chronic diseases [273], [22]. However, many problems remain to be solved. On the one hand, more experimental data are needed to elucidate the mechanisms of a considerable number of natural products. Therefore, the development of a comprehensive research method is very important to capture the specific mechanism by which natural products regulate the circadian rhythm to restore biological functions. In addition, the circadian rhythms differ between humans and laboratory animals. When natural products are introduced into an application or recommended diet based on the circadian rhythm, additional assessments are needed to ensure their efficacy and safety.
Table 1.
Protective effects of monomers on diseases through regulation of the circadian rhythm.
| Disease | Natural product | Experimental model | Natural active ingredient dose or concentration/route of exposure | Toxic compound dose or concentration/route of exposure | Finding(s) | Reference(s) |
|---|---|---|---|---|---|---|
| Dysglycemia | Genistein | Female C57BL/6 mice | 2 g/kg in food for 8 weeks | High-fat diet for 8 weeks | Recovery amplitude | [117] |
| Dyslipidemia | Epigallocatechin gallate | HepG2 cells | 50 μM for 18 h | High-sugar medium | Recovery amplitude | [148], [147] |
| C57BL/6J mice | 2 g/L in drinking water for 16 weeks | High-fat diet and 10 % fructose in drinking water for 16 weeks | Recovery amplitude | |||
| Capsaicin | HepG2 cells | 50 μM capsaicin for 18 h | 20 mM glucosamine | Recovery amplitude | [153] | |
| Chicoric acid | HepG2 cells | 200 μM for 24 h | 100 µM free fatty acid (OA:PA = 2:1) for 24 h | Recovery amplitude | [154] | |
| Berberine | C3H10T1/2 cells | 10 μM for 20 h | Compound C (AMPK inhibitor) for 15 min | Recovery amplitude | [156] | |
| Male C57BL/6 mice | 5 mg/kg daily | High-fat diet | Recovery amplitude | |||
| Nobiletin | Primary hepatocytes | 200 μM for 4 h | 0.4 mM palmitate for 18 h | Recovery amplitude | [162] | |
| Resveratrol | HepG2 cells | 100 µM for 6 h | 100 µM free fatty acid (OA: PA = 2:1) for 20 h | Recovery amplitude | [168], [167] | |
| Piperine | HepG2 cells | 25, 50, 75 µM for 24 h | 40 mM oleic acid | Recovery amplitude, recovery phase | [172] | |
| Hypertension | Puerarin | Wild-type male C57BL/6 mice | 50, 100, 200 mg/kg, daily for 1 week, i.p. | 0.5 % methionine in drinking water for 8 weeks | Recovery amplitude | [211], [212] |
| Isorhynchophylline | Male Wistar Kyoto rats and rats with spontaneous hypertension | 0.3 mg/kg for 6 weeks, i.p. | / | Recovery phase, recovery cycle | [216] | |
| Arteriosclerosis | Bavachalcone | Human umbilical vein endothelial cells | 20 µM for 6 h | 0.3 % serum DMEM for 16 h | Recovery amplitude | [224] |
| Depression | β-Carotene | Male albino rats | 10 mg/kg for 21 days, i.p. | chronic unpredictable stress for 21 days | Recovery amplitude, recovery phase | [255] |
Abbreviations: AMPK, intracellular cyclic adenosine 5′-monophosphate-activated protein kinase; OA, oleic acid; PA, palmitic acid; DMEM, Dulbecco’s modified Eagle’s medium.
Table 2.
Protective effects of extracts on diseases through regulation of the circadian rhythm.
| Disease | Natural product | Source | Experimental model | Natural active ingredient dose or concentration/route of exposure | Toxic compound dose or concentration/route of exposure | Finding(s) | Reference |
|---|---|---|---|---|---|---|---|
| Dysglycemia | Lycium barbarum polysaccharide | Fruits of Lycium. L barbarum | Male Wistar rats | 5, 10 mg/kg, daily for 4 weeks, oral | High-sucrose/high-fat diet for 4 weeks, intraperitoneal injection of 0.5 % streptozotocin solution at a dose of 50 mg/kg weight | Recovery amplitude | [126] |
| Tilapia collagen peptide mixture TY001 | / | Male C57BL/6 mice | 30 g/L in drinking water for 30 days | 0.25 mg/kg LPS daily for 9 days, i.p. | Recovery amplitude, recovery phase | [132] | |
| Pomegranate juice | Mature fruits of Punica granatum L. | Humans (impaired fasting glucose) | 1.5 ml/kg | / | Recovery amplitude, recovery phase | [136] | |
| Dyslipidemia | Grape seed procyanidin extract | Grape seeds and skin | Male Fischer 344 rats | 25 mg/k, daily for 4 weeks, oral | Cafeteria diet | Recovery amplitude | [176] |
| Apple polyphenol | Mature fruits of Malus pumila Mill. | Male C57BL/6 mice | 500 mg/kg, daily for 5 weeks, oral | Daytime feeding | Recovery amplitude | [179] | |
| Tea polyphenol | Leaves of Camellia sinensis (L.) O. Kuntze | HepG2 cells | 40 μg/mL for 12 h | 200 μM H2O2 for 12 h | Recovery amplitude, recovery phase | [183] | |
| Sea cucumber saponin | Body wall of Stichopus japonicus | Male ICR mice | 0.03 % in food for 2 weeks | Night feeding for 2 weeks | Recovery amplitude, recovery phase | [187] | |
| Oat dietary fiber | Seed of Avena sativa L. | Male C57BL/6 mice | 0.8 % in food for 21 weeks | High-fat diet for 21 weeks | Recovery amplitude | [189] | |
| Cyclocarya paliurus (Batal.) Ijinskaja aqueous extract | / | Germ-free male C57BL/6 mice | 100 mg/kg, daily for 4 weeks, oral | Constant darkness for 4 weeks | Recovery amplitude, recovery phase | [192] | |
| Poria cocos extract | Dried sclerotium of Poria cocos (Schw.) Wolf | Male C57BL/6 mice | 666.67 mg/kg (food), daily for 10 weeks | High-fat diet for 8 weeks | Recovery amplitude, recovery phase | [194] | |
| Black ginger extract | Rhizome of Kaempferia parviflora | NIH3T3 cells | 0.5 mg/mL for 4 h | / | Recovery amplitude, recovery phase | [198] | |
| Male C57BL/6 mice | 100 mg/kg, daily for 12 weeks | High-fat diet for 12 weeks | Recovery amplitude, recovery phase | ||||
| Arteriosclerosis | Asparagus extract | Bottom-stem part of Asparagus officinalis L. | Human umbilical vein endothelial cells | 5 ng/ml for 24 h | 200 μM TMAO for 24 h | Recovery amplitude | [227], [228] |
| Alzheimer’s disease | Asparagus extract | Bottom-stem parts of Asparagus officinalis L. | Male SAMP8 and senescence-accelerated-resistant mice | 200, 1000 mg/kg, daily for 12 weeks | / | Recovery amplitude | [240] |
| Peach polysaccharide | Mature fruits of Prunus persica (L.) Batsch | Male C57BL/6 mice | 100 mg/kg (food), daily for 4 weeks, oral | Constant darkness for 4 weeks | Recovery amplitude | [242] | |
| Dendrobium officinale polysaccharide | Fresh stems of Dendrobium officinale Kimura & Migo | Male C57BL/6 mice | 200 mg/kg, daily for 4 weeks, oral | Constant darkness for 4 weeks | Recovery amplitude | [246] | |
| Depression | Oak extract | Quercus spp. | Mouse embryonic fibroblasts | 10 ∼ 100 μM urolithin A or 10 ∼ 50 μM ellagic acid for 30 min | / | Recovery amplitude, recovery phase | [257] |
| Male C57BL/6 mice | 0.05 %, 0.5 % oak extracts in food | Constant darkness for 15 days | Recovery phase | [262] | |||
| Saffron extract | Stigma of Crocus sativus L. | Humans (sleep disorders) | 14 or 28 mg, daily for 4 weeks, oral | / | Recovery amplitude, recovery phase | ||
| Agarwood water extract | Aquilaria agallocha | AB strain zebrafish (Danio rerio) | 100 ppm for 8 weeks | Exposure to predator stress | Recovery amplitude | [264] |
Abbreviations: LPS, lipopolysaccharide; TMAO, trimethylamine oxide.
Relevant studies have shown that the gut microbiota may be the key to regulating the circadian rhythms of certain natural products [21], [228], [189], [242]. In normal mice, the abundance of Firmicutes and Anaeroplasma shows a clear circadian rhythm, with Firmicutes peaking at the end of the feeding phase and Anaeroplasma peaking during the fasting phase [274], [275]. However, when the circadian rhythm of the gut microbiota structure is disrupted, the circadian rhythm of gut microbiota metabolites is reset [276], [277], [278], [279]. Through the blood circulation, gut microbiota metabolites further disrupt the rhythm of gene expression in the host’s proximal and distal intestinal organs, promoting the development of a variety of diseases, such as cardiovascular disease [280], [281], [282], [283]. Therefore, it is reasonable to believe that the gut microbiota may be the key to the regulation of the circadian rhythm throughout the whole organism by certain natural products.
As an under-researched field, the circadian rhythm may become an important research topic in the future, and the related findings provide a new molecular basis for the treatment of chronic diseases using natural products. This work offers a reference for exploring the molecular mechanism of natural products targeting the circadian rhythm, provides a feasible strategy for artificially intervening with the circadian rhythm to restore biological functions, and provides a new direction as well as perspective for the treatment of various chronic diseases, including those of the metabolic system, cardiovascular system, and nervous system.
Compliance with ethics requirements
This article does not describe any studies with human or animal subjects.
Funding
This work was supported by National Key R&D Program of China (NO. 2022YFC2010104, China), Shandong Province Key R&D Program (NO. 2021SFGC1205, China), the Taishan School Foundation of Shandong Province (NO. tsqn202312184, China), the Shenzhen Longgang District Science and Technology Development Special Fund (NO. LGWJ2021-142, China), the Natural Science Foundation of Shandong Province (NO. ZR2022MH306, ZR2023MH263, China), the China Postdoctoral Science Foundation (NO. 2023M733918, 2023T160729, China), and the University Youth Innovation Team of Shandong Province (NO. 2022KJ229, China).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Biographies

Meiling Xin is currently a master’s student in the School of Life and Medicine, Shandong University of Technology. She focuses on the pharmacodynamic analysis of natural products; the separation, extraction and identification of active ingredients of traditional Chinese medicine; and the multi-component analysis of endocrine disease treatment.

Fangjie Bi is the Deputy Chief Physician of Zibo Central Hospital, the Assistant Director of the Second Ward of Cardiovascular Internal Medicine, a doctor of medicine, and a master's supervisor. He has been involved in the clinical diagnosis, treatment and scientific research of cardiovascular diseases for a long time and has expertise in coronary intervention treatment, permanent pacemaker implantation, etc.

Chao Wang received his PhD from KyungHee University and is a teacher in the School of Life Sciences and Medicine, Shandong University of Technology. His research direction is the biotransformation of natural active substances and the biosynthesis and application of metal nanoparticles.

Yuhong Huang is currently a master's student in the School of Life Sciences of Yangtze University. She is committed to the development of active substances in traditional Chinese medicine and the study of the pathogenesis of metabolic diseases.

Yujia Xu received his MD from Jiamusi University and works in Zibo Central Hospital. His research direction is the transformation and application of ultrasonic medicine in the prevention and treatment of different diseases.

Shufei Liang is an academic master’s student of the College of Life and Medicine, Shandong University of Technology. She is mainly focused on the pharmacodynamic analysis of natural products; the isolation, extraction and identification of active ingredients in Chinese medicine; and mechanistic research and analyses of metabolic disease treatment.

Tianqi Cai is a master's degree student in the College of Life and Medicine, Shandong University of Technology. She is mainly focused on the pharmacodynamic analysis of natural products; the isolation, extraction and identification of active ingredients in Chinese medicine; and mechanistic research and analyses of metabolic disease treatment.

Xiaoxue Xu, master's degree student in College of Life and Medicine, Shandong University of Technology. Her research areas are the pharmacodynamic analysis of natural products; the isolation, extraction and identification of active ingredients in Chinese medicine; and mechanistic research and analyses of metabolic disease treatment.

Ling Dong is a master's degree student at the College of Life and Medicine, Shandong University of Technology. She is mainly focused on the pharmacodynamic analysis of natural products; the isolation, extraction and identification of active ingredients in Chinese medicine; and mechanistic research and analyses of metabolic disease treatment.

Tianxing Li is a doctoral student of the Institute of Basic Theory for Chinese Medicine, China Academy of Chinese Medical Sciences, Beijing 100700, China. Tianxing is mainly interested in constitution intervention and evaluation, the correlation between constitution and disease and the prevention and control of chronic diseases, as well as mechanistic research and analyses of metabolic disease treatment.

Xueke Wang is associated with the Second Clinical Medical College of Henan University of Traditional Chinese Medicine, majoring in internal medicine of traditional Chinese medicine. She is mainly focused on mechanistic research of the effective components of traditional Chinese medicine in the treatment of metabolic diseases.

Yini Fang is a doctoral student of the Basic Medical College, Zhejiang Chinese Medical University. Yini is mainly focused on constitution intervention and evaluation, the correlation between constitution and disease and the prevention and control of chronic diseases, as well as mechanistic research and analyses of metabolic disease treatment.

Zhengbao Xu received his PhD from Lanzhou University and is a teacher in the School of Life Sciences and Medicine, Shandong University of Technology. He is committed to organic synthesis methodology and drug synthesis chemistry and exploratory research on green drug synthesis methods.

Meng Wang received his PhD from Zhejiang University and is a teacher in the School of Life Sciences and Medicine, Shandong University of Technology. His research mainly focuses on the construction of new nano drug delivery systems; drug delivery, diagnosis and treatment of tumors; and overcoming multidrug resistance in tumors.

Xinhua Song is a master’s supervisor and associate professor in the School of Life Sciences and Medicine, Shandong University of Technology. She took a refresher course in the Animal Laboratory of the School of Life Sciences, Peking University, and the State Key Laboratory of Fungi, Institute of Microbiology, Chinese Academy of Sciences. She is a National Secondary Public Dietitian and the director of the Shandong Special Medical Food Alliance.

Yanfei Zheng received his PhD from Beijing University of Chinese Medicine and is an associate professor in the National Institute of Constitution and Prevention of Diseases of Traditional Chinese Medicine, Beijing University of Chinese Medicine. His research direction is the transformation and application of traditional Chinese medicine constitutions and the molecular mechanisms of traditional Chinese medicine in treating reproductive diseases.

Wenlong Sun received his PhD from Dalian University of Technology and is an associate professor in the School of Life Sciences and Medicine, Shandong University of Technology. His research direction is the transformation and application of traditional Chinese medicine constitutions and the molecular mechanisms of traditional Chinese medicine in treating endocrine diseases.

Lingru Li is a professor in the National Institute of Constitution and Prevention of Diseases of Traditional Chinese Medicine, Beijing University of Chinese Medicine. She has presided over two National Natural Science Foundations of China, and one National Key Research and Development Program of China. Her research direction is the prevention and treatment of metabolic diseases and cardiovascular and cerebrovascular diseases. She is the guest editor of FRONT ENDOCRINOL and an editorial board member of J Tradit Chin Med Sci.
Contributor Information
Xinhua Song, Email: 892442572@qq.com.
Yanfei Zheng, Email: yanfei_z@163.com.
Wenlong Sun, Email: 512649113@qq.com.
Lingru Li, Email: lilingru912@163.com.
References
- 1.Albrecht U., Eichele G. The mammalian circadian clock. Curr Opin Genet Dev. 2003;13:271–277. doi: 10.1016/s0959-437x(03)00055-8. [DOI] [PubMed] [Google Scholar]
- 2.Turek F.W. Circadian rhythms. Horm Res. 1998;49:109–113. doi: 10.1159/000023155. [DOI] [PubMed] [Google Scholar]
- 3.Asher G., Schibler U. Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab. 2011;13:125–137. doi: 10.1016/j.cmet.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Shimizu I., Yoshida Y., Minamino T. A role for circadian clock in metabolic disease. Hypertens Res. 2016;39:483–491. doi: 10.1038/hr.2016.12. [DOI] [PubMed] [Google Scholar]
- 5.Gnocchi D., Bruscalupi G. Circadian Rhythms and Hormonal Homeostasis: Pathophysiological Implications. Biology (Basel) 2017:6. doi: 10.3390/biology6010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laothamatas I., Rasmussen E.S., Green C.B., Takahashi J.S. Metabolic and chemical architecture of the mammalian circadian clock. Cell Chem Biol. 2023;30:1033–1052. doi: 10.1016/j.chembiol.2023.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaz J.R., Silva L.M., Stergiou N. Stride-to-Stride Fluctuations of Human Gait Are Affected By Chronobiology: An Exploratory Study. Adv Biol (Weinh) 2023;7:e2200235. doi: 10.1002/adbi.202200235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buijs R.M., Kalsbeek A. Hypothalamic integration of central and peripheral clocks. Nat Rev Neurosci. 2001;2:521–526. doi: 10.1038/35081582. [DOI] [PubMed] [Google Scholar]
- 9.Manoogian E.N.C., Panda S. Circadian rhythms, time-restricted feeding, and healthy aging. Ageing Res Rev. 2017;39:59–67. doi: 10.1016/j.arr.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie M., Tang Q., Nie J., Zhang C., Zhou X., Yu S., et al. BMAL1-Downregulation Aggravates Porphyromonas Gingivalis-Induced Atherosclerosis by Encouraging Oxidative Stress. Circ Res. 2020;126:e15–e29. doi: 10.1161/CIRCRESAHA.119.315502. [DOI] [PubMed] [Google Scholar]
- 11.Niu L., Zhang F., Xu X., Yang Y., Li S., Liu H., et al. Chronic sleep deprivation altered the expression of circadian clock genes and aggravated Alzheimer's disease neuropathology. Brain Pathol. 2022;32:e13028. doi: 10.1111/bpa.13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jokl E., Llewellyn J., Simpson K., Adegboye O., Pritchett J., Zeef L., et al. Circadian Disruption Primes Myofibroblasts for Accelerated Activation as a Mechanism Underpinning Fibrotic Progression in Non-Alcoholic Fatty Liver Disease. Cells. 2023:12. doi: 10.3390/cells12121582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khosravipour M., Khanlari P., Khazaie S., Khosravipour H., Khazaie H. A systematic review and meta-analysis of the association between shift work and metabolic syndrome: The roles of sleep, gender, and type of shift work. Sleep Med Rev. 2021;57 doi: 10.1016/j.smrv.2021.101427. [DOI] [PubMed] [Google Scholar]
- 14.Xiao Z., Xu C., Liu Q., Yan Q., Liang J., Weng Z., et al. Night Shift Work, Genetic Risk, and Hypertension. Mayo Clin Proc. 2022;97:2016–2027. doi: 10.1016/j.mayocp.2022.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Stowe T.A., McClung C.A. How Does Chronobiology Contribute to the Development of Diseases in Later Life. Clin Interv Aging. 2023;18:655–666. doi: 10.2147/CIA.S380436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rehman S.U., Ikram M., Ullah N., Alam S.I., Park H.Y., Badshah H., et al. Neurological Enhancement Effects of Melatonin against Brain Injury-Induced Oxidative Stress, Neuroinflammation, and Neurodegeneration via AMPK/CREB Signaling. Cells. 2019:8. doi: 10.3390/cells8070760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bottalico L.N., Weljie A.M. Cross-species physiological interactions of endocrine disrupting chemicals with the circadian clock. Gen Comp Endocrinol. 2021;301 doi: 10.1016/j.ygcen.2020.113650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dutcher C.D., Dowd S.M., Zalta A.K., Taylor D.J., Rosenfield D., Perrone A., et al. Sleep quality and outcome of exposure therapy in adults with social anxiety disorder. Depress Anxiety. 2021;38:1182–1190. doi: 10.1002/da.23167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eide P.K., Vinje V., Pripp A.H., Mardal K.A., Ringstad G. Sleep deprivation impairs molecular clearance from the human brain. Brain. 2021;144:863–874. doi: 10.1093/brain/awaa443. [DOI] [PubMed] [Google Scholar]
- 20.Vijayakrishnan Nair V., Kish B.R., Chong P.L., Yang H.S., Wu Y.C., Tong Y., et al. Neurofluid coupling during sleep and wake states. Sleep Med. 2023;110:44–53. doi: 10.1016/j.sleep.2023.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Satyanarayanan S.K., Su H., Lin Y.W., Su K.P. Circadian Rhythm and Melatonin in the Treatment of Depression. Curr Pharm Des. 2018;24:2549–2555. doi: 10.2174/1381612824666180803112304. [DOI] [PubMed] [Google Scholar]
- 22.Flanagan A., Bechtold D.A., Pot G.K., Johnston J.D. Chrono-nutrition: From molecular and neuronal mechanisms to human epidemiology and timed feeding patterns. J Neurochem. 2021;157:53–72. doi: 10.1111/jnc.15246. [DOI] [PubMed] [Google Scholar]
- 23.Lages M., Barros R., Carmo-Silva S., Guarino M.P. Linking dietary intake, circadian biomarkers, and clock genes on obesity: A study protocol. Front Nutr. 2023;10:1134789. doi: 10.3389/fnut.2023.1134789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galloway G.P., Buscemi R., Coyle J.R., Flower K., Siegrist J.D., Fiske L.A., et al. A randomized, placebo-controlled trial of sustained-release dextroamphetamine for treatment of methamphetamine addiction. Clin Pharmacol Ther. 2011;89:276–282. doi: 10.1038/clpt.2010.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voican C.S., Corruble E., Naveau S., Perlemuter G. Antidepressant-induced liver injury: a review for clinicians. Am J Psychiatry. 2014;171:404–415. doi: 10.1176/appi.ajp.2013.13050709. [DOI] [PubMed] [Google Scholar]
- 26.Koehn F.E., Carter G.T. The evolving role of natural products in drug discovery. Nat Rev Drug Discov. 2005;4:206–220. doi: 10.1038/nrd1657. [DOI] [PubMed] [Google Scholar]
- 27.Liu P., Chen Y., Xiao J., Zhu W., Yan X., Chen M. Protective effect of natural products in the metabolic-associated kidney diseases via regulating mitochondrial dysfunction. Front Pharmacol. 2022;13:1093397. doi: 10.3389/fphar.2022.1093397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng J., Zheng Y., Guo M., Ares I., Martínez M., Lopez-Torres B., et al. Oxidative stress, the blood–brain barrier and neurodegenerative diseases: The critical beneficial role of dietary antioxidants. Acta Pharm Sin B. 2023 doi: 10.1016/j.apsb.2023.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Machado I.F., Miranda R.G., Dorta D.J., Rolo A.P., Palmeira C.M. Targeting Oxidative Stress with Polyphenols to Fight Liver Diseases. Antioxidants (Basel) 2023:12. doi: 10.3390/antiox12061212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narishige S., Kuwahara M., Shinozaki A., Okada S., Ikeda Y., Kamagata M., et al. Effects of caffeine on circadian phase, amplitude and period evaluated in cells in vitro and peripheral organs in vivo in PER2::LUCIFERASE mice. Br J Pharmacol. 2014;171:5858–5869. doi: 10.1111/bph.12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stowie A.C., Amicarelli M.J., Prosser R.A., Glass J.D. Chronic cocaine causes long-term alterations in circadian period and photic entrainment in the mouse. Neuroscience. 2015;284:171–179. doi: 10.1016/j.neuroscience.2014.08.057. [DOI] [PubMed] [Google Scholar]
- 32.Shin J.C., Jung H.Y., Harikishore A., Kwon O.D., Yoon H.S., Kim K.T., et al. The flavonoid myricetin reduces nocturnal melatonin levels in the blood through the inhibition of serotonin N-acetyltransferase. Biochem Biophys Res Commun. 2013;440:312–316. doi: 10.1016/j.bbrc.2013.09.076. [DOI] [PubMed] [Google Scholar]
- 33.He B., Nohara K., Park N., Park Y.S., Guillory B., Zhao Z., et al. The Small Molecule Nobiletin Targets the Molecular Oscillator to Enhance Circadian Rhythms and Protect against Metabolic Syndrome. Cell Metab. 2016;23:610–621. doi: 10.1016/j.cmet.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohawk J.A., Green C.B., Takahashi J.S. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohawk J.A., Takahashi J.S. Cell autonomy and synchrony of suprachiasmatic nucleus circadian oscillators. Trends Neurosci. 2011;34:349–358. doi: 10.1016/j.tins.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peschke E., Muhlbauer E., Musshoff U., Csernus V.J., Chankiewitz E., Peschke D. Receptor (MT(1)) mediated influence of melatonin on cAMP concentration and insulin secretion of rat insulinoma cells INS-1. J Pineal Res. 2002;33:63–71. doi: 10.1034/j.1600-079x.2002.02919.x. [DOI] [PubMed] [Google Scholar]
- 37.Alonso-Vale M.I., Andreotti S., Mukai P.Y., Borges-Silva C., Peres S.B., Cipolla-Neto J., et al. Melatonin and the circadian entrainment of metabolic and hormonal activities in primary isolated adipocytes. J Pineal Res. 2008;45:422–429. doi: 10.1111/j.1600-079X.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 38.Muhlbauer E., Albrecht E., Hofmann K., Bazwinsky-Wutschke I., Peschke E. Melatonin inhibits insulin secretion in rat insulinoma beta-cells (INS-1) heterologously expressing the human melatonin receptor isoform MT2. J Pineal Res. 2011;51:361–372. doi: 10.1111/j.1600-079X.2011.00898.x. [DOI] [PubMed] [Google Scholar]
- 39.Slominski R.M., Reiter R.J., Schlabritz-Loutsevitch N., Ostrom R.S., Slominski A.T. Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol Cell Endocrinol. 2012;351:152–166. doi: 10.1016/j.mce.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peschke E., Bach A.G., Muhlbauer E. Parallel signaling pathways of melatonin in the pancreatic beta-cell. J Pineal Res. 2006;40:184–191. doi: 10.1111/j.1600-079X.2005.00297.x. [DOI] [PubMed] [Google Scholar]
- 41.Borjigin J., Zhang L.S., Calinescu A.A. Circadian regulation of pineal gland rhythmicity. Mol Cell Endocrinol. 2012;349:13–19. doi: 10.1016/j.mce.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chuang J.I., Chen S.S., Lin M.T. Melatonin decreases brain serotonin release, arterial pressure and heart rate in rats. Pharmacology. 1993;47:91–97. doi: 10.1159/000139083. [DOI] [PubMed] [Google Scholar]
- 43.Liu R., Fu A., Hoffman A.E., Zheng T., Zhu Y. Melatonin enhances DNA repair capacity possibly by affecting genes involved in DNA damage responsive pathways. BMC Cell Biol. 2013;14:1. doi: 10.1186/1471-2121-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petkova Z., Tchekalarova J., Pechlivanova D., Moyanova S., Kortenska L., Mitreva R., et al. Treatment with melatonin after status epilepticus attenuates seizure activity and neuronal damage but does not prevent the disturbance in diurnal rhythms and behavioral alterations in spontaneously hypertensive rats in kainate model of temporal lobe epilepsy. Epilepsy Behav. 2014;31:198–208. doi: 10.1016/j.yebeh.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 45.Somalo-Barranco G., Pagano Zottola A.C., Abdulrahman A.O., El Zein R.M., Cannich A., Munoz L., et al. Mitochondria-targeted melatonin photorelease supports the presence of melatonin MT1 receptors in mitochondria inhibiting respiration. Cell Chem Biol. 2023;30(920–932):e927. doi: 10.1016/j.chembiol.2023.07.009. [DOI] [PubMed] [Google Scholar]
- 46.Minokoshi Y., Alquier T., Furukawa N., Kim Y.B., Lee A., Xue B., et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 47.Yi C.X., van der Vliet J., Dai J., Yin G., Ru L., Buijs R.M. Ventromedial arcuate nucleus communicates peripheral metabolic information to the suprachiasmatic nucleus. Endocrinology. 2006;147:283–294. doi: 10.1210/en.2005-1051. [DOI] [PubMed] [Google Scholar]
- 48.Kubota N., Yano W., Kubota T., Yamauchi T., Itoh S., Kumagai H., et al. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007;6:55–68. doi: 10.1016/j.cmet.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 49.Um J.H., Pendergast J.S., Springer D.A., Foretz M., Viollet B., Brown A., et al. AMPK regulates circadian rhythms in a tissue- and isoform-specific manner. PLoS One. 2011;6:e18450. doi: 10.1371/journal.pone.0018450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lopez M., Nogueiras R., Tena-Sempere M., Dieguez C. Hypothalamic AMPK: a canonical regulator of whole-body energy balance. Nat Rev Endocrinol. 2016;12:421–432. doi: 10.1038/nrendo.2016.67. [DOI] [PubMed] [Google Scholar]
- 51.Honma K., Hikosaka M., Mochizuki K., Goda T. Loss of circadian rhythm of circulating insulin concentration induced by high-fat diet intake is associated with disrupted rhythmic expression of circadian clock genes in the liver. Metabolism. 2016;65:482–491. doi: 10.1016/j.metabol.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 52.Tognini P., Murakami M., Liu Y., Eckel-Mahan K.L., Newman J.C., Verdin E., et al. Distinct Circadian Signatures in Liver and Gut Clocks Revealed by Ketogenic Diet. Cell Metab. 2017;26(523–538):e525. doi: 10.1016/j.cmet.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 53.de Assis L.V.M., Demir M., Oster H. Nonalcoholic Steatohepatitis Disrupts Diurnal Liver Transcriptome Rhythms in Mice. Cell Mol Gastroenterol Hepatol. 2023;16:341–354. doi: 10.1016/j.jcmgh.2023.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mukherji A., Kobiita A., Damara M., Misra N., Meziane H., Champy M.F., et al. Shifting eating to the circadian rest phase misaligns the peripheral clocks with the master SCN clock and leads to a metabolic syndrome. Proc Natl Acad Sci U S A. 2015;112:E6691–E6698. doi: 10.1073/pnas.1519807112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tahara Y., Yamazaki M., Sukigara H., Motohashi H., Sasaki H., Miyakawa H., et al. Gut Microbiota-Derived Short Chain Fatty Acids Induce Circadian Clock Entrainment in Mouse Peripheral Tissue. Sci Rep. 2018;8:1395. doi: 10.1038/s41598-018-19836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goh G.H., Blache D., Mark P.J., Kennington W.J., Maloney S.K. Daily temperature cycles prolong lifespan and have sex-specific effects on peripheral clock gene expression in Drosophila melanogaster. J Exp Biol. 2021;224 doi: 10.1242/jeb.233213. [DOI] [PubMed] [Google Scholar]
- 57.Brown SA, Azzi A. Peripheral circadian oscillators in mammals. Handb Exp Pharmacol 201345-66. doi: 10.1007/978-3-642-25950-0_3. [DOI] [PubMed]
- 58.Yi J.S., Diaz N.M., D'Souza S., Buhr E.D. The molecular clockwork of mammalian cells. Semin Cell Dev Biol. 2022;126:87–96. doi: 10.1016/j.semcdb.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Preitner N., Damiola F., Lopez-Molina L., Zakany J., Duboule D., Albrecht U., et al. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 60.Guillaumond F., Dardente H., Giguere V., Cermakian N. Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. J Biol Rhythms. 2005;20:391–403. doi: 10.1177/0748730405277232. [DOI] [PubMed] [Google Scholar]
- 61.Trott A.J., Menet J.S. Regulation of circadian clock transcriptional output by CLOCK:BMAL1. PLoS Genet. 2018;14:e1007156. doi: 10.1371/journal.pgen.1007156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rabinovich-Nikitin I., Rasouli M., Reitz C.J., Posen I., Margulets V., Dhingra R., et al. Mitochondrial autophagy and cell survival is regulated by the circadian Clock gene in cardiac myocytes during ischemic stress. Autophagy. 2021;17:3794–3812. doi: 10.1080/15548627.2021.1938913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharma D, Partch CL. PAS Dimerization at the Nexus of the Mammalian Circadian Clock. J Mol Biol 2023168341. doi: 10.1016/j.jmb.2023.168341. [DOI] [PMC free article] [PubMed]
- 64.Giorgio M., Migliaccio E., Orsini F., Paolucci D., Moroni M., Contursi C., et al. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 65.Zhu Z., Hua B., Xu L., Yuan G., Li E., Li X., et al. CLOCK promotes 3T3-L1 cell proliferation via Wnt signaling. IUBMB Life. 2016;68:557–568. doi: 10.1002/iub.1512. [DOI] [PubMed] [Google Scholar]
- 66.Curtis A.M., Cheng Y., Kapoor S., Reilly D., Price T.S., Fitzgerald G.A. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci U S A. 2007;104:3450–3455. doi: 10.1073/pnas.0611680104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dyar K.A., Ciciliot S., Wright L.E., Bienso R.S., Tagliazucchi G.M., Patel V.R., et al. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol Metab. 2014;3:29–41. doi: 10.1016/j.molmet.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McKee C.A., Polino A.J., King M.W., Musiek E.S. Circadian clock protein BMAL1 broadly influences autophagy and endolysosomal function in astrocytes. Proc Natl Acad Sci U S A. 2023;120 doi: 10.1073/pnas.2220551120. e2220551120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang G., Chen L., Grant G.R., Paschos G., Song W.L., Musiek E.S., et al. Timing of expression of the core clock gene Bmal1 influences its effects on aging and survival. Sci Transl Med. 2016;8:324ra316. doi: 10.1126/scitranslmed.aad3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ono D., Honma S., Honma K. Cryptochromes are critical for the development of coherent circadian rhythms in the mouse suprachiasmatic nucleus. Nat Commun. 2013;4:1666. doi: 10.1038/ncomms2670. [DOI] [PubMed] [Google Scholar]
- 71.Kriebs A., Jordan S.D., Soto E., Henriksson E., Sandate C.R., Vaughan M.E., et al. Circadian repressors CRY1 and CRY2 broadly interact with nuclear receptors and modulate transcriptional activity. Proc Natl Acad Sci U S A. 2017;114:8776–8781. doi: 10.1073/pnas.1704955114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hatori M., Panda S. CRY links the circadian clock and CREB-mediated gluconeogenesis. Cell Res. 2010;20:1285–1288. doi: 10.1038/cr.2010.152. [DOI] [PubMed] [Google Scholar]
- 73.Lamia K.A., Papp S.J., Yu R.T., Barish G.D., Uhlenhaut N.H., Jonker J.W., et al. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature. 2011;480:552–556. doi: 10.1038/nature10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qin B., Deng Y. Overexpression of circadian clock protein cryptochrome (CRY) 1 alleviates sleep deprivation-induced vascular inflammation in a mouse model. Immunol Lett. 2015;163:76–83. doi: 10.1016/j.imlet.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 75.Yang L., Chu Y., Wang L., Wang Y., Zhao X., He W., et al. Overexpression of CRY1 protects against the development of atherosclerosis via the TLR/NF-kappaB pathway. Int Immunopharmacol. 2015;28:525–530. doi: 10.1016/j.intimp.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 76.Gotoh T., Vila-Caballer M., Liu J., Schiffhauer S., Finkielstein C.V. Association of the circadian factor Period 2 to p53 influences p53's function in DNA-damage signaling. Mol Biol Cell. 2015;26:359–372. doi: 10.1091/mbc.E14-05-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hustedt N., Durocher D. The control of DNA repair by the cell cycle. Nat Cell Biol. 2016;19:1–9. doi: 10.1038/ncb3452. [DOI] [PubMed] [Google Scholar]
- 78.Solocinski K., Holzworth M., Wen X., Cheng K.Y., Lynch I.J., Cain B.D., et al. Desoxycorticosterone pivalate-salt treatment leads to non-dipping hypertension in Per1 knockout mice. Acta Physiol (Oxf) 2017;220:72–82. doi: 10.1111/apha.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Griffin P., Dimitry J.M., Sheehan P.W., Lananna B.V., Guo C., Robinette M.L., et al. Circadian clock protein Rev-erbalpha regulates neuroinflammation. Proc Natl Acad Sci U S A. 2019;116:5102–5107. doi: 10.1073/pnas.1812405116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Y., Kuang Z., Yu X., Ruhn K.A., Kubo M., Hooper L.V. The intestinal microbiota regulates body composition through NFIL3 and the circadian clock. Science. 2017;357:912–916. doi: 10.1126/science.aan0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Delerive P., Monte D., Dubois G., Trottein F., Fruchart-Najib J., Mariani J., et al. The orphan nuclear receptor ROR alpha is a negative regulator of the inflammatory response. EMBO Rep. 2001;2:42–48. doi: 10.1093/embo-reports/kve007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yoshitane H., Asano Y., Sagami A., Sakai S., Suzuki Y., Okamura H., et al. Functional D-box sequences reset the circadian clock and drive mRNA rhythms. Commun Biol. 2019;2:300. doi: 10.1038/s42003-019-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao Y., Wu Y., Wang J., Liao C., Mi X., Chen F. Circadian transcription factor Dbp promotes rat calvarial osteoprogenitors osteogenic differentiation through Kiss1/GnRH/E2 signaling pathway loop. J Cell Biochem. 2021;122:166–179. doi: 10.1002/jcb.29836. [DOI] [PubMed] [Google Scholar]
- 84.Kamizono S., Duncan G.S., Seidel M.G., Morimoto A., Hamada K., Grosveld G., et al. Nfil3/E4bp4 is required for the development and maturation of NK cells in vivo. J Exp Med. 2009;206:2977–2986. doi: 10.1084/jem.20092176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yu X., Rollins D., Ruhn K.A., Stubblefield J.J., Green C.B., Kashiwada M., et al. TH17 cell differentiation is regulated by the circadian clock. Science. 2013;342:727–730. doi: 10.1126/science.1243884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Keniry M., Dearth R.K., Persans M., Parsons R. New Frontiers for the NFIL3 bZIP Transcription Factor in Cancer. Metabolism and Beyond Discoveries (Craiova) 2014;2:e15. doi: 10.15190/d.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hermida R.C., Crespo J.J., Dominguez-Sardina M., Otero A., Moya A., Rios M.T., et al. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial. Eur Heart J. 2020;41:4565–4576. doi: 10.1093/eurheartj/ehz754. [DOI] [PubMed] [Google Scholar]
- 88.Amiama-Roig A, Verdugo-Sivianes EM, Carnero A, Blanco JR. Chronotherapy: Circadian Rhythms and Their Influence in Cancer Therapy. Cancers (Basel) 2022;14. doi: 10.3390/cancers14205071. [DOI] [PMC free article] [PubMed]
- 89.Yu F., Liu Y., Zhang R., Zhu L., Zhang T., Shi Y. Recent advances in circadian-regulated pharmacokinetics and its implications for chronotherapy. Biochem Pharmacol. 2022;203 doi: 10.1016/j.bcp.2022.115185. [DOI] [PubMed] [Google Scholar]
- 90.Weger M., Weger B.D., Gachon F. Understanding circadian dynamics: current progress and future directions for chronobiology in drug discovery. Expert Opin Drug Discov. 2023;18:893–901. doi: 10.1080/17460441.2023.2224554. [DOI] [PubMed] [Google Scholar]
- 91.Watanabe H., Nakano S., Nagai K., Ogawa N. Time-dependent absorption of theophylline in man. J Clin Pharmacol. 1984;24:509–514. doi: 10.1002/j.1552-4604.1984.tb02760.x. [DOI] [PubMed] [Google Scholar]
- 92.Kamali F., Fry J.R., Bell G.D. Temporal variations in paracetamol absorption and metabolism in man. Xenobiotica. 1987;17:635–641. doi: 10.3109/00498258709043970. [DOI] [PubMed] [Google Scholar]
- 93.White W.B., Anders R.J., MacIntyre J.M., Black H.R., Sica D.A. Nocturnal dosing of a novel delivery system of verapamil for systemic hypertension. Verapamil Study Group Am J Cardiol. 1995;76:375–380. doi: 10.1016/s0002-9149(99)80104-0. [DOI] [PubMed] [Google Scholar]
- 94.Oishi K., Konishi T., Hashimoto C., Yamamoto S., Takahashi Y., Shiina Y. Dietary fish oil differentially ameliorates high-fructose diet-induced hepatic steatosis and hyperlipidemia in mice depending on time of feeding. J Nutr Biochem. 2018;52:45–53. doi: 10.1016/j.jnutbio.2017.09.024. [DOI] [PubMed] [Google Scholar]
- 95.Yoshizaki T., Tada Y., Hida A., Sunami A., Yokoyama Y., Togo F., et al. Influence of dietary behavior on the circadian rhythm of the autonomic nervous system as assessed by heart rate variability. Physiol Behav. 2013;118:122–128. doi: 10.1016/j.physbeh.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 96.Yoshizaki T., Tada Y., Hida A., Sunami A., Yokoyama Y., Yasuda J., et al. Effects of feeding schedule changes on the circadian phase of the cardiac autonomic nervous system and serum lipid levels. Eur J Appl Physiol. 2013;113:2603–2611. doi: 10.1007/s00421-013-2702-z. [DOI] [PubMed] [Google Scholar]
- 97.Palla L, Almoosawi S. Diurnal Patterns of Energy Intake Derived via Principal Component Analysis and Their Relationship with Adiposity Measures in Adolescents: Results from the National Diet and Nutrition Survey RP (2008(-)2012). Nutrients 2019;11. doi: 10.3390/nu11020422. [DOI] [PMC free article] [PubMed]
- 98.Jiang P., Xu C., Zhang P., Ren J., Mageed F., Wu X., et al. Epigallocatechin-3-gallate inhibits self-renewal ability of lung cancer stem-like cells through inhibition of CLOCK. Int J Mol Med. 2020;46:2216–2224. doi: 10.3892/ijmm.2020.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sarma A., Sharma V.P., Sarkar A.B., Sekar M.C., Samuel K., Geusz M.E. The circadian clock modulates anti-cancer properties of curcumin. BMC Cancer. 2016;16:759. doi: 10.1186/s12885-016-2789-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Onishi Y., Kawano Y., Yamazaki Y. Lycorine, a candidate for the control of period length in mammalian cells. Cell Physiol Biochem. 2012;29:407–416. doi: 10.1159/000338495. [DOI] [PubMed] [Google Scholar]
- 101.Lopresti A.L., Smith S.J., Malvi H., Kodgule R. An investigation into the stress-relieving and pharmacological actions of an ashwagandha (Withania somnifera) extract: A randomized, double-blind, placebo-controlled study. Medicine (Baltimore) 2019;98:e17186. doi: 10.1097/MD.0000000000017186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kukkemane K., Jagota A. Therapeutic effects of hydro-alcoholic leaf extract of Withania somnifera on age-induced changes in daily rhythms of Sirt1, Nrf2 and Rev-erbalpha in the SCN of male Wistar rats. Biogerontology. 2020;21:593–607. doi: 10.1007/s10522-020-09875-x. [DOI] [PubMed] [Google Scholar]
- 103.Kambe D., Kotani M., Yoshimoto M., Kaku S., Chaki S., Honda K. Effects of quercetin on the sleep-wake cycle in rats: involvement of gamma-aminobutyric acid receptor type A in regulation of rapid eye movement sleep. Brain Res. 2010;1330:83–88. doi: 10.1016/j.brainres.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 104.Numata M., Hirano A., Yamamoto Y., Yasuda M., Miura N., Sayama K., et al. Metastasis of Breast Cancer Promoted by Circadian Rhythm Disruption due to Light/Dark Shift and its Prevention by Dietary Quercetin in Mice. J Circadian Rhythms. 2021;19:2. doi: 10.5334/jcr.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Menek M.Y., Budak M. Effect of exercises according to the circadian rhythm in type 2 diabetes: Parallel-group, single-blind, crossover study. Nutr Metab Cardiovasc Dis. 2022;32:1742–1752. doi: 10.1016/j.numecd.2022.04.017. [DOI] [PubMed] [Google Scholar]
- 106.Joost H.G., Bell G.I., Best J.D., Birnbaum M.J., Charron M.J., Chen Y.T., et al. Nomenclature of the GLUT/SLC2A family of sugar/polyol transport facilitators. Am J Physiol Endocrinol Metab. 2002;282:E974–E976. doi: 10.1152/ajpendo.00407.2001. [DOI] [PubMed] [Google Scholar]
- 107.Wright E.M., Turk E. The sodium/glucose cotransport family SLC5. Pflugers Arch. 2004;447:510–518. doi: 10.1007/s00424-003-1063-6. [DOI] [PubMed] [Google Scholar]
- 108.Zhao F.Q., Keating A.F. Functional properties and genomics of glucose transporters. Curr Genomics. 2007;8:113–128. doi: 10.2174/138920207780368187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kuo T., McQueen A., Chen T.C., Wang J.C. Regulation of Glucose Homeostasis by Glucocorticoids. Advances in Experimental Medicine and Biology. 2015;872:99–126. doi: 10.1007/978-1-4939-2895-8_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Klein S., Gastaldelli A., Yki-Jarvinen H., Scherer P.E. Why does obesity cause diabetes? Cell Metab. 2022;34:11–20. doi: 10.1016/j.cmet.2021.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Perelis M., Marcheva B., Ramsey K.M., Schipma M.J., Hutchison A.L., Taguchi A., et al. Pancreatic beta cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science. 2015;350:aac4250. doi: 10.1126/science.aac4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Alexander R.K., Liou Y.H., Knudsen N.H., Starost K.A., Xu C., Hyde A.L., et al. Bmal1 integrates mitochondrial metabolism and macrophage activation. Elife. 2020;9 doi: 10.7554/eLife.54090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li E., Li X., Huang J., Xu C., Liang Q., Ren K., et al. BMAL1 regulates mitochondrial fission and mitophagy through mitochondrial protein BNIP3 and is critical in the development of dilated cardiomyopathy. Protein Cell. 2020;11:661–679. doi: 10.1007/s13238-020-00713-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gabriel B.M., Altintas A., Smith J.A.B., Sardon-Puig L., Zhang X., Basse A.L., et al. Disrupted circadian oscillations in type 2 diabetes are linked to altered rhythmic mitochondrial metabolism in skeletal muscle. Sci Adv. 2021;7:eabi9654. doi: 10.1126/sciadv.abi9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yu F., Wang Z., Zhang T., Chen X., Xu H., Wang F., et al. Deficiency of intestinal Bmal1 prevents obesity induced by high-fat feeding. Nat Commun. 2021;12:5323. doi: 10.1038/s41467-021-25674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.George J., Banik N.L., Ray S.K. Genistein induces receptor and mitochondrial pathways and increases apoptosis during BCL-2 knockdown in human malignant neuroblastoma SK-N-DZ cells. J Neurosci Res. 2010;88:877–886. doi: 10.1002/jnr.22244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhou L., Xiao X., Zhang Q., Zheng J., Li M., Yu M., et al. Dietary Genistein Could Modulate Hypothalamic Circadian Entrainment, Reduce Body Weight, and Improve Glucose and Lipid Metabolism in Female Mice. Int J Endocrinol. 2019;2019:2163838. doi: 10.1155/2019/2163838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.de Vries M.J., Nunes Cardozo B., van der Want J., de Wolf A., Meijer J.H. Glutamate immunoreactivity in terminals of the retinohypothalamic tract of the brown Norwegian rat. Brain Res. 1993;612:231–237. doi: 10.1016/0006-8993(93)91665-f. [DOI] [PubMed] [Google Scholar]
- 119.Ebling F.J. The role of glutamate in the photic regulation of the suprachiasmatic nucleus. Prog Neurobiol. 1996;50:109–132. doi: 10.1016/s0301-0082(96)00032-9. [DOI] [PubMed] [Google Scholar]
- 120.Engin A. Circadian Rhythms in Diet-Induced Obesity. Advances in Experimental Medicine and Biology. 2017;960:19–52. doi: 10.1007/978-3-319-48382-5_2. [DOI] [PubMed] [Google Scholar]
- 121.Potterat O. Goji (Lycium barbarum and L. chinense): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med. 2010;76:7–19. doi: 10.1055/s-0029-1186218. [DOI] [PubMed] [Google Scholar]
- 122.Deng X., Li X., Luo S., Zheng Y., Luo X., Zhou L. Antitumor activity of Lycium barbarum polysaccharides with different molecular weights: an in vitro and in vivo study. Food Nutr Res. 2017;61:1399770. doi: 10.1080/16546628.2017.1399770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tian X., Liang T., Liu Y., Ding G., Zhang F., Ma Z. Extraction, Structural Characterization, and Biological Functions of Lycium Barbarum Polysaccharides. A Review Biomolecules. 2019;9 doi: 10.3390/biom9090389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Feng L., Xiao X., Liu J., Wang J., Zhang N., Bing T., et al. Immunomodulatory Effects of Lycium barbarum Polysaccharide Extract and Its Uptake Behaviors at the Cellular Level. Molecules. 2020;25 doi: 10.3390/molecules25061351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li R., Qu S., Qin M., Huang L., Huang Y., Du Y., et al. Immunomodulatory and antiviral effects of Lycium barbarum glycopeptide on influenza a virus infection. Microb Pathog. 2023;176 doi: 10.1016/j.micpath.2023.106030. [DOI] [PubMed] [Google Scholar]
- 126.Zhao R, Gao X, Zhang T, Li X. Effects of Lycium barbarum. polysaccharide on type 2 diabetes mellitus rats by regulating biological rhythms. Iran J Basic Med Sci 2016;19:1024-1030. doi. Available from https://www.ncbi.nlm.nih.gov/pubmed/27803791. [PMC free article] [PubMed]
- 127.Unger R.H. Lipotoxic diseases. Annu Rev Med. 2002;53:319–336. doi: 10.1146/annurev.med.53.082901.104057. [DOI] [PubMed] [Google Scholar]
- 128.Myers M.G., Jr., Leibel R.L., Seeley R.J., Schwartz M.W. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab. 2010;21:643–651. doi: 10.1016/j.tem.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Markova-Car E.P., Jurisic D., Ilic N., Kraljevic P.S. Running for time: circadian rhythms and melanoma. Tumour Biol. 2014;35:8359–8368. doi: 10.1007/s13277-014-1904-2. [DOI] [PubMed] [Google Scholar]
- 130.Xiong X.Y., Liu Y., Shan L.T., Xu Y.Q., Liang J., Lai Y.H., et al. Evaluation of collagen mixture on promoting skin wound healing in zebrafish caused by acetic acid administration. Biochem Biophys Res Commun. 2018;505:516–522. doi: 10.1016/j.bbrc.2018.09.148. [DOI] [PubMed] [Google Scholar]
- 131.Xiong X., Liang J., Xu Y., Liu J., Liu Y. The wound healing effects of the Tilapia collagen peptide mixture TY001 in streptozotocin diabetic mice. J Sci Food Agric. 2020;100:2848–2858. doi: 10.1002/jsfa.10104. [DOI] [PubMed] [Google Scholar]
- 132.Xiong X.Y., Liang J., Xu Y.Q., Liu Y. The Tilapia collagen peptide mixture TY001 protects against LPS-induced inflammation, disruption of glucose metabolism, and aberrant expression of circadian clock genes in mice. Chronobiol Int. 2019;36:1013–1023. doi: 10.1080/07420528.2019.1606821. [DOI] [PubMed] [Google Scholar]
- 133.Danesi F., Ferguson L.R. Could Pomegranate Juice Help in the Control of Inflammatory Diseases? Nutrients. 2017;9 doi: 10.3390/nu9090958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Banihani S.A., Shuaibu S.M., Al-Husein B.A., Makahleh S.S. Fresh Pomegranate Juice Decreases Fasting Serum Erythropoietin in Patients with Type 2 Diabetes. Int J Food Sci. 2019;2019:1269341. doi: 10.1155/2019/1269341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Di Stefano V., Pitonzo R., Novara M.E., Bongiorno D., Indelicato S., Gentile C., et al. Antioxidant activity and phenolic composition in pomegranate (Punica granatum L.) genotypes from south Italy by UHPLC-Orbitrap-MS approach. J Sci Food Agric. 2019;99:1038–1045. doi: 10.1002/jsfa.9270. [DOI] [PubMed] [Google Scholar]
- 136.Banihani S.A., Fashtaky R.A., Makahleh S.M., El-Akawi Z.J., Khabour O.F., Saadeh N.A. Effect of fresh pomegranate juice on the level of melatonin, insulin, and fasting serum glucose in healthy individuals and people with impaired fasting glucose. Food Sci Nutr. 2019;8:567–574. doi: 10.1002/fsn3.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Boden G., Ruiz J., Urbain J.L., Chen X. Evidence for a circadian rhythm of insulin secretion. Am J Physiol. 1996;271:E246–E252. doi: 10.1152/ajpendo.1996.271.2.E246. [DOI] [PubMed] [Google Scholar]
- 138.Peschke E., Hofmann K., Bahr I., Streck S., Albrecht E., Wedekind D., et al. The insulin-melatonin antagonism: studies in the LEW.1AR1-iddm rat (an animal model of human type 1 diabetes mellitus) Diabetologia. 2011;54:1831–1840. doi: 10.1007/s00125-011-2138-0. [DOI] [PubMed] [Google Scholar]
- 139.Li Y., Ma J., Yao K., Su W., Tan B., Wu X., et al. Circadian rhythms and obesity: Timekeeping governs lipid metabolism. J Pineal Res. 2020;69:e12682. doi: 10.1111/jpi.12682. [DOI] [PubMed] [Google Scholar]
- 140.Sinha R.A., Singh B.K., Yen P.M. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat Rev Endocrinol. 2018;14:259–269. doi: 10.1038/nrendo.2018.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pereira S., Cline D.L., Glavas M.M., Covey S.D., Kieffer T.J. Tissue-Specific Effects of Leptin on Glucose and Lipid Metabolism. Endocr Rev. 2021;42:1–28. doi: 10.1210/endrev/bnaa027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xie X., Yang S., Zou Y., Cheng S., Wang Y., Jiang Z., et al. Influence of the core circadian gene “Clock” on obesity and leptin resistance in mice. Brain Res. 2013;1491:147–155. doi: 10.1016/j.brainres.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 143.Han S.G., Han S.S., Toborek M., Hennig B. EGCG protects endothelial cells against PCB 126-induced inflammation through inhibition of AhR and induction of Nrf2-regulated genes. Toxicol Appl Pharmacol. 2012;261:181–188. doi: 10.1016/j.taap.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shin Y.S., Kang S.U., Park J.K., Kim Y.E., Kim Y.S., Baek S.J., et al. Anti-cancer effect of (-)-epigallocatechin-3-gallate (EGCG) in head and neck cancer through repression of transactivation and enhanced degradation of beta-catenin. Phytomedicine. 2016;23:1344–1355. doi: 10.1016/j.phymed.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 145.Liu B., Yan W. Lipophilization of EGCG and effects on antioxidant activities. Food Chem. 2019;272:663–669. doi: 10.1016/j.foodchem.2018.08.086. [DOI] [PubMed] [Google Scholar]
- 146.Zhang Y., Zhang Y., Ma R., Sun W., Ji Z. Antibacterial Activity of Epigallocatechin Gallate (EGCG) against Shigella flexneri. Int J Environ Res Public Health. 2023;20 doi: 10.3390/ijerph20064676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mi Y, Qi G, Gao Y, Li R, Wang Y, Li X, et al. (-)-Epigallocatechin-3-gallate Ameliorates Insulin Resistance and Mitochondrial Dysfunction in HepG2 Cells: Involvement of Bmal1. Mol Nutr Food Res 2017;61. doi: 10.1002/mnfr.201700440. [DOI] [PubMed]
- 148.Mi Y., Qi G., Fan R., Ji X., Liu Z., Liu X. EGCG ameliorates diet-induced metabolic syndrome associating with the circadian clock. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1575–1589. doi: 10.1016/j.bbadis.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 149.Cannon D.T., Wu Y. Topical capsaicin as an adjuvant analgesic for the treatment of traumatic amputee neurogenic residual limb pain. Arch Phys Med Rehabil. 1998;79:591–593. doi: 10.1016/s0003-9993(98)90080-6. [DOI] [PubMed] [Google Scholar]
- 150.Kim C.S., Kawada T., Kim B.S., Han I.S., Choe S.Y., Kurata T., et al. Capsaicin exhibits anti-inflammatory property by inhibiting IkB-a degradation in LPS-stimulated peritoneal macrophages. Cell Signal. 2003;15:299–306. doi: 10.1016/s0898-6568(02)00086-4. [DOI] [PubMed] [Google Scholar]
- 151.Zheng J., Zheng S., Feng Q., Zhang Q., Xiao X. Dietary capsaicin and its anti-obesity potency: from mechanism to clinical implications. Biosci Rep. 2017;37 doi: 10.1042/BSR20170286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shomorony A., Santamaria C.M., Zhao C., Rwei A.Y., Mehta M., Zurakowski D., et al. Prolonged Duration Local Anesthesia by Combined Delivery of Capsaicin- and Tetrodotoxin-Loaded Liposomes. Anesth Analg. 2019;129:709–717. doi: 10.1213/ANE.0000000000004108. [DOI] [PubMed] [Google Scholar]
- 153.Li R., Xiao J., Cao Y., Huang Q., Ho C.T., Lu M. Capsaicin Attenuates Oleic Acid-Induced Lipid Accumulation via the Regulation of Circadian Clock Genes in HepG2 Cells. J Agric Food Chem. 2022;70:794–803. doi: 10.1021/acs.jafc.1c06437. [DOI] [PubMed] [Google Scholar]
- 154.Guo R., Zhao B., Wang Y., Wu D., Wang Y., Yu Y., et al. Cichoric Acid Prevents Free-Fatty-Acid-Induced Lipid Metabolism Disorders via Regulating Bmal1 in HepG2 Cells. J Agric Food Chem. 2018;66:9667–9678. doi: 10.1021/acs.jafc.8b02147. [DOI] [PubMed] [Google Scholar]
- 155.Wang S., Xu Z., Cai B., Chen Q. Berberine as a Potential Multi-Target Agent for Metabolic Diseases: A Review of Investigations for Berberine. Endocr Metab Immune Disord Drug Targets. 2021;21:971–979. doi: 10.2174/1871530320666200910105612. [DOI] [PubMed] [Google Scholar]
- 156.Hirai T., Mitani Y., Kurumisawa K., Nomura K., Wang W., Nakashima K.I., et al. Berberine stimulates fibroblast growth factor 21 by modulating the molecular clock component brain and muscle Arnt-like 1 in brown adipose tissue. Biochem Pharmacol. 2019;164:165–176. doi: 10.1016/j.bcp.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 157.Kharitonenkov A., Shiyanova T.L., Koester A., Ford A.M., Micanovic R., Galbreath E.J., et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.El-Masry S.A., Farid M.N., Hassan N.E., Soliman M.A., Mekkawy L.H., Elashry G.I., et al. Fibroblast growth factor-21 and Visfatin as potential predictors for metabolic risk factors in obese children. Sci Rep. 2024;14:1190. doi: 10.1038/s41598-024-51394-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hagenlocher Y., Gommeringer S., Held A., Feilhauer K., Koninger J., Bischoff S.C., et al. Nobiletin acts anti-inflammatory on murine IL-10(-/-) colitis and human intestinal fibroblasts. Eur J Nutr. 2019;58:1391–1401. doi: 10.1007/s00394-018-1661-x. [DOI] [PubMed] [Google Scholar]
- 160.Kim E., Kim Y.J., Ji Z., Kang J.M., Wirianto M., Paudel K.R., et al. ROR activation by Nobiletin enhances antitumor efficacy via suppression of IkappaB/NF-kappaB signaling in triple-negative breast cancer. Cell Death Dis. 2022;13:374. doi: 10.1038/s41419-022-04826-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jahan S., Ansari U.A., Srivastava A.K., Aldosari S., Alabdallat N.G., Siddiqui A.J., et al. A protein-miRNA biomic analysis approach to explore neuroprotective potential of nobiletin in human neural progenitor cells (hNPCs) Front Pharmacol. 2024;15:1343569. doi: 10.3389/fphar.2024.1343569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Qi G., Guo R., Tian H., Li L., Liu H., Mi Y., et al. Nobiletin protects against insulin resistance and disorders of lipid metabolism by reprogramming of circadian clock in hepatocytes. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863:549–562. doi: 10.1016/j.bbalip.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 163.Alexandre E.C., Calmasini F.B., de Oliveira M.G., Silva F.H., da Silva C.P.V., Andre D.M., et al. Chronic treatment with resveratrol improves overactive bladder in obese mice via antioxidant activity. Eur J Pharmacol. 2016;788:29–36. doi: 10.1016/j.ejphar.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 164.Tao L., Ding Q., Gao C., Sun X. Resveratrol attenuates neuropathic pain through balancing pro-inflammatory and anti-inflammatory cytokines release in mice. Int Immunopharmacol. 2016;34:165–172. doi: 10.1016/j.intimp.2016.02.033. [DOI] [PubMed] [Google Scholar]
- 165.Zou T., Chen D., Yang Q., Wang B., Zhu M.J., Nathanielsz P.W., et al. Resveratrol supplementation of high-fat diet-fed pregnant mice promotes brown and beige adipocyte development and prevents obesity in male offspring. J Physiol. 2017;595:1547–1562. doi: 10.1113/JP273478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wu J., Li Y., Yu J., Gan Z., Wei W., Wang C., et al. Resveratrol Attenuates High-Fat Diet Induced Hepatic Lipid Homeostasis Disorder and Decreases m(6)A RNA Methylation. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.568006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Spaleniak W., Cuendet M. Resveratrol as a circadian clock modulator: mechanisms of action and therapeutic applications. Mol Biol Rep. 2023;50:6159–6170. doi: 10.1007/s11033-023-08513-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Li J., Wei L., Zhao C., Li J., Liu Z., Zhang M., et al. Resveratrol Maintains Lipid Metabolism Homeostasis via One of the Mechanisms Associated with the Key Circadian Regulator Bmal1. Molecules. 2019;24 doi: 10.3390/molecules24162916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gunasekaran V., Elangovan K., Niranjali D.S. Targeting hepatocellular carcinoma with piperine by radical-mediated mitochondrial pathway of apoptosis: An in vitro and in vivo study. Food Chem Toxicol. 2017;105:106–118. doi: 10.1016/j.fct.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 170.Jaisin Y., Ratanachamnong P., Wongsawatkul O., Watthammawut A., Malaniyom K., Natewong S. Antioxidant and anti-inflammatory effects of piperine on UV-B-irradiated human HaCaT keratinocyte cells. Life Sci. 2020;263 doi: 10.1016/j.lfs.2020.118607. [DOI] [PubMed] [Google Scholar]
- 171.Saetang J., Tedasen A., Sangkhathat S., Sangkaew N., Dokduang S., Prompat N., et al. Low Piperine Fractional Piper nigrum Extract Enhanced the Antitumor Immunity via Regulating the Th1/Th2/Treg Cell Subsets on NMU-Induced Tumorigenesis Rats. Planta Med. 2022;88:527–537. doi: 10.1055/a-1458-5646. [DOI] [PubMed] [Google Scholar]
- 172.Zhang W., Ho C.T., Lu M. Piperine Improves Lipid Dysregulation by Modulating Circadian Genes Bmal1 and Clock in HepG2 Cells. Int J Mol Sci. 2022;23 doi: 10.3390/ijms23105611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sun J., Tang B., Ho C.T., Lu M. Piperine Attenuates Bmal1-Mediated Glucose Metabolism Disorder in a Trpv1-Dependent Manner in HepG2 Cells. J Agric Food Chem. 2023;71:19581–19591. doi: 10.1021/acs.jafc.3c06683. [DOI] [PubMed] [Google Scholar]
- 174.Zhang Y., Huang N., Chen M., Jin H., Nie J., Shi J., et al. Procyanidin protects against 6-hydroxydopamine-induced dopaminergic neuron damage via the regulation of the PI3K/Akt signalling pathway. Biomed Pharmacother. 2019;114 doi: 10.1016/j.biopha.2019.108789. [DOI] [PubMed] [Google Scholar]
- 175.Manocchio F., Soliz-Rueda J.R., Ribas-Latre A., Bravo F.I., Arola-Arnal A., Suarez M., et al. Grape Seed Proanthocyanidins Modulate the Hepatic Molecular Clock via MicroRNAs. Mol Nutr Food Res. 2022;66:e2200443. doi: 10.1002/mnfr.202200443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cortes-Espinar AJ, Ibarz-Blanch N, Soliz-Rueda JR, Bonafos B, Feillet-Coudray C, Casas F, et al. Rhythm and ROS: Hepatic Chronotherapeutic Features of Grape Seed Proanthocyanidin Extract Treatment in Cafeteria Diet-Fed Rats. Antioxidants (Basel) 2023;12. doi: 10.3390/antiox12081606. [DOI] [PMC free article] [PubMed]
- 177.Hua C., Zhao J., Wang H., Chen F., Meng H., Chen L., et al. Apple polyphenol relieves hypoxia-induced pulmonary arterial hypertension via pulmonary endothelium protection and smooth muscle relaxation: In vivo and in vitro studies. Biomed Pharmacother. 2018;107:937–944. doi: 10.1016/j.biopha.2018.08.080. [DOI] [PubMed] [Google Scholar]
- 178.Liu F., Wang X., Li D., Cui Y., Li X. Apple polyphenols extract alleviated dextran sulfate sodium-induced ulcerative colitis in C57BL/6 male mice by restoring bile acid metabolism disorder and gut microbiota dysbiosis. Phytother Res. 2021;35:1468–1485. doi: 10.1002/ptr.6910. [DOI] [PubMed] [Google Scholar]
- 179.Cui Y., Yin Y., Li S., Xie Y., Wu Z., Yang H., et al. Apple polyphenol extract targets circadian rhythms to improve liver biological clock and lipid homeostasis in C57BL/6 male mice with mistimed high-fat diet feeding. J Funct Foods. 2022;92 doi: 10.1016/j.jff.2022.105051. [DOI] [Google Scholar]
- 180.Kulshrestha S., Devkar R. Circadian control of Nocturnin and its regulatory role in health and disease. Chronobiol Int. 2023;40:970–981. doi: 10.1080/07420528.2023.2231081. [DOI] [PubMed] [Google Scholar]
- 181.Lecumberri E., Dupertuis Y.M., Miralbell R., Pichard C. Green tea polyphenol epigallocatechin-3-gallate (EGCG) as adjuvant in cancer therapy. Clin Nutr. 2013;32:894–903. doi: 10.1016/j.clnu.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 182.Keske M.A., Ng H.L., Premilovac D., Rattigan S., Kim J.A., Munir K., et al. Vascular and metabolic actions of the green tea polyphenol epigallocatechin gallate. Curr Med Chem. 2015;22:59–69. doi: 10.2174/0929867321666141012174553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Qi G., Wu W., Mi Y., Shi R., Sun K., Li R., et al. Tea polyphenols direct Bmal1-driven ameliorating of the redox imbalance and mitochondrial dysfunction in hepatocytes. Food Chem Toxicol. 2018;122:181–193. doi: 10.1016/j.fct.2018.10.031. [DOI] [PubMed] [Google Scholar]
- 184.Musiek E.S., Lim M.M., Yang G., Bauer A.Q., Qi L., Lee Y., et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest. 2013;123:5389–5400. doi: 10.1172/JCI70317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Antle M.C., Steen N.M., Mistlberger R.E. Adenosine and caffeine modulate circadian rhythms in the Syrian hamster. Neuroreport. 2001;12:2901–2905. doi: 10.1097/00001756-200109170-00029. [DOI] [PubMed] [Google Scholar]
- 186.Wen M., Cui J., Xu J., Xue Y., Wang J., Xue C., et al. Effects of dietary sea cucumber saponin on the gene expression rhythm involved in circadian clock and lipid metabolism in mice during nighttime-feeding. J Physiol Biochem. 2014;70:801–808. doi: 10.1007/s13105-014-0349-9. [DOI] [PubMed] [Google Scholar]
- 187.Androulakis I.P. Circadian rhythms and the HPA axis: A systems view. WIREs Mech Dis. 2021;13:e1518. doi: 10.1002/wsbm.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Decker E.A., Rose D.J., Stewart D. Processing of oats and the impact of processing operations on nutrition and health benefits. Br J Nutr. 2014;112(Suppl 2):S58–S64. doi: 10.1017/S000711451400227X. [DOI] [PubMed] [Google Scholar]
- 189.Han S., Gao H., Song R., Zhang W., Li Y., Zhang J. Oat Fiber Modulates Hepatic Circadian Clock via Promoting Gut Microbiota-Derived Short Chain Fatty Acids. J Agric Food Chem. 2021;69:15624–15635. doi: 10.1021/acs.jafc.1c06130. [DOI] [PubMed] [Google Scholar]
- 190.Shang X., Tan J.N., Du Y., Liu X., Zhang Z. Environmentally-Friendly Extraction of Flavonoids from Cyclocarya paliurus (Batal.) Iljinskaja Leaves with Deep Eutectic Solvents and Evaluation of Their Antioxidant Activities. Molecules. 2018;23 doi: 10.3390/molecules23092110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Liu W., Deng S., Zhou D., Huang Y., Li C., Hao L., et al. 3,4-seco-Dammarane Triterpenoid Saponins with Anti-Inflammatory Activity Isolated from the Leaves of Cyclocarya paliurus. J Agric Food Chem. 2020;68:2041–2053. doi: 10.1021/acs.jafc.9b06898. [DOI] [PubMed] [Google Scholar]
- 192.Xu G, Yoshitomi H, Sun W, Guo X, Wu L, Guo X, et al. Cyclocarya paliurus (Batal.) Ijinskaja Aqueous Extract (CPAE) Ameliorates Obesity by Improving Insulin Signaling in the Hypothalamus of a Metabolic Syndrome Rat Model. Evid Based Complement Alternat Med 2017;2017:4602153. doi: 10.1155/2017/4602153. [DOI] [PMC free article] [PubMed]
- 193.Wang J., Zheng D., Huang F., Zhao A., Kuang J., Ren Z., et al. Theabrownin and Poria cocos Polysaccharide Improve Lipid Metabolism via Modulation of Bile Acid and Fatty Acid Metabolism. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.875549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Xie Q., Jia X., Zhang W., Xu Y., Zhu M., Zhao Z., et al. Effects of Poria cocos extract and protein powder mixture on glucolipid metabolism and rhythm changes in obese mice. Food Sci Nutr. 2023;11:2356–2371. doi: 10.1002/fsn3.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Jin S., Lee M.Y. Kaempferia parviflora Extract as a Potential Anti-Acne Agent with Anti-Inflammatory, Sebostatic and Anti-Propionibacterium acnes Activity. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19113457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lee M.H., Han A.R., Jang M., Choi H.K., Lee S.Y., Kim K.T., et al. Antiskin Inflammatory Activity of Black Ginger (Kaempferia parviflora) through Antioxidative Activity. Oxid Med Cell Longev. 2018;2018:5967150. doi: 10.1155/2018/5967150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hairunisa I, Bakar MFA, Da'i M, Bakar FIA, Syamsul ES. Cytotoxic Activity, Anti-Migration and In Silico Study of Black Ginger (Kaempferia parviflora) Extract against Breast Cancer Cell. Cancers (Basel) 2023;15. doi: 10.3390/cancers15102785. [DOI] [PMC free article] [PubMed]
- 198.Yoshida I., Kumagai M., Ide M., Horigome S., Takahashi Y., Mishima T., et al. Polymethoxyflavones in black ginger (Kaempferia parviflora) regulate the expression of circadian clock genes. J Funct Foods. 2020;68 doi: 10.1016/j.jff.2020.103900. [DOI] [Google Scholar]
- 199.Yoshida I., Mishima T., Kumagai M., Takahashi Y., Fujita K., Igarashi T. Black ginger (Kaempferia parviflora) extract enhances circadian rhythm and promotes lipolysis in mice fed a high-fat diet. J Funct Foods. 2023;107 doi: 10.1016/j.jff.2023.105649. [DOI] [Google Scholar]
- 200.Smolensky M.H., Hermida R.C., Portaluppi F. Circadian mechanisms of 24-hour blood pressure regulation and patterning. Sleep Med Rev. 2017;33:4–16. doi: 10.1016/j.smrv.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 201.Sowers J.R., Brickman A.S. Circadian blood pressure and renin, aldosterone, cortisol, and prolactin levels in hypertensive pseudohypoparathyroid patients. J Clin Endocrinol Metab. 1982;55:1202–1208. doi: 10.1210/jcem-55-6-1202. [DOI] [PubMed] [Google Scholar]
- 202.Schewe J., Seidel E., Forslund S., Marko L., Peters J., Muller D.N., et al. Elevated aldosterone and blood pressure in a mouse model of familial hyperaldosteronism with ClC-2 mutation. Nat Commun. 2019;10:5155. doi: 10.1038/s41467-019-13033-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Tanaka S., Ueno T., Tsunemi A., Nagura C., Tahira K., Fukuda N., et al. The adrenal gland circadian clock exhibits a distinct phase advance in spontaneously hypertensive rats. Hypertens Res. 2019;42:165–173. doi: 10.1038/s41440-018-0148-8. [DOI] [PubMed] [Google Scholar]
- 204.Liu H., Zhang X., Zhong X., Li Z., Cai S., Yang P., et al. Puerarin inhibits vascular calcification of uremic rats. Eur J Pharmacol. 2019;855:235–243. doi: 10.1016/j.ejphar.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 205.Su A.S., Zhang J.W., Zou J. The anxiolytic-like effects of puerarin on an animal model of PTSD. Biomed Pharmacother. 2019;115 doi: 10.1016/j.biopha.2019.108978. [DOI] [PubMed] [Google Scholar]
- 206.Demuth K., Atger V., Borderie D., Benoit M.O., Sauvaget D., Lotersztajn S., et al. Homocysteine decreases endothelin-1 production by cultured human endothelial cells. Eur J Biochem. 1999;263:367–376. doi: 10.1046/j.1432-1327.1999.00496.x. [DOI] [PubMed] [Google Scholar]
- 207.Liu LS, Writing Group of Chinese Guidelines for the Management of H. 2010 Chinese guidelines for the management of hypertension. Zhonghua Xin Xue Guan Bing Za Zhi 2011;39:579-615. doi. Available from https://www.ncbi.nlm.nih.gov/pubmed/22088239. [PubMed]
- 208.Liu J., Xu Y., Zhang H., Gao X., Fan H., Wang G. Coronary flow velocity reserve is impaired in hypertensive patients with hyperhomocysteinemia. J Hum Hypertens. 2014;28:743–747. doi: 10.1038/jhh.2014.22. [DOI] [PubMed] [Google Scholar]
- 209.Zheng H., Li Y., Xie N., Xu H., Huang J., Luo M. Echocardiographic assessment of hypertensive patients with or without hyperhomocysteinemia. Clin Exp Hypertens. 2014;36:181–186. doi: 10.3109/10641963.2013.804542. [DOI] [PubMed] [Google Scholar]
- 210.Zhang T., Chen M., Guo L., Yu F., Zhou C., Xu H., et al. Reverse Erythroblastosis Virus alpha Antagonism Promotes Homocysteine Catabolism and Ammonia Clearance. Hepatology. 2019;70:1770–1784. doi: 10.1002/hep.30675. [DOI] [PubMed] [Google Scholar]
- 211.Chen M., Zhou C., Xu H., Zhang T., Wu B. Chronopharmacological targeting of Rev-erbalpha by puerarin alleviates hyperhomocysteinemia in mice. Biomed Pharmacother. 2020;125 doi: 10.1016/j.biopha.2020.109936. [DOI] [PubMed] [Google Scholar]
- 212.Liu J., Xu H., Zhang L., Wang S., Lu D., Chen M., et al. Chronoeffects of the Herbal Medicines Puerariae radix and Coptidis rhizoma in Mice: A Potential Role of REV-ERBalpha. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.707844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Xian Y.F., Fan D., Ip S.P., Mao Q.Q., Lin Z.X. Antidepressant-Like Effect of Isorhynchophylline in Mice. Neurochem Res. 2017;42:678–685. doi: 10.1007/s11064-016-2124-5. [DOI] [PubMed] [Google Scholar]
- 214.Li Y., Yu R., Zhang D., Yang W., Hou Q., Li Y., et al. Deciphering the Mechanism of the Anti-Hypertensive Effect of Isorhynchophylline by Targeting Neurotransmitters Metabolism of Hypothalamus in Spontaneously Hypertensive Rats. ACS Chem Neurosci. 2020;11:1563–1572. doi: 10.1021/acschemneuro.9b00699. [DOI] [PubMed] [Google Scholar]
- 215.Li Z., Shi H., Li Y., Wang W., Li Z., Chen B., et al. Isorhynchophylline ameliorates the progression of osteoarthritis by inhibiting the NF-kappaB pathway. Eur J Pharmacol. 2022;924 doi: 10.1016/j.ejphar.2022.174971. [DOI] [PubMed] [Google Scholar]
- 216.Wang D., Sun M., Liu Y., Wang L., Li C., Li Y., et al. Isorhynchophylline Regulates the Circadian Rhythm of the Hypothalamus in Spontaneously Hypertensive Rats to Treat Hypertension. Curr Pharm Des. 2023;29:139–148. doi: 10.2174/1381612829666221222115134. [DOI] [PubMed] [Google Scholar]
- 217.Sun M., Wang H., Gong L., Qi D., Wang X., Li Y., et al. A novel time-dimension and circadian rhythm-dependent strategy for pharmacodynamic evaluation of Uncaria in the regulation of neurotransmitter circadian metabolic homeostasis in spontaneously hypertensive rats. Biomed Pharmacother. 2020;131 doi: 10.1016/j.biopha.2020.110704. [DOI] [PubMed] [Google Scholar]
- 218.Pourcet B., Duez H. Circadian Control of Inflammasome Pathways: Implications for Circadian Medicine. Front Immunol. 2020;11:1630. doi: 10.3389/fimmu.2020.01630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Huang T., Mariani S., Redline S. Sleep Irregularity and Risk of Cardiovascular Events: The Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2020;75:991–999. doi: 10.1016/j.jacc.2019.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wu X., Chen L., Zeb F., Li C., Jiang P., Chen A., et al. Clock-Bmal1 mediates MMP9 induction in acrolein-promoted atherosclerosis associated with gut microbiota regulation. Environ Pollut. 2019;252:1455–1463. doi: 10.1016/j.envpol.2019.06.042. [DOI] [PubMed] [Google Scholar]
- 221.Schilperoort M., van den Berg R., Bosmans L.A., van Os B.W., Dolle M.E.T., Smits N.A.M., et al. Disruption of circadian rhythm by alternating light-dark cycles aggravates atherosclerosis development in APOE*3-Leiden.CETP mice. J Pineal Res. 2020;68:e12614. doi: 10.1111/jpi.12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Young M.E., Brewer R.A., Peliciari-Garcia R.A., Collins H.E., He L., Birky T.L., et al. Cardiomyocyte-specific BMAL1 plays critical roles in metabolism, signaling, and maintenance of contractile function of the heart. J Biol Rhythms. 2014;29:257–276. doi: 10.1177/0748730414543141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Rodrigo G.C., Herbert K.E. Regulation of vascular function and blood pressure by circadian variation in redox signalling. Free Radic Biol Med. 2018;119:115–120. doi: 10.1016/j.freeradbiomed.2017.10.381. [DOI] [PubMed] [Google Scholar]
- 224.Dang Y., Ling S., Ma J., Ni R., Xu J.W. Bavachalcone Enhances RORalpha Expression, Controls Bmal1 Circadian Transcription, and Depresses Cellular Senescence in Human Endothelial Cells. Evid Based Complement Alternat Med. 2015;2015 doi: 10.1155/2015/920431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Alcorta D.A., Xiong Y., Phelps D., Hannon G., Beach D., Barrett J.C. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc Natl Acad Sci U S A. 1996;93:13742–13747. doi: 10.1073/pnas.93.24.13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Orjalo A.V., Bhaumik D., Gengler B.K., Scott G.K., Campisi J. Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc Natl Acad Sci U S A. 2009;106:17031–17036. doi: 10.1073/pnas.0905299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wu X., Chen L., Zeb F., Huang Y., An J., Ren J., et al. Regulation of circadian rhythms by NEAT1 mediated TMAO-induced endothelial proliferation: A protective role of asparagus extract. Exp Cell Res. 2019;382 doi: 10.1016/j.yexcr.2019.05.032. [DOI] [PubMed] [Google Scholar]
- 228.Chen L., Wu X., Zeb F., Huang Y., An J., Jiang P., et al. Acrolein-induced apoptosis of smooth muscle cells through NEAT1-Bmal1/Clock pathway and a protection from asparagus extract. Environ Pollut. 2020;258 doi: 10.1016/j.envpol.2019.113735. [DOI] [PubMed] [Google Scholar]
- 229.Scheltens P., Blennow K., Breteler M.M., de Strooper B., Frisoni G.B., Salloway S., et al. Alzheimer's disease. Lancet. 2016;388:505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 230.Holth J.K., Fritschi S.K., Wang C., Pedersen N.P., Cirrito J.R., Mahan T.E., et al. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science. 2019;363:880–884. doi: 10.1126/science.aav2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Liguori C., Spanetta M., Izzi F., Franchini F., Nuccetelli M., Sancesario G.M., et al. Sleep-Wake Cycle in Alzheimer's Disease Is Associated with Tau Pathology and Orexin Dysregulation. J Alzheimers Dis. 2020;74:501–508. doi: 10.3233/JAD-191124. [DOI] [PubMed] [Google Scholar]
- 232.Cullell N, Carcel-Marquez J, Gallego-Fabrega C, Muino E, Llucia-Carol L, Lledos M, et al. Sleep/wake cycle alterations as a cause of neurodegenerative diseases: A Mendelian randomization study. Neurobiol Aging 2021;106:320 e321-320 e312. doi: 10.1016/j.neurobiolaging.2021.05.008. [DOI] [PubMed]
- 233.Benedict C., Blennow K., Zetterberg H., Cedernaes J. Effects of acute sleep loss on diurnal plasma dynamics of CNS health biomarkers in young men. Neurology. 2020;94:e1181–e1189. doi: 10.1212/WNL.0000000000008866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Huang J., Peng X., Fan R., Dong K., Shi X., Zhang S., et al. Disruption of Circadian Clocks Promotes Progression of Alzheimer's Disease in Diabetic Mice. Mol Neurobiol. 2021;58:4404–4412. doi: 10.1007/s12035-021-02425-7. [DOI] [PubMed] [Google Scholar]
- 235.Song H., Moon M., Choe H.K., Han D.H., Jang C., Kim A., et al. Abeta-induced degradation of BMAL1 and CBP leads to circadian rhythm disruption in Alzheimer's disease. Mol Neurodegener. 2015;10:13. doi: 10.1186/s13024-015-0007-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Cronin P., McCarthy M.J., Lim A.S.P., Salmon D.P., Galasko D., Masliah E., et al. Circadian alterations during early stages of Alzheimer's disease are associated with aberrant cycles of DNA methylation in BMAL1. Alzheimers Dement. 2017;13:689–700. doi: 10.1016/j.jalz.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zhu X., Zhang W., Zhao J., Wang J., Qu W. Hypolipidaemic and hepatoprotective effects of ethanolic and aqueous extracts from Asparagus officinalis L. by-products in mice fed a high-fat diet. J Sci Food Agric. 2010;90:1129–1135. doi: 10.1002/jsfa.3923. [DOI] [PubMed] [Google Scholar]
- 238.Zhao J., Zhang W., Zhu X., Zhao D., Wang K., Wang R., et al. The aqueous extract of Asparagus officinalis L. by-product exerts hypoglycaemic activity in streptozotocin-induced diabetic rats. J Sci Food Agric. 2011;91:2095–2099. doi: 10.1002/jsfa.4429. [DOI] [PubMed] [Google Scholar]
- 239.Wang J., Liu Y., Zhao J., Zhang W., Pang X. Saponins extracted from by-product of Asparagus officinalis L. suppress tumour cell migration and invasion through targeting Rho GTPase signalling pathway. J Sci Food Agric. 2013;93:1492–1498. doi: 10.1002/jsfa.5922. [DOI] [PubMed] [Google Scholar]
- 240.Chan Y.C., Wu C.S., Wu T.C., Lin Y.H., Chang S.J. A Standardized Extract of Asparagus officinalis Stem (ETAS((R))) Ameliorates Cognitive Impairment, Inhibits Amyloid beta Deposition via BACE-1 and Normalizes Circadian Rhythm Signaling via MT1 and MT2. Nutrients. 2019:11. doi: 10.3390/nu11071631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Mihaylova D., Popova A., Vrancheva R., Dincheva I. HS-SPME-GC-MS Volatile Profile Characterization of Peach (Prunus persica L. Batsch) Varieties Grown in the Eastern Balkan Peninsula. Plants (Basel) 2022:11. doi: 10.3390/plants11020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Sun Q., Xu W., Liu Y., Zhan S., Shao X., Wu Z., et al. Single-Cell Transcriptomic Analysis Demonstrates the Regulation of Peach Polysaccharides on Circadian Rhythm Disturbance. Mol Nutr Food Res. 2022;66:e2101170. doi: 10.1002/mnfr.202101170. [DOI] [PubMed] [Google Scholar]
- 243.Zhao M., Han J. Dendrobium Officinale Kimura et Migo Ameliorates Insulin Resistance in Rats with Diabetic Nephropathy. Med Sci Monit Basic Res. 2018;24:84–92. doi: 10.12659/MSMBR.909242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Li S., Wang J., Zhang L., Zheng Y., Ma G., Sun X., et al. Preparation of Dendrobium officinale Flower Anthocyanin and Extended Lifespan in Caenorhabditis elegans. Molecules. 2022:27. doi: 10.3390/molecules27238608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Sun S.J., Deng P., Peng C.E., Ji H.Y., Mao L.F., Peng L.Z. Extraction, Structure and Immunoregulatory Activity of Low Molecular Weight Polysaccharide from Dendrobium officinale. Polymers (Basel) 2022:14. doi: 10.3390/polym14142899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sun Y., Zeng X., Liu Y., Zhan S., Wu Z., Zheng X., et al. Dendrobium officinale polysaccharide attenuates cognitive impairment in circadian rhythm disruption mice model by modulating gut microbiota. Int J Biol Macromol. 2022;217:677–688. doi: 10.1016/j.ijbiomac.2022.07.090. [DOI] [PubMed] [Google Scholar]
- 247.Park M., Kim S.A., Yee J., Shin J., Lee K.Y., Joo E.J. Significant role of gene-gene interactions of clock genes in mood disorder. J Affect Disord. 2019;257:510–517. doi: 10.1016/j.jad.2019.06.056. [DOI] [PubMed] [Google Scholar]
- 248.Costa C., Mondello S., Micali E., Indelicato G., Licciardello A.A., Vitale E., et al. Night shift work in resident physicians: does it affect mood states and cognitive levels? J Affect Disord. 2020;272:289–294. doi: 10.1016/j.jad.2020.03.139. [DOI] [PubMed] [Google Scholar]
- 249.Cheng W.J., Puttonen S., Vanttola P., Koskinen A., Kivimaki M., Harma M. Association of shift work with mood disorders and sleep problems according to chronotype: a 17-year cohort study. Chronobiol Int. 2021;38:518–525. doi: 10.1080/07420528.2021.1885431. [DOI] [PubMed] [Google Scholar]
- 250.Spencer S., Falcon E., Kumar J., Krishnan V., Mukherjee S., Birnbaum S.G., et al. Circadian genes Period 1 and Period 2 in the nucleus accumbens regulate anxiety-related behavior. Eur J Neurosci. 2013;37:242–250. doi: 10.1111/ejn.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Crouse J.J., Carpenter J.S., Song Y.J.C., Hockey S.J., Naismith S.L., Grunstein R.R., et al. Circadian rhythm sleep-wake disturbances and depression in young people: implications for prevention and early intervention. Lancet Psychiatry. 2021;8:813–823. doi: 10.1016/S2215-0366(21)00034-1. [DOI] [PubMed] [Google Scholar]
- 252.Dallaspezia S., Locatelli C., Lorenzi C., Pirovano A., Colombo C., Benedetti F. Sleep homeostatic pressure and PER3 VNTR gene polymorphism influence antidepressant response to sleep deprivation in bipolar depression. J Affect Disord. 2016;192:64–69. doi: 10.1016/j.jad.2015.11.039. [DOI] [PubMed] [Google Scholar]
- 253.Difrancesco S., Lamers F., Riese H., Merikangas K.R., Beekman A.T.F., van Hemert A.M., et al. Sleep, circadian rhythm, and physical activity patterns in depressive and anxiety disorders: A 2-week ambulatory assessment study. Depress Anxiety. 2019;36:975–986. doi: 10.1002/da.22949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Chellian R., Pandy V., Mohamed Z. Pharmacology and toxicology of alpha- and beta-Asarone: A review of preclinical evidence. Phytomedicine. 2017;32:41–58. doi: 10.1016/j.phymed.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 255.Mohammed M.M., Sallam A.E., Hussein A.A., Marrez D.A., Ibrahim Z.N. The cyanobacteriumOscillatoria brevisβ-carotene extract modulates alterations of biochemical and hematological circadian patterns in stress-induced rat. Biol Rhythm Res. 2015;47:339–352. doi: 10.1080/09291016.2015.1116740. [DOI] [Google Scholar]
- 256.Soriano A., Alanon M.E., Alarcon M., Garcia-Ruiz A., Diaz-Maroto M.C., Perez-Coello M.S. Oak wood extracts as natural antioxidants to increase shelf life of raw pork patties in modified atmosphere packaging. Food Res Int. 2018;111:524–533. doi: 10.1016/j.foodres.2018.05.055. [DOI] [PubMed] [Google Scholar]
- 257.Haraguchi A., Du Y., Shiraishi R., Takahashi Y., Nakamura T.J., Shibata S. Oak extracts modulate circadian rhythms of clock gene expression in vitro and wheel-running activity in mice. Sleep Biol Rhythms. 2022;20:255–266. doi: 10.1007/s41105-021-00365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Xu Z., Lin S., Gong J., Feng P., Cao Y., Li Q., et al. Exploring the Protective Effects and Mechanism of Crocetin From Saffron Against NAFLD by Network Pharmacology and Experimental Validation. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.681391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Siddiqui S.A., Ali Redha A., Snoeck E.R., Singh S., Simal-Gandara J., Ibrahim S.A., et al. Anti-Depressant Properties of Crocin Molecules in Saffron. Molecules. 2022;27 doi: 10.3390/molecules27072076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Chen W., Su J., Liu Y., Gao T., Ji X., Li H., et al. Crocin Ameliorates Diabetic Nephropathy through Regulating Metabolism, CYP4A11/PPARgamma, and TGF-beta/Smad Pathways in Mice. Curr Drug Metab. 2023;24:709–722. doi: 10.2174/0113892002257928231031113337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Xiao L., Sun R., Han Y., Xia L., Lin K., Fu W., et al. NAMPT-NAD(+) is involved in the senescence-delaying effects of saffron in aging mice. Exp Ther Med. 2024;27:123. doi: 10.3892/etm.2024.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Lopresti A.L., Smith S.J., Drummond P.D. An investigation into an evening intake of a saffron extract (affron(R)) on sleep quality, cortisol, and melatonin concentrations in adults with poor sleep: a randomised, double-blind, placebo-controlled, multi-dose study. Sleep Med. 2021;86:7–18. doi: 10.1016/j.sleep.2021.08.001. [DOI] [PubMed] [Google Scholar]
- 263.Tsai Y.C., Wang S.L., Wu M.Y., Liao C.H., Lin C.H., Chen J.J., et al. Pilloin, A Flavonoid Isolated from Aquilaria sinensis, Exhibits Anti-Inflammatory Activity In Vitro and In Vivo. Molecules. 2018;23 doi: 10.3390/molecules23123177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Kankaynar M., Ceyhun H.A., Baran A., Sulukan E., Yildirim S., Bolat I., et al. The anxiolytic and circadian regulatory effect of agarwood water extract and its effects on the next generation; zebrafish modelling. Comp Biochem Physiol C Toxicol Pharmacol. 2023;269 doi: 10.1016/j.cbpc.2023.109621. [DOI] [PubMed] [Google Scholar]
- 265.Kawamoto N., Ito H., Tokuda I.T., Iwasaki H. Damped circadian oscillation in the absence of KaiA in Synechococcus. Nat Commun. 2020;11:2242. doi: 10.1038/s41467-020-16087-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Gutierrez-Martinez P., Rossi D.J., Beerman I. DNA Damage and Aging Around the Clock. Trends Mol Med. 2016;22:635–637. doi: 10.1016/j.molmed.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Wang N., Sun Y., Zhang H., Wang B., Chen C., Wang Y., et al. Long-term night shift work is associated with the risk of atrial fibrillation and coronary heart disease. Eur Heart J. 2021;42:4180–4188. doi: 10.1093/eurheartj/ehab505. [DOI] [PubMed] [Google Scholar]
- 268.Chen JH, Zhou MY, Huang RF, Xin HR, Cheng ST, Li MD, et al. [Feeding rhythm entrains circadian metabolism genes, but not the circadian clock, in brown adipose tissue]. Sheng Li Xue Bao 2022;74:726-736. doi. Available from https://www.ncbi.nlm.nih.gov/pubmed/36319096. [PubMed]
- 269.He R., Zhang S., Yu J., Yu X., Wang J., Qiu Y., et al. Per1/Per2 knockout Affects Spleen Immune Function in Elderly Mice via Inducing Spleen Lymphocyte Ferroptosis. Int J Mol Sci. 2022;23 doi: 10.3390/ijms232112962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Aye L., Wang Z., Chen F., Xiong Y., Zhou J., Wu F., et al. Circadian Regulator-Mediated Molecular Subtypes Depict the Features of Tumor Microenvironment and Indicate Prognosis in Head and Neck Squamous Cell Carcinoma. J Immunol Res. 2023;2023:9946911. doi: 10.1155/2023/9946911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Perez-Villa A., Echeverria-Garces G., Ramos-Medina M.J., Prathap L., Martinez-Lopez M., Ramirez-Sanchez D., et al. Integrated multi-omics analysis reveals the molecular interplay between circadian clocks and cancer pathogenesis. Sci Rep. 2023;13:14198. doi: 10.1038/s41598-023-39401-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Hua Y., Dai X., Xu Y., Xing G., Liu H., Lu T., et al. Drug repositioning: Progress and challenges in drug discovery for various diseases. Eur J Med Chem. 2022;234 doi: 10.1016/j.ejmech.2022.114239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Martino T.A., Harrington M.E. The Time for Circadian Medicine. J Biol Rhythms. 2020;35:419–420. doi: 10.1177/0748730420946501. [DOI] [PubMed] [Google Scholar]
- 274.Zarrinpar A., Chaix A., Yooseph S., Panda S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 2014;20:1006–1017. doi: 10.1016/j.cmet.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Liang X., Bushman F.D., FitzGerald G.A. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc Natl Acad Sci U S A. 2015;112:10479–10484. doi: 10.1073/pnas.1501305112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Thaiss C.A., Levy M., Korem T., Dohnalova L., Shapiro H., Jaitin D.A., et al. Microbiota Diurnal Rhythmicity Programs Host Transcriptome Oscillations. Cell. 2016;167(1495–1510):e1412. doi: 10.1016/j.cell.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 277.Brooks J.F., 2nd, Behrendt C.L., Ruhn K.A., Lee S., Raj P., Takahashi J.S., et al. The microbiota coordinates diurnal rhythms in innate immunity with the circadian clock. Cell. 2021;184(4154–4167):e4112. doi: 10.1016/j.cell.2021.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Fawad J.A., Luzader D.H., Hanson G.F., Moutinho T.J., Jr., McKinney C.A., Mitchell P.G., et al. Histone Deacetylase Inhibition by Gut Microbe-Generated Short-Chain Fatty Acids Entrains Intestinal Epithelial Circadian Rhythms. Gastroenterology. 2022;163(1377–1390):e1311. doi: 10.1053/j.gastro.2022.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Kang H., Zhang W., Jing J., Huang D., Zhang L., Wang J., et al. The gut-brain axis involved in polystyrene nanoplastics-induced neurotoxicity via reprogramming the circadian rhythm-related pathways. J Hazard Mater. 2023;458 doi: 10.1016/j.jhazmat.2023.131949. [DOI] [PubMed] [Google Scholar]
- 280.Mencarelli A., Cipriani S., Renga B., Bruno A., D'Amore C., Distrutti E., et al. VSL#3 resets insulin signaling and protects against NASH and atherosclerosis in a model of genetic dyslipidemia and intestinal inflammation. PLoS One. 2012;7:e45425. doi: 10.1371/journal.pone.0045425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Sanduzzi Zamparelli M., Compare D., Coccoli P., Rocco A., Nardone O.M., Marrone G., et al. The Metabolic Role of Gut Microbiota in the Development of Nonalcoholic Fatty Liver Disease and Cardiovascular Disease. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17081225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Demir M., Lang S., Hartmann P., Duan Y., Martin A., Miyamoto Y., et al. The fecal mycobiome in non-alcoholic fatty liver disease. J Hepatol. 2022;76:788–799. doi: 10.1016/j.jhep.2021.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Zhang Q., Xing W., Wang Q., Tang Z., Wang Y., Gao W. Gut microbiota-mitochondrial inter-talk in non-alcoholic fatty liver disease. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.934113. [DOI] [PMC free article] [PubMed] [Google Scholar]