Graphical abstract
Keywords: Dry eye disease, Depression, Meta-analysis, Genome-wide association study, Mendelian randomization, Multimodality Biobank
Highlights
-
•
Our meta-analysis confirmed the positive correlation between DED and DEP.
-
•
The genetic architecture similarity between DED-DEP was 0.19 in TW biobank and 0.109 in UK biobank.
-
•
Transcriptome study underlined immune activation at the transaction of DED-DEP concurrence.
-
•
Bi-direction DED-to-DEP and DEP-to-DED disease Causation was detected by IVW-MR analysis.
-
•
Disease causation inference remains valid after COJO selected LD-free IVSNPs for MR experiments.
Abstract
Introduction
The clinical presentations of dry eye disease (DED) and depression (DEP) often comanifest. However, the robustness and the mechanisms underlying this association were undetermined.
Objectives
To this end, we set up a three-segment study that employed multimodality results (meta-analysis, genome-wide association study [GWAS] and Mendelian randomization [MR]) to elucidate the association, common pathways and causality between DED and DEP.
Methods
A meta-analysis comprising 26 case−control studies was first conducted to confirm the DED-DEP association. Next, we performed a linkage disequilibrium (LD)-adjusted GWAS and targeted phenotype association study (PheWAS) in East Asian TW Biobank (TWB) and European UK Biobank (UKB) populations. Single-nucleotide polymorphisms (SNPs) were further screened for molecular interactions and common pathways at the functional gene level. To further elucidate the activated pathways in DED and DEP, a systemic transcriptome review was conducted on RNA sequencing samples from the Gene Expression Omnibus. Finally, 48 MR experiments were implemented to examine the bidirectional causation between DED and DEP.
Results
Our meta-analysis showed that DED patients are associated with an increased DEP prevalence (OR = 1.83), while DEP patients have a concurrent higher risk of DED (OR = 2.34). Notably, cross-disease GWAS analysis revealed that similar genetic architecture (rG = 0.19) and pleiotropic functional genes contributed to phenotypes in both diseases. Through protein−protein interaction and ontology convergence, we summarized the pleiotropic functional genes under the ontology of immune activation, which was further validated by a transcriptome systemic review. Importantly, the inverse variance-weighted (IVW)-MR experiments in both TWB and UKB populations (p value <0.001) supported the bidirectional exposure-outcome causation for DED-to-DEP and DEP-to-DED. Despite stringent LD-corrected instrumental variable re-selection, the bidirectional causation between DED and DEP remained.
Conclusion
With the multi-modal evidence combined, we consolidated the association and causation between DED and DEP.
Introduction
Clinical association between DED and DEP
Dry eye disease (DED) and depression (DEP) are two prevalent health conditions that trouble modern society. The prevalence of DED and DEP varies form 1.5–65.0 % and 2.7–37.4 % respectively across different nations (Table 1 and Supplement 1, Fig. S1) [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28]. Although distinguished as two independent diseases, DED and DEP are highly comanifested in real-world populations, for which the underlying mechanisms remain uncharted.
Table 1.
Prevalence of Dry Eye Disease and Depression in Various Countries.
| Continent | Country |
Prevalence |
||
|---|---|---|---|---|
| Dry eye disease | Depression | |||
| Africa | North | Egyptian | 34.9 % [1] | 2.7 % [2] |
| South | South Africa | 54.9 % [1] | 9.7 % [3] | |
| West | Ghana | 38.3 % [1] | 25.2 % [4] | |
| Asia | North | Russia | 8.3 % [5] | 41.5 % [6] |
| Northeast | Japan | 17.4 % [7] | 5.7 % [8] | |
| South | India | 32.0 % [9] | 5.3 % [10] | |
| Southeast | Indonesia | 27.5 % [11] | 21.8 % [12] | |
| East | Taiwan | 33.7 % [13] | 1.9 % [14] | |
| West | Saudi Arabia | 36.7 % [15] | 37.4 % [16] | |
| America | North | United State | 14.6 % [17] | 9.2 % [18] |
| South | Brazil | 12.8 % [19] | 10.8 % [20] | |
| Australia | Australia | 1.5–16.3 % [21] | 21.3 % [22] | |
| Europe | North | Sweden | 65.0 % [23] | 17.2 % [24] |
| South | Spain | 11.0 % [25] | 4.7 % [26] | |
| West | United Kingdom | 32.1 % [27] | 15.5 % [28] | |
Notably, a secondary cross-sectional analysis [29] of the DRy Eye Assessment and Management (DREAM) study demonstrated that patients with DEP conditions had poor DED characteristics, including reduced tear break-up time (TBUT), increased corneal epithelium defect, and worse dry-eye composite grading index scores. Significantly, objective DED tear biomarkers were also reduced in newly diagnosed DEP patients who were antidepressant-free [30]. This finding suggested that the association between DED and DEP is valid regardless of the DED-causing drug effects of various anti-depressants. On the other hand, a meta-analysis enrolling three million individuals of multiple ethnicities also concluded that patients with DED have an increased risk (odds ratio (OR) = 2.92) of DEP with a worse DEP severity[31]. These independent but inter-connected evidence indicated that DED-DEP was associated in terms of both disease prevalence and disease severity.
Despite the positive evidence that supports the DED-DEP association, the claim has been challenged by negative results in some cross-sectional[32] and naturalistic observational studies[33], Specifically, the contradictory findings suggested that the DED-DEP association was an epiphenomenon caused by psychiatric subjectiveness and unmeasurable confounding factors, such as chronic inflammation[34] and medication adverse events[35], [36]. While the clinical insights into the DED-DEP association constantly advance our intervention strategies, more avant-garde studies that employ genetic and multimodal data have been conducted to expand the landscape of disease-disease associations[37].
Genetic and pathway similarity between DED and DEP
Measuring cross-disease genetic architecture similarity has emerged as a robust method to evaluate disease-disease associations. Given that both DED and DEP are multifaceted diseases that involve genetic x environmental (GxE) interactions, it is sensible to elucidate the genetic contributions underlying the clinical DED-DEP comanifestation[38]. Genetic pleiotropic analysis has been adopted to confirm the shared genetic architecture between a stress-related disease network including but not limited to the psychiatric and ocular disease entries[39]. Meanwhile the brain and the eye were both densely neuron composite organs, in which a genetic defect of the eye could lead to a parallel brain disease[40], [41]. Hence by decoding the brain-eye intersected mutation profile may uncover valuable molecular hotspots accountable for the pathogenesis of DED and DEP.
According to the UK Adult Twin Registry (TwinsUK) and meta-analysis, the heritability (h2) of DED and DEP were estimated to be approximately 37 %[42] and 41 %[43] respectively. This indicated that genetic predisposition plays an important role in DED and DEP. To date, clinical biomarker discovery, transcriptome analysis and genome-wide association studies (GWASs) have shown overlapping pathways in DED/DEP, including inflammatory cytokine cascade[34] and immune cell activation[44]. Enzyme-linked immunosorbent assays have confirmed that inflammatory mediators such as the cytokine interleukin-1 (IL-1) are highly expressed in the basal tears of DED patients[44] as well as in the plasma of DEP patients[45].
A DED clinical trial demonstrated that IL-1 blockade could improve DED symptoms[46], while another pharmacogenetic study identified single-nucleotide polymorphisms (SNPs: rs16944, rs1143643, and rs1143634) in the IL-1b gene as indicators of DED treatment refractoriness[47]. On the other hand, the InCHIANTI study[45] revealed that increased IL-1 receptor antagonist (IL-1Ra) levels were associated with depressive symptoms in elderly individuals. A concurrent study also showed prominent proinflammatory cytokine (IL-1beta, TNF-alpha, IFN-gamma, IL-6 and IL-17A) elevation in antidepressant nonresponders[48]. In fact, a systemic review indicated that cytokine-blocking biologics such as anti-TNF alpha (adalimumab, etanercept, infliximab) and anti-IL-6 (tocilizumab) demonstrated antidepressant activities in clinical patients[49]. These collective studies indicated a converging interest in targeting the inflammatory axis as a novel treatment for DED and DEP.
Nevertheless, the cytokine association does not explain the cause of the DED-DEP comanifestation; hence, further causative analyses, such as Mendelian randomization (MR) studies, were conducted in this study.
Investigate disease variant causations by MR
Mendelian randomization (MR) is a causation analysis method that employs instrument variables (IVs) to examine the relations between disease exposure and outcome. Among all implemented MR IVs, trait-associated SNPs stood out as a robust disease proxy, given that randomized SNP segregation in meiosis could be employed to eliminate confounding factors in causal inference. The selection of IVs should satisfy three assumptions[50]: (i) relevance assumption: IVs should be related to the exposure disease; (ii) independence assumption: IVs should be unrelated to confounders of both the exposure and the outcome; and (iii) exclusion restriction assumption: IVs do not directly influence the outcome of interest. If any of the assumptions are violated, an extended spectrum of the modified MR can be applied to ensure causative validity[51].
MR-based studies have advanced our knowledge of the causation of DED and DEP; for instance, dysregulation of gut microbiota[52] may cause dry eye symptoms in Sjögren’s syndrome patients, while increased IL-6/C-reactive protein axis activity[53] and less physical activity[54] cause DEP. Through MR studies, investigators identified modifiable risk factors that have the potential to treat DED and DEP patients. However, in terms of the complex DED-DEP clinical comanifestation, whether DED and DEP can directly cause one another is a question yet to be determined.
Albeit a nested body of evidence had affirmed the concurrence between DED and DEP, the overwhelming amount of studies had not brought forth a general consensus, hence it is imperative to digest the hitherto findings with a systemic approach. To this end, we performed an up to date meta-analysis to consolidate the DED-DEP association, then conducted a transcriptome analysis to find the molecular pathways at the transaction of DED-DEP co-axis. Importantly, we employed the multiethnic genetic data from the Taiwan Biobank (TWB) and UK Biobank (UKB) to (i) characterize the disease-associated SNPs and the targeted phenome-wide SNP association; (ii) evaluate the genetic architecture similarity and its contribution to DED-DEP cooccurrence; and (iii) identify genetic causations of a cooccurring disease in a sequential manner (Fig. 1).
Fig. 1.
Study Design Overview. To evaluate the underlying relationship between dry eye disease (DED) and depression (DEP), we performed a three-segment study. First, we reviewed the disease association by literature meta-analysis. Second, we confirmed a two-disease genetic correlation by a genome-wide association study (GWAS) in Taiwan Biobank (TWB) and UK Biobank (UKB), clarifying pathway correlation by gene ontology (GO) and protein−protein interaction (PPI) analysis. Finally, we identified bidirectional causation by regarding single nucleotide polymorphisms (SNPs) as instrumental variables (IVs) in Mendelian randomization (MR).
Material and methods
This is a three-segment study that included meta-analysis, GWAS-ontology and MR analysis, each part addressed the clinical correlation, genetic similarity and causation effects between DED and DEP.
Ethics statement
This study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Institutional Review Board of Taipei Veterans General Hospital (ID no. 2023–01-006AC). All participants in TWB and UKB signed written informed consent before starting the study. All articles selected for systemic transcriptome review involving animals were conducted according to the ethical policies and procedures approved by the ethics committee of the researchers’ institute.
Meta-analysis for DED-DEPs clinical association
The meta-analysis was completed according to the Meta-analyses Of Observational Studies in Epidemiology (MOOSE) checklist (Supplement 1, Table S1) with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) completeness evaluation (Supplement 1, Table S2) and Newcastle–Ottawa Scale (NOS) literature quality check (Supplement 1, Table S3)[55]. the experimental details are specified in Supplement 1. The final search was conducted on 24 August 2022, and elusive information was clarified by inquiring author responses; the last inquiry was replied to on 31 December 2022. The eligibility criteria for study inclusion were based on the Cochrane Handbook for Systematic Review of Interventions. We included all studies documenting DED (xerophthalmi, xerophthalmia, keratoconjuctivitis sicca or dry eye) and DEP (depression or depressive). We included only original studies. Disease prevalence or graded severity were not documented will be excluded from subsequent analysis (details are specified in Supplement 1, Fig. S2). We gathered the data from studies that fulfilled our inclusion criteria. We utilized a standardized form to document the details of the authors, year of publication, study design, sample size, age, gender, the tool or scale employed to evaluate and rate DED and DEP, the occurrence rate of DED and DEP, and the score of DED and DEP based on the scale or instrument used. The included studies were assessed independently by two reviewers (H-Y Wu and T-H Yu). In case of disagreements, a third party (H-J Dai) would mediate until mutual agreement was reached.
In this study, DED severity was graded by a subjective questionnaire (Ocular Surface Disease Index [OSDI] and 5-Item Dry Eye Questionnaire [DEQS]) or objective measures (ST and TBUT). Meanwhile, DEP severity was evaluated by scales including but not limited to the Patient Health Questionnaire-9 (PHQ-9), Hamilton Depression Rating Scale (HDRS) and Major Depression Inventory (MDI).
Twenty-six articles were finally selected, including 1 cross-sectional study[56], 2 cohort studies[57], [58], and 23 case-control studies[35], [36], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79]. The total number of patients was 515,389 for DED and 571,518 for DEP, and the demographic descriptions are provided in Supplement 1, Table S4.
Review Manager software (RevMan) was used for the analysis, and the primary outcome was the Mantel–Haenszel (M−H) OR between the occurrence of DED and DEP, and the secondary outcome was the disease severity standardized mean difference (SMD) under the effect of disease co-occurrence. A random-effect model was employed if heterogeneity was significant according to Cochran’s Q-statistics χ2 test and I2 statistic (p value <0.1), and subgroup analysis was conducted to evaluate the DED-DEP association in distinct subpopulations.
Genetic correlation analysis in TWB and UKB
Genetic association results were established based on the genotype-phenotype data from selected subjects from TWB (https://www.twbiobank.org.tw/) and UKB (https://www.ukbiobank.ac.uk/) (Supplement 2, Table S1). Approximately 130,000 Asians and 502,000 European were collected from UKB and TWB, respectively. The disease cases in UKB were curated by International Classification of Disease (ICD) information, whereas the disease labels in TWB relied on self-report questionnaires. The control group was collected by screening individuals above 60 years old who were disease-free.
Genetic data quality control (QC) was processed on PLINK (version 1.9 downloaded from http://zzz.bwh.harvard.edu/plink/), in which we set SNPs and participant QC thresholds by the following conditions: (i) SNPs missing in more than 2 % of participants; (ii) individuals missing more than 5 % SNP data; (iii) SNPs with minor allele frequency (MAF) <0.05; (iv) SNPs deviating from Hardy–Weinberg equilibrium (p value <0.05); and (v) individuals with identity by descent (IBD) over 0.125. The subsequent analysis used 460,9691 SNPs (n = 6066) linked to DEP from TWB1 participants, 268,481 SNPs (n = 24745) linked to DED from TWB2 participants, and 296,724 and 297,473 SNPs linked to DED and DEP, respectively, from UKB. In total, 7,684/27,737 individuals in TWB1, 26,866/68,978 individuals in TWB2, and 215063/215957 individuals in UKB were enrolled for further analysis (Table 2). Age, sex, education, marital status, race, BMI, habits, and addiction were considered risk factors for DED and DEP and were adjusted for in the analysis. For individuals listed in both TWB1 and TWB2, our study only utilized the information from TWB2 to ensure that there was no duplication in the data calculations.
Table 2.
Demographic Information of the Participants.
| Characteristic | Taiwan Biobank 1 | Taiwan Biobank 2 | UK Biobank | p value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dry Eye Disease |
Case (n = 2564) |
Control (n = 4479) |
p value |
Case (n = 8757) |
Control (n = 15988) |
p value |
Case (n = 311) |
Control (n = 39223) |
p value | |
| Age (years) | 53 ± 11 | 64 ± 3 | <0.001+ | 55 ± 9 | 64 ± 3 | <0.001+ | 62 ± 6 | 64 ± 3 | <0.001+ | <0.001+ |
| Male sex (%) | 713 (27.8) | 2452 (54.7) | <0.001* | 1430 (16.3) | 6573 (41.1) | <0.001* | 74 (23.8) | 18,448 (47.0) | <0.001* | 0.905‡ |
| Depression | 141 (5.5) | 124 (2.8) | <0.001* | 657 (7.3) | 509 (3.2) | <0.001* | 32 (10.3) | 1811 (4.6) | <0.001* | <0.001‡ |
| Depression |
Case (n = 832) |
Control (n = 5234) |
p value |
Case (n = 2999) |
Control (n = 18625) |
p value |
Case (n = 3449) |
Control (n = 11293) |
p value | |
| Age (years) | 49 ± 11 | 64 ± 3 | <0.001+ | 52 ± 10 | 64 ± 3 | <0.001+ | 56 ± 8 | 64 ± 3 | <0.001+ | <0.001+ |
| Male sex (%) | 308 (37.0) | 2646 (50.6) | <0.001* | 621 (20.7) | 7074 (38.0) | <0.001* | 1139 (33.0) | 5433 (48.1) | <0.001* | 0.994‡ |
| Dry eye disease | 142 (17.1) | 836 (16.0) | 0.4551* | 678 (22.6) | 3255 (17.3) | <0.001* | 8 (0.2) | 23 (0.2) | 0.9163* | <0.001‡ |
Note: + Wilcox test, + Chi-square test of independence, ‡ Chi-square goodness of fit test.
Variant-disease association (VDA) studies focus on the linkages between SNPs and disease phenotypes through GWASs, scalable and accurate implementation of generalized mixed model (SAIGE), and conditional and joint association analysis (COJO). As the primary outcome of VDA, the results of the GWAS were presented via a Manhattan plot. Because single-variant score statistics did not follow a Gaussian distribution in DED with an unbalanced case−control ratio of DED in UKB (0.008)[80], SAIGE based on a logistic mixed model was applied to avoid inflation of the type I error rate[81]. COJO was applied to interpret joint effects of multiple SNPs owing to overestimated effects of different SNPs on a given phenotype caused by linkage disequilibrium (LD)[82]. We used the -cojo-cond function in the Genome-wide Complex Trait Analysis (GCTA)—COJO program (version 1.91.1 beta)[83].
Disease-disease association (DDA) studies focus on the genetic correlation among different disease phenotypes according to LD score regression. To calculate the overall genetic correlation of DDA, LD score regression used the product of two Z statistics from GWAS, each taken from a GWAS of both diseases[84], with the advantages of being robust to sample overlap bias and not requiring multiple individual genotypes or LD pruning[85].
Gene Ontology (GO) analysis was conducted in the Enrichr web server (https://maayanlab.cloud/Enrichr/)[86], [87], [88] to identify DED and DEP targeted phenotype association studies (PheWASs) between the databases (TWB and UKB) based on UK Biobank GWAS v1. Protein−protein interaction (PPI) network analysis was evaluated in the Search Tool for Retrieval of Interacting Genes/Proteins (STRING version 11.5 download from http://string-db.org/) with a confidence level of 0.400 (medium) based on experiments, databases, coexpression, neighborhood, gene function, and cooccurrence [88]. The activated pathways of functional genes within the PPI network were further analyzed in the Enrichr web server based on Reactome 2022. To further elucidate the activated pathways in DED and DEP, a systemic transcriptome review was conducted on RNA-seq samples from the Gene Expression Omnibus[89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109], [110], [111], [112], [113], [114], [115], [116], [117].
Bidirectional causation by MR
MR analysis was completed according to Strengthening the reporting of observational studies in epidemiology using mendelian randomization (STROBE-MR) (Supplement 4, Table S1)[118], [119], the experimental details are specified in Supplement 4.
To evaluate bidirectional causation between DED and DEP, MR analysis was conducted with SNPs from VDA studies using the TwoSampleMR package in R software (version 4.2.0 downloaded from https://github.com/MRCIEU/TwoSampleMR). However, SNPs may influence the expression of different genes owing to LD, and the issue of pleiotropy becomes nonnegligible, including horizontal (multiple independent effects of a given SNP causing multiple phenotypes) and vertical pleiotropy (multiple effects in a cascade causing multiple causally related phenotypes)[120]. To rule out pleiotropy, sensitivity analyses were conducted by reproducing the results through 6 different MR methods, including MR pleiotropy residual sum and outlier (MR-PRESSO)[121], inverse variance-weighted (IVW)[122], MR-Egger[123], two model-based estimation (MBE) methods[124], and weighted median (WM)[125].
In the absence of pleiotropy, the inverse variance weighted (IVW) method was preferred by weighing the effects of SNPs with the reciprocal of the intercept variance to estimate robust causal effects in the absence of directional pleiotropy[126]. In the presence of pleiotropy, the MR−Egger method was conducted by accepting the presence of an intercept under the assumption that the pleiotropic associations are independent[121]. The model-based estimation (MBE) simple mode and weighted mode methods allow the maximum proportion of information from invalid IVs to less and more than half, respectively, under the zero modal pleiotropy assumption (ZEMPA) that the most frequent value of horizontal pleiotropy is zero[124]. Weighted median methods combine data on multiple IVs into a single causal estimate by adopting a median after giving different weights to every IV based on the empirical distribution function[125].
Results
Applying a meta-analysis to confirm the clinical association between DED and DEP
Studies with diagnostic instruments and severity scales were included in this meta-analysis to assess the incidence and severity association between DED and DEP. Nineteen of the 26 included studies reported DEP symptoms in DED cohorts (19 reported the prevalence[8], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46] and 6 graded the severity[56], [61], [63], [69], [70], [71], [73]), while 10 studies reported DED symptoms in DEP cohorts (6 reported the prevalence[35], [56], [59], [64], [74], [75], 7 graded the severity[56], [74], [75], [76], [77], [78], [79], and 3 reported both[56], [74], [75]) (Supplement 1, Table S4). Our meta-analysis confirmed that DED patients, in comparison to healthy controls, had an increased DEP prevalence (summary OR = 1.83, 95 % CI: 1.60–2.10, I2 = 0 %) and DEP severity (SMD = 0.45, 95 % CI: 0.25–0.65, I2 = 0 %) (Fig. 2, Fig. 3). Meanwhile, DEP patients manifested with increased DED prevalence (summary OR = 2.34, 95 % CI: 1.58–3.46, I2 = 40.5 %), but were not linked to significant subjective (OSDI and DEQS) or objective DED severity (ST and TBUT) (Supplement 1, Fig. S3 and S4). Subgroup analyses are presented in Supplement 1, Fig. S5.
Fig. 2.
Forest plots illustrating associations between dry eye disease (DED) and depression (DEP) in terms of prevalence odds ratio (OR). (A) Association between the prevalence of depression in DED patients versus the control group. (B) Association between the prevalence of DED in depression patients versus the control group. CI, confidence interval; M−H, Mantel–Haenszel.
Fig. 3.
Meta-analysis results for the association between dry eye disease (DED) and depression (DEP). (A) Association between the prevalence of depression in DED patients versus the control group. (B) Association between the prevalence of DED in depression patients versus the control group. (C) Association between depression severity in DED patients versus the control group. Since the depression scales varied from study to study, the severity of depression was standardized into a Z score. (D) Association between the Schirmer test (ST) of DED severity in depression patients versus the control group. (E) Association between tear break-up time (TBUT) and DED severity in depression patients versus the control group. NS, not significant (p value >0.05); *, statistically significant with p value <0.05; **, statistically significant with p value <0.01; ****, statistically significant with p value <0.0001.
To validate the meta-analysis findings in an independent population-based cohort, we examined the disease association in TWB (Table 2). The results showed that increased DEP prevalence was observed in DED patients within TWB1 (OR = 2.04, 95 % CI: 1.60–2.61, p value <0.00001) and TWB2 (OR = 2.38, 95 % CI: 2.11–2.68, p value <0.00001). However, increased DED prevalence was observed only in DEP patients in TWB2 (OR = 1.39, 95 % CI: 1.27–1.53, p value <0.00001) but not TWB1 (Table 2). In brief, our meta-analysis and TWB validation supported the positive association between DED and DEP (Table 2).
Genetic similarity and shared disease ontology between DED and DEP
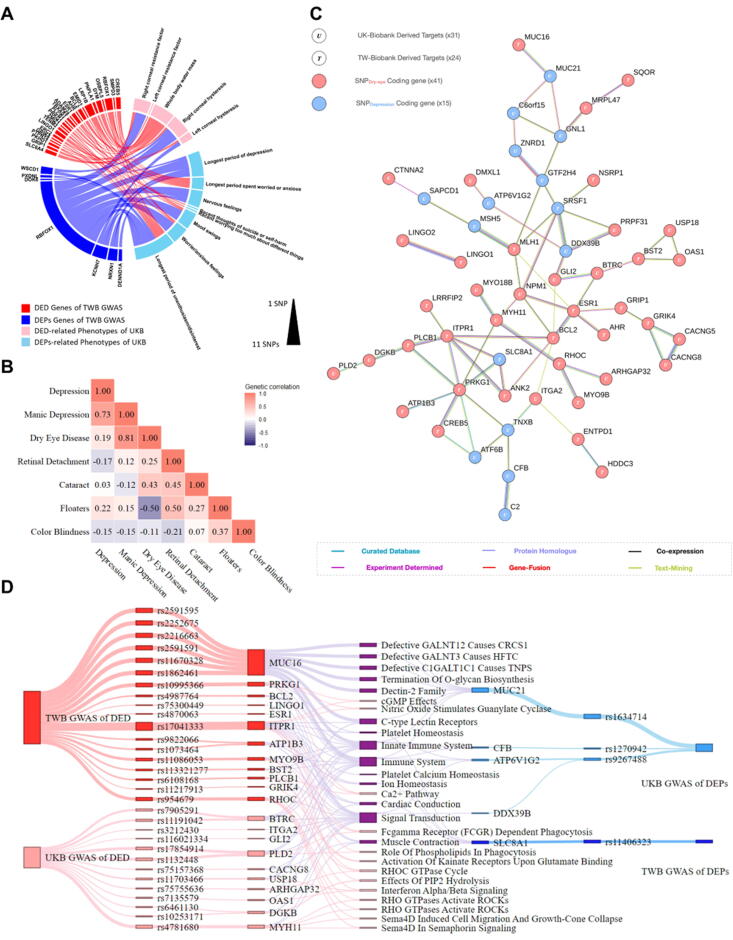
To explore whether genetic factors contributed to the DED and DEP association, we performed PheWAS and a chord-interacting diagram analysis to find the shared functional SNPs in the DED-DEP UKB phoneme. With the input of the top 500 DED and DEP SNPs, we retrieved 87 DED and 62 DEP significant phenotypes (Supplement 2, Table S2) from the UKB phoneme. Among the significant phenotypes, 5 DED-relevant phenotypes, including corneal hysteresis, corneal resistance factor, and whole body water mass (OR = 2.060–3.887), were selected, whereas 6 DEP-relevant phenotypes, including time and frequency of medical experiences (OR = 2.537–9.405), were selected for subsequent analysis. Interestingly, through a chord diagram with the top 200 DED and DEP SNPs, we identified 24 DED and 7 DEP functional SNPs that jointly constitute both DED and DEP phenotypes (Fig. 4A). These results indicate that some functional hotspots were shared between the two diseases.
Fig. 4.
Investigation of dry eye disease (DED) and depression (DEP) association through genetic approaches. (A) Chord diagram showing crosswalks between the phoneme and functional single-nucleotide polymorphisms (SNPs) of DED and DEP. (B) Heatmap of the genetic correlation scores between listed ocular and depressive diseases in Taiwan Biobank (TWB). (C) The protein−protein interaction (PPI) network of the functional SNPDED and SNPDEP genes derived from TWB and UK Biobank (UKB). (D) The converging ontology flow chart analyzing the functional SNPDED and SNPDEP genes.
We next performed genetic correlation (rG) score analysis in both UKB and TWB to assess whether global SNP patterns are similar between DED and DEP (Fig. 4B). Notably, in the TWB Asian population, DED shared an rG = 0.19 genetic correlation with DEP. Cataract disease, which has a less heritable genetic component, was parallel enrolled as a negative control (Cataract-DEP rG = 0.03) to the DED-DEP genetic correlation. Concordantly, in the UKB, common variant SNP analysis showed a positive genetic correlation (rG = 0.109) between DED and DEP, indicating that genetic architecture similarity was evident regardless of human ethnicity (Supplement 2, Fig. S1).
Disease-associated variant analysis in this study was performed in tandem with SAIGE or COJO adjustment, thereby mitigating the false-positive SNP findings that were subject to imbalanced case control ratio and LD. After SAIGE and COJO correction, 71 DED SNPs within 38 functional genes and 53 DEP SNPs within 33 genes survived the adjustment (Supplement 2, Fig. S2 and S3) (Supplement 2, Tables S3 - S12). Although there were no identical SNPs found between DED and DEP, some of the top 100 DED and DEP SNPs were found in common functional genes such as MUC16 (DED: rs1862461, p value = 4.5x10−4; rs2591595, p value = 2.67x10−4; rs22522675, p value = 2.69x10−4; rs2216663, p value = 2.77x10−4; and DEP: rs11707526, p value = 4.5x10−4) and TENM2 (DED: rs73801227, p value = 2.76x10−4, and DEP: rs13185961, p value = 7.22x10−5) in TWB2 (Supplement 2, Tables 3 and 13). Given the overlap of the functional SNP genes, we further investigated whether the top 200 functional gene lists, SNPDED and SNPDEP, exhibited interactions at the biochemical level regarding molecular-molecular interactions, protein homologs, gene fusions and gene coexpression (Fig. 4C). From the UKB and TWB combined functional SNP gene dot and line network, we demonstrated crosstalk between DED and DEP (DDX39B-PRPF31; PRKG1- SLC8A1 and MLH1-MSH5). Meanwhile, some protein hotspots (ESR1, SRSF1, DDX39B, RHOC and MYH11) were found to connect functional SNP genes across UKB and TWB. Together, our results indicated that individual DED and DEP functional SNP genes were biochemically connected.
Meanwhile, our protein–protein interaction (PPI) analysis showed that the top 200 SNPDED and SNPDEP functional genes formed a functional network that converged on pathway ontologies of immune system, innate immune system and signal transduction (Fig. 4D). The converged pathway was validated on public GEO datasets that consist of clinical and animal transcriptome sequencing samples. By screening 1072 DED accessions and 4212 DEP accessions indexed within the GEO database, 15 DED datasets (blood cells, cornea, conjunctiva and lacrimal glands) and 15 DEP datasets (blood cells, subregion cortexes, hippocampus and amygdala) were included and analyzed over a target-expanded spectrum of Reactome-2022 HSA ontologies (Fig. 5) (Supplement 3, Tables S1-S30).
Fig. 5.
Heatmap of the activated common pathways in published dry eye disease (DED) and depression (DEP) transcriptome databases. R-HAS, Reactome Homo sapiens; GSE, Gene Expression Omnibus Series; DC, dendritic cell; SjS, Sjögren's syndrome; PBMC, peripheral blood mononuclear cells; IL, interleukin; and TLR, Toll-like receptors.
By screening through the detailed entry of the immune ontology, we determined that the immune activations are patterned and asymmetric between DED and DEP samples. Specifically, while the immune system (R-HSA-168256) and cytokine signaling in immune system (R-HSA-1280215) were broadly activated in both DED and DEP samples, the infectious disease (R-HSA-5663205) was primarily activated in the tissues of the ocular surface and in the peripheral blood of DEP samples, but not in the majority brain tissue accessions. Meanwhile, the interferon signaling (R-HSA-913531) and innate immune system (R-HSA-168249) pathways were activated more in DED than in DEP. Together, these differences indicated that the pathogen-elicited immune response may affect DED through direct tissue infection, but influence DEP through an indirect blood stream effect.
Through analyzing the pathway activation patterns, we noticed that the ulcerative cornea of the DED model (GSE195962)[98] and the hippocampal tissue of the stress-induced DEP model (GSE148579)[109] showed pan-activation across immune pathways (cytokine-related, infectious-related and innate immunity pathways). Whereas, the innate immunity category that comprises toll-like receptor cascades and MyD88 cascades was robust in specific DED (dendritic cell, plasmacytoid dendritic cell, monocyte, conjunctiva and cornea) and DEP (hippocampus and pre-frontal cortex) specimen subsets. This highlighted the heterogenous inflammatory etiology of DED and DEP, and echoed to the inconsistent immune modulatory treatment effects observed from the real world DED [127] and DEP [128] trials.
Notably, signal transduction pathways (R-HSA-162582, R-HSA-194315, R-HSA-9716542 and R-HSA-9006934) were also activated in specified DED (cornea Aire knockout GSE208297)[99] and DEP (whole blood GSE182193[102] and dentate gyrus GSE84183[113]) samples; therefore, the significance of signal transduction ontologies requires further validation. In summary, through systemic analysis on transcriptome, we addressed the shared features of immune activation in both DED and DEP pathways.
Bidirectional causation by MR in TWB and UKB
Studying the exposure–outcome causations in DED and DEP is challenging, given the confounding effects of multifactorial environmental and life incidences. Randomized trials for causation investigation remain the gold standard but are often expensive and impossible to carry out. MR is a genetic-based technique that employs SNP IVs to distinguish exposure-outcome causations from observational data. Instead of using summarized statistics, we performed GWASs and confounding IV exclusions to approximate optimal MR assumptions. A total of 48 MR conditions comprising 6 MR methods (IVW, MR-PRESSO, MR−Egger, simple mode, weighted mode, weighted median) with 2 SNP sets (GWAS and COJO) were performed in 2 genetic databases (TWB and UKB), and each MR experiment was carried out in two directions, designated either DED-to-DEP or DEP-to-DED. In the absence of directional pleiotropy (p value: MR-PRESSO >0.05; test of Q >0.05; MR−Egger intercept >0.05) (Supplement 4, Table 2, Table 3), inverse-variant weighted (IVW) modified-MR were selected as the primary instrument to detect disease causal detection in this study.
Table 3.
Mendelian Randomization Results of the Tested Conditions.
| Mendelian Randomization |
TWB |
UKB |
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
DED-to-DEP |
DEP-to-DED |
DED-to-DEP |
DEP-to-DED |
||||||
| OR [95 % CI] | p value | OR [95 % CI] | p value | OR [95 % CI] | p value | OR [95 % CI] | p value | ||
| Genome-wide Association Study (GWAS) | |||||||||
| One-sample | IVW | 1.388 [1.144–1.683] | 0.0009 | 1.212 [1.109–1.324] | <0.0001 | 1.049 [1.020–1.078] | <0.0001 | 2.547 [1.773–3.661] | <0.0001 |
| MR-Egger | 1.715 [0.820–3.588] | 0.1756 | 0.994 [0.611–1.615] | 0.9803 | 1.013 [0.916–1.121] | 0.7922 | 1.772 [0.400–7.850] | 0.4553 | |
| Simple mode | 1.460 [0.951–2.242] | 0.1054 | 1.059 [0.844–1.329] | 0.6267 | 1.028 [0.961–1.100] | 0.4287 | 2.285 [0.892–5.856] | 0.0930 | |
| Weighted mode | 1.463 [0.968–2.212] | 0.0925 | 1.055 [0.861–1.294] | 0.6129 | 1.028 [0.974–1.084] | 0.3245 | 2.367 [0.937–5.982] | 0.0760 | |
| Weighted median | 1.444 [1.111–1.879] | 0.0061 | 1.099 [0.968–1.247] | 0.1432 | 1.035 [0.999–1.072] | 0.0528 | 2.446 [1.492–4.012] | 0.0004 | |
| Two-sample | IVW | 1.847 [1.428–2.389] | <0.0001 | 1.089 [1.036–1.144] | 0.0008 | 1.105 [1.048–1.165] | 0.0002 | 1.599 [1.215–2.105] | 0.0008 |
| MR-Egger | 2.124 [0.732–6.163] | 0.1738 | 1.127 [0.839–1.513] | 0.4444 | 1.409 [1.021–1.945] | 0.0511 | 1.202 [0.372–3.877] | 0.7627 | |
| Simple mode | 1.289 [0.613–2.711] | 0.5076 | 1.088 [0.984–1.202] | 0.1229 | 1.069 [0.958–1.194] | 0.2461 | 1.268 [0.704–2.284] | 0.4417 | |
| Weighted mode | 1.262 [0.620–2.571] | 0.5252 | 1.084 [0.975–1.206] | 0.1602 | 1.072 [0.962–1.195] | 0.2204 | 1.280 [0.724–2.262] | 0.4094 | |
| Weighted median | 1.593 [1.122–2.262] | 0.0093 | 1.090 [1.017–1.168] | 0.0146 | 1.090 [1.017–1.169] | 0.0138 | 1.417 [0.974–2.060] | 0.0678 | |
| Conditional and Joint Association Analysis (COJO) | |||||||||
| One-sample | IVW | 1.362 [1.147–1.616] | 0.0004 | 1.221 [1.114–1.339] | <0.0001 | 1.050 [1.021–1.080] | <0.0001 | 2.497 [1.714–3.637] | <0.0001 |
| MR-Egger | 1.712 [0.899–3.259] | 0.1189 | 0.925 [0.572–1.497] | 0.756 | 1.014 [0.912–1.128] | 0.7930 | 1.470 [0.293–7.381] | 0.6419 | |
| Simple mode | 1.445 [0.967–2.159] | 0.088 | 1.061 [0.840–1.340] | 0.6272 | 1.026 [0.964–1.091] | 0.4234 | 2.136 [0.810–5.633] | 0.1330 | |
| Weighted mode | 1.445 [0.969–2.156] | 0.0869 | 1.056 [0.855–1.306] | 0.6191 | 1.024 [0.962–1.091] | 0.4591 | 2.080 [0.768–5.628] | 0.1573 | |
| Weighted median | 1.406 [1.126–1.756] | 0.0026 | 1.097 [0.959–1.256] | 0.1758 | 1.033 [0.994–1.073] | 0.0938 | 2.319 [1.378–3.903] | 0.0015 | |
| Two-sample | IVW | 1.877 [1.447–2.435] | <0.0001 | 1.104 [1.049–1.162] | 0.0001 | 1.113 [1.055–1.175] | <0.0001 | 1.659 [1.301–2.116] | <0.0001 |
| MR-Egger | 2.219 [0.773–6.371] | 0.1468 | 1.097 [0.821–1.466] | 0.5422 | 1.285 [0.943–1.751] | 0.1286 | 1.338 [0.460–3.890] | 0.5986 | |
| Simple mode | 1.347 [0.649–2.793] | 0.4289 | 1.090 [0.975–1.220] | 0.1551 | 1.067 [0.937–1.216] | 0.3363 | 1.275 [0.735–2.212] | 0.3965 | |
| Weighted mode | 1.300 [0.640–2.639] | 0.4728 | 1.088 [0.979–1.208] | 0.1412 | 1.067 [0.958–1.189] | 0.2504 | 1.281 [0.750–2.188] | 0.3742 | |
| Weighted median | 1.623 [1.130–2.332] | 0.0088 | 1.093 [1.018–1.175] | 0.0145 | 1.086 [1.008–1.169] | 0.0282 | 1.422 [1.011–1.998] | 0.0426 | |
Note: DED, dry eye disease; DEP, depression; IVW, inverse variance-weighted; TWB, Taiwan Biobank; UKB, UK Biobank; OR, odds ratio; and CI, confidence interval.
The adjusted p-value of both the IVW-MR (DEDexposure to DEPoutcome and DEPexposure to DEDoutcome) were <0.05/48≈0.001 after Bonferroni’s correction (Table 3). Notably, the results still stood when we applied COJO correction to select LD-free sanity IVSNPs. In two-sample MR experiments with no case overlaps, the causative OR of DED-to-DEP was 1.877 (95 % CI = 1.447–2.435) in TWB and 1.113 (95 % CI = 1.055–1.175) in UKB. The ORs of DEP-to-DED were 1.104 (95 % CI = 1.049–1.162) in TWB and 1.659 (95 % CI = 1.301–2.116) in UKB.
To assess the consistency of directional causation, the OR of each MR experiment was plotted and examined (Fig. 6) (Supplement 4, Fig. S1 and S2), confirming the directional causation between DED-to-DEP and DEP-to-DED in both the UKB and TWB populations. IVSNP selection bias and minimum necessary SNP counts were investigated by replicative MR studies using different SNP numbers (n = 5, 10, 15, 20, 40, 60), whereby a number of approximately 40 can attain statistical significance (p <0.001) with a stable OR.
Fig. 6.
Validation of dry eye disease (DED)-depression (DEP) bidirectional causation using different two-sample Mendelian randomization (TSMR) methods in replicative practices. Scatter plots of (A) TSMR experiments using genome-wide association study (GWAS)-selected SNPs as instrumental variables. Experiments were repeated in both the Taiwan Biobank (TWB) and UK Biobank (UKB). (B) TSMR instrument variables were replaced by conditional and joint association analysis (COJO) and scalable and accurate implementation of generalized mixed model (SAIGE)-corrected GWAS single-nucleotide polymorphisms (SNPs) in UKB. (C) The SNP number needed for TSMR significance and its corresponding effects on the TSMR odds ratio was investigated.
In the heterogeneity analysis, Cochran's Q value implied no substantial heterogeneity with a Q value smaller than a degree of freedom in all models. Sequentially omitting each SNP, leave-one-out (LOO) analysis showed that most of the ORs fell within the 95 % CI of the IVW model, which indicated that the bidirectional causation between DED and DEP is robust without any particular IV influencing the causal inference of GWAS, SAIGE and COJO (Supplement 4, Fig. S3 and S4). Collectively, our MR-LOO sensitivity analysis indicated that a cluster of small effect IVSNP rather than singular large effect IVSNPs accounted for the bidirectional causal effect underlying DED and DEP.
Discussion
In this study, we sought to investigate the disease relation between dry-eye disease (DED) and depression (DEP). By employing multimodal analysis and genetic data in UK biobank and TW biobank, we conducted meta-analysis, GWAS, transcriptome systematic review and COJO- adjusted MR experiments to reconstitute the interacting relations between DED and DEP. Sequentially, we demonstrated that DED and DEP were tightly linked in terms of clinical co-morbidity and shared a common pathway of inflammation activation. Moreover, our COJO-adjusted MR experiments suggested that a bidirectional genetic causation was detected in the DED-DEP relation.
Clinical association between DED and DEP
This is an updated meta-analysis that confirmed the clinical association between DED and DEP. From the current literature evidence, DED patients were associated with increased DEP prevalence and worse DEP symptoms, whereas DEP patients were associated only with an increased DED prevalence, no increased DED severity was detected. The non-linear discrepancy between the high DED prevalence but no increased DED severity was also reported in DED-associated diseases such as: thyroid dysfunction, osteoarthritis, diabetes, irritable bowel syndrome, hypercholesterolemia, hypertension, and hypertriglyceridemia[129]. Nevertheless, the exact mechanism regarding why certain disease associates do not combine severity association may be context dependent, and requires further cell-biology studies on stratified clinical specimens.
Genetic linkage between DED and DEP
Genetic pleiotropy, defined as one SNP affecting both DED and DEP, was not detected in TWB or UKB. Nevertheless, MUC16 and TENM2 mutations at the functional gene levels were found to contribute to both DED and DEP in TWB.
Our study reported one novel MUC16 SNPDED (rs7260214) and four synonymous MUC16 SNPDEP (rs1862461, rs2591595, rs2252675, and rs2216663) in DED and DEP patients in TWB2, respectively. MUC16, a key transmembrane mucin protein that maintains the hydrophilic glycocalyx layer of the ocular surface, exhibits altered glycosylation in DED patients[130]. Concordantly, an observation study also reported that patients with lower MUC16 expression levels at the ocular surface are prone to suffer from DED[131]. MUC16 is a tumor marker also known as CA-125[132], and recent studies have shown that DEP predisposition is associated with higher MUC16 expression in a population with ovarian cancer[133]. Notably, in a hospitalized observation study enrolling young adolescents without underlying ovarian cancer, DEP patients with self-harm behavior had higher MUC16 levels in their peripheral blood. In addition, incorporating MUC16 levels into the Hamilton Depression Rating Scale (HDRS) can improve self-harm behavior prediction in DEP patients[134]. These studies indicated that mutations affecting either the expression level or the posttranslational modification of MUC16 contribute to the cooccurrence of DED and DEP.
Mutations in TENM2, a highly expressed cell–cell adhesion molecule in the central nervous system, were discovered to be accountable for DED (rs73801227) and DEP (rs13185961) in this study. The expression of TENM2 was significantly correlated with DEP gene sets in the postmortem parietal cortex of DEP patients[135] and mutations of TENM2 were also found in the cornea of keratoconus[136] and astigmatism[137] patients. Although the exact functions of the TENM2 intron SNPs are yet unknown, an adaptive evolution GWAS using primate phylogeny and the modern human genome has reported that mutations of TENM2 with 16 other DEP genes were positively selected in East Asian ancestry evolution[138], notably, most of the positive selected variants resided in the untranslated regions (UTRs) and noncoding exons of TENM2. While TENM2 functioned as a presynapse transmembrane protein that guides retinal axon migration[139] and orchestrates synaptogenesis excitatory/inhibitory balance[140], SNPs of TENM2 may disrupt the potential role of TENM2 in the pathogenesis of DED and DEP.
In the PPI network analysis, several interesting hotspots were identified as protein hubs (ESR1 and DDX39B) for DED-DEP transactions. A genetic polymorphism of ESR1, a key estrogen receptor, was associated with both DED[141] and DEP[142]. The expression of ESR1 has been connected to hormone regulation with corneal homeostasis[143]. Meanwhile, estrogen dysregulation and usage of hormone replacement therapies[144] and aromatase inhibitors[145] have all been reported to cause MG dysfunction in the evaporative subtype of DED[146].
Our study recapitulated the canonical disease pathophysiology hotspots and elucidated the extensive molecular networks that account for the co-occurrence of DED and DEP.
Common underlying pathways
For the first time, we proposed and addressed the significance of immune-related pathways at the intersection of DED and DEP pathophysiology. By indexing our converging flow ontology SNPs (Supplement 2, Tables 3-12) in the GWAS catalogue[147], we identified rs17854914 (PLD2), a C-reactive protein (CRP)-associated SNP, that linked DED to the immune ontology. On the other hand, the DEP-associated rs1270942 (CFB) was related to various autoimmune diseases, such as type 1 diabetic mellitus (T1DM), systemic lupus erythematosus (SLE) and autoimmune thyroiditis. Although the immune response and host defense systems have been studied from the respective DED[148] and DEP[149] perspectives, whether immune crosstalk exists between the two diseases has not been fully explored.
In our transcriptome meta-analysis results, we observed that a compelling ontology similarity, mainly immune pathways, was shared across independent DED and DEP GEO datasets. The finding of an elevated cytokine signaling transcriptome was supported by a DED systematic review, which reported elevated cytokine (IL-1β, IL-6, IL-8, IL-10, INF-γ and TNF-α) levels in DED tear samples[150]. The significance of IL-6 in DEP was addressed by another meta-analysis using a longitudinal DEP cohort, which revealed that higher IL-6 levels in the peripheral blood are associated with future DEP[149], and antidepressant treatment can reduce blood IL-6 levels[151]. Although IFN-gamma signaling was not upregulated in the DEP samples, interferon signaling was critical to DED onset. IFN-γ was reported to drive squamous metaplasia in corneal epithelium[152] and to block goblet cell differentiation[153], and both mechanisms may aggravate DED conditions.
These independent but complementary meta-analysis results collectively indicate the significance of cytokine signaling in both DED and DEP pathophysiology.
Causal effects
Compared to using summary statistics, the in-house SNP discovery and pruning process allowed us to combine COJO and SAIGE correction in IV selections, which resulted in better fitness of IVs to the classic MR assumptions.
Our bidirectional IVW-MR results identified both DED and DEP as the risk exposure for the disease. This finding implied that treating either DED or DEP has the potential to resolve the entangled clinical co-occurrence of DED and DEP. Although studies have reported that treating DEP with selective serotonin reuptake inhibitors (SSRIs) may cause DED aggravation through NF-kB inflammation[154], contrasting results have demonstrated that treating DEP with serotonin norepinephrine reuptake inhibitors (SNRIs) does not aggravate DED[155]. Meanwhile, a case−control study reported that effective DED treatment can reduce depressive symptoms[156]. Together, the genetic causation findings of this study provide a theoretical and strategic background to block the DED-DEP vicious cycle in the clinical setting.
Limitations
Meta-analysis
While our results supported the positive correlation between DED and DEP, analytic limitations were noted in this meta-analysis. To be specific, the available published DED-DEP studies scored 15–20 out of 22 in the scale of STROBE checklist (Supplement 1, Table S2), which indicates the majority of current DED-DEP studies had not make full addressment in either the sources of bias, showing sensitivity analysis of the results, adjust confounders or perform adequate subgroups analysis. Therefore, limited the statistical power of the overall meta-analysis. Noteworthily, despite our subgroup meta-analysis and SMD statistics demonstrated that DED-DEP clinical association holds independent to the selected ethnicity, the inevitable heterogeneity (clinical, methodological, and statistical features) of the studies may have restricted the statement, unless further DED-DEP study characterization opted a standardized; uniform protocol.
Genetic correlation
In spite of selecting strongly associated SNPs, common SNPs might not explain the diseases completely since both DED and DEP are multi-genetic-derived. Furthermore, since coding genes only account for 1–––2 % of the whole human genome, SNPs outside coding gene regions were not explained in this research, however, they are likely to affect disease if they are located within functionally important non-coding regions such as enhancers, promoters, silencers, or insulators. Moreover, genetic data were predominantly of Chinese origin, so the results might not apply to other regions.
Causal effects
Albeit we employed COJO and two-sample MR to mitigate LD-inflated instrument bias and prevent over-estimated beta value respectively, the correlated horizontal pleiotropy (CHP) of the underlying immune pathway was not fully considered in current MR experiments. For conditions in which a CHP has violated MR assumptions.
Future prospectives and clinical impacts
The pathognomonic resonance between brain-eye comorbidity were recently proposed and in-depth reviewed from both clinical [157], [158] and biomolecular standpoints [159], [160]. While this study adds DED-DEP relations to the brain-eye comorbidity clusters, we investigated the patternized immune activation in detailed tissue origins, which may guide immune modulatory drug usage on target-specific compartments. Notably, brain atrophy at pre-frontal cortex and hippocampus region were symbolic DEP traits [161], and concordantly our transcriptome analysis showed activated TLR and MyD88 cascades in the pre-frontal and hippocampus segments. These information whereby suggest TLR [162] and MyD88 [163] antagonists may have a role in the DED-DEP blockade.
Conclusion
Collectively, our meta-analysis confirmed the association between DED and DEP; and the GWAS target-PheWAS attributed immune activation under the common ground of DED-DEP co-axis. Last but not the least, we detected DED-DEP bi-directional causation by COJO-adjusted IVW-MR. With the multi-modal evidence combined, we consolidated the association and causation between DED and DEP.
CRediT authorship contribution statement
Kao-Jung Chang: Conceptualization, Methodology, Investigation, Writing – original draft. Hsin-Yu Wu: Conceptualization, Methodology, Investigation, Writing – original draft. Pin-Hsuan Chiang: Software, Formal analysis, Project administration, Data curation. Yu-Tien Hsu: Software, Formal analysis, Project administration, Data curation. Pei-Yu Weng: Software, Formal analysis, Project administration, Data curation. Ting-Han Yu: Validation, Visualization. Cheng-Yi Li: Validation, Visualization. Yu-Hsiang Chen: Validation, Visualization. He-Jhen Dai: Validation, Visualization. Han-Ying Tsai: Formal analysis, Investigation, Project administration. Yu-Jung Chang: Formal analysis, Investigation, Project administration. You-Ren Wu: Writing – review & editing, Supervision. Yi-Ping Yang: Software, Formal analysis, Project administration, Data curation. Cheng-Ta Li: Writing – review & editing, Supervision. Chih-Chien Hsu: Writing – review & editing, Supervision. Shih-Jen Chen: Writing – review & editing, Supervision. Yu-Chun Chen: Writing – review & editing, Supervision. Ching-Yu Cheng: Writing – review & editing, Supervision. Ai-Ru Hsieh: Resources, Writing – review & editing, Supervision, Funding acquisition. Shih-Hwa Chiou: Resources, Writing – review & editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Funded by grants of the Taiwan National Science and Technology Council (NSTC 112-2321-B-A49-007, NSTC 111-2320-B-A49-028-MY3, NSTC 112-2124-M-038-001, and NSTC 112-2314-B-032-001) and Taipei Veterans General Hospital (V112C-026 and 112VACS-007), this study is based in part on data from the Big Data Center, Taipei Veterans General Hospital (BDC, TPEVGH). The interpretations and conclusions contained herein do not represent the position of Taipei Veterans General Hospital.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2024.03.015.
Contributor Information
Ai-Ru Hsieh, Email: 142438@mail.tku.edu.tw.
Shih-Hwa Chiou, Email: shchiou@vghtpe.gov.tw.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Akowuah P.K., Kobia-Acquah E. Prevalence of dry eye disease in Africa: a systematic review and meta-analysis. Optom Vis Sci. 2020;97(12):1089–1098. doi: 10.1097/OPX.0000000000001610. [DOI] [PubMed] [Google Scholar]
- 2.Ghanem M., Gadallah M., Meky F.A., Mourad S., El-Kholy G. National Survey of prevalence of mental Disorders in Egypt: preliminary survey. East Mediterr Health J. 2009;15(1):65–75. [PubMed] [Google Scholar]
- 3.Tomlinson M., Grimsrud A.T., Stein D.J., Williams D.R., Myer L. The epidemiology of major depression in South Africa: results from the south african stress and health study. S Afr Med J. 2009;99(5 Pt 2):367–373. [PMC free article] [PubMed] [Google Scholar]
- 4.Amu H., Osei E., Kofie P., et al. Prevalence and predictors of depression, anxiety, and stress among adults in Ghana: a community-based cross-sectional study. PLoS One. 2021;16(10):e0258105. doi: 10.1371/journal.pone.0258105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bikbov M.M., Gilmanshin T.R., Zainullin R.M., et al. Prevalence and associations of dry eye disease and meibomian gland dysfunction in the ural eye and medical study. Sci Rep. 2022;12(1):18849. doi: 10.1038/s41598-022-22580-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bikbov M.M., Gilmanshin T.R., Kazakbaeva G.M., et al. Prevalence of depression, anxiety and suicidal ideas and associated factors, in particular sensory impairments, in a population of Bashkortostan in Russia. Sci Rep. 2023;13(1):17256. doi: 10.1038/s41598-023-44561-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uchino M., Nishiwaki Y., Michikawa T., et al. Prevalence and risk factors of dry eye disease in Japan: koumi study. Ophthalmology. 2011;118(12):2361–2367. doi: 10.1016/j.ophtha.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 8.Ishikawa H., Tachimori H., Takeshima T., et al. Prevalence, treatment, and the correlates of common mental disorders in the mid 2010's in Japan: the results of the world mental health Japan 2nd survey. J Affect Disord. 2018;241:554–562. doi: 10.1016/j.jad.2018.08.050. [DOI] [PubMed] [Google Scholar]
- 9.Titiyal J.S., Falera R.C., Kaur M., Sharma V., Sharma N. Prevalence and risk factors of dry eye disease in North India: Ocular surface disease index-based cross-sectional hospital study. Indian J Ophthalmol. 2018;66(2):207–211. doi: 10.4103/ijo.IJO_698_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arvind B.A., Gururaj G., Loganathan S., et al. Prevalence and socioeconomic impact of depressive disorders in India: multisite population-based cross-sectional study. BMJ Open. 2019;9(6):e027250. doi: 10.1136/bmjopen-2018-027250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee A.J., Lee J., Saw S.M., et al. Prevalence and risk factors associated with dry eye symptoms: a population based study in Indonesia. Br J Ophthalmol. 2002;86(12):1347–1351. doi: 10.1136/bjo.86.12.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peltzer K., Pengpid S. High prevalence of depressive symptoms in a national sample of adults in Indonesia: childhood adversity, sociodemographic factors and health risk behaviour. Asian J Psychiatr. 2018;33:52–59. doi: 10.1016/j.ajp.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Lin P.Y., Tsai S.Y., Cheng C.Y., Liu J.H., Chou P., Hsu W.M. Prevalence of dry eye among an elderly chinese population in Taiwan: the shihpai eye study. Ophthalmology. 2003;110(6):1096–1101. doi: 10.1016/S0161-6420(03)00262-8. [DOI] [PubMed] [Google Scholar]
- 14.Wang H.H., Chang C.M., Chang S.S., et al. Ten-year trends in depression care in Taiwan. J Formos Med Assoc. 2022;121(10):2001–2011. doi: 10.1016/j.jfma.2022.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Alamri A., Bakri S., Alqahtani R., et al. Prevalence of depression among people with dry eye disease: empirical analysis from the southern region of Saudi Arabia. Cureus. 2023;15(5):e39253. doi: 10.7759/cureus.39253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nour M.O., Alharbi K.K., Hafiz T.A., et al. Prevalence of depression and associated factors among adults in Saudi Arabia: systematic review and meta-analysis (2000–2022) Depress Anxiety. 2023;2023 [Google Scholar]
- 17.Schein O.D., Munoz B., Tielsch J.M., Bandeen-Roche K., West S. Prevalence of dry eye among the elderly. Am J Ophthalmol. 1997;124(6):723–728. doi: 10.1016/s0002-9394(14)71688-5. [DOI] [PubMed] [Google Scholar]
- 18.Goodwin R.D., Dierker L.C., Wu M., Galea S., Hoven C.W., Weinberger A.H. Trends in U.S. depression prevalence from 2015 to 2020: the widening treatment gap. Am J Prev Med. 2022;63(5):726–733. doi: 10.1016/j.amepre.2022.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castro J.S., Selegatto I.B., Castro R.S., et al. Prevalence and risk factors of self-reported dry eye in Brazil using a short symptom questionnaire. Sci Rep. 2018;8(1):2076. doi: 10.1038/s41598-018-20273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopes C.S., Gomes N.L., Junger W.L., Menezes P.R. Trend in the prevalence of depressive symptoms in Brazil: results from the brazilian National Health Survey. Cad Saude Publica. 2022;38(Suppl 1):e00123421. doi: 10.1590/0102-311X00123421. 2013 and 2019. [DOI] [PubMed] [Google Scholar]
- 21.McCarty C.A., Bansal A.K., Livingston P.M., Stanislavsky Y.L., Taylor H.R. The epidemiology of dry eye in Melbourne. Australia, Ophthalmology. 1998;105(6):1114–1119. doi: 10.1016/S0161-6420(98)96016-X. [DOI] [PubMed] [Google Scholar]
- 22.Kasturi S., Oguoma V.M., Grant J.B., Niyonsenga T., Mohanty I. Prevalence rates of depression and anxiety among young rural and urban australians: a systematic review and meta-analysis. Int J Environ Res Public Health. 2023;20(1) doi: 10.3390/ijerph20010800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roth J., Nilsson I., Melin J., Macedo A.F. Dry eye symptoms using the Ocular Surface disease index in Sweden: a short report from a pilot study, scandinavian journal of optometry and visual. Science. 2022;15(1) [Google Scholar]
- 24.Johansson R., Carlbring P., Heedman A., Paxling B., Andersson G. Depression, anxiety and their comorbidity in the swedish general population: point prevalence and the effect on health-related quality of life. PeerJ. 2013;1:e98. doi: 10.7717/peerj.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viso E., Rodriguez-Ares M.T., Gude F. Prevalence of and associated factors for dry eye in a spanish adult population (the salnes eye study) Ophthalmic Epidemiol. 2009;16(1):15–21. doi: 10.1080/09286580802228509. [DOI] [PubMed] [Google Scholar]
- 26.Vieta E., Alonso J., Perez-Sola V., et al. Epidemiology and costs of depressive disorder in Spain: the EPICO study. Eur Neuropsychopharmacol. 2021;50:93–103. doi: 10.1016/j.euroneuro.2021.04.022. [DOI] [PubMed] [Google Scholar]
- 27.Vidal-Rohr M., Craig J.P., Davies L.N., Wolffsohn J.S. The epidemiology of dry eye disease in the UK: the Aston dry eye study. Cont Lens Anterior Eye. 2023;46(3) doi: 10.1016/j.clae.2023.101837. [DOI] [PubMed] [Google Scholar]
- 28.Arias de la Torre J., Vilagut G., Ronaldson A., et al. Prevalence and age patterns of depression in the United Kingdom. a population-based study. J Affect Disord. 2021;279:164–172. doi: 10.1016/j.jad.2020.09.129. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Y., Murrough J., Yu Y., et al. Association between depression and severity of dry eye symptoms signs, and inflammatory Markers in the DREAM study. JAMA Ophthalmol. 2022;140(4):392–399. doi: 10.1001/jamaophthalmol.2022.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ulusoy M.O., Isik-Ulusoy S., Kivanc S.A. Evaluation of dry eye disease in newly diagnosed anxiety and depression patients using anterior segment optical coherence tomography. Eye Vis (Lond) 2019;6:25. doi: 10.1186/s40662-019-0149-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wan K.H., Chen L.J., Young A.L. Depression and anxiety in dry eye disease: a systematic review and meta-analysis. Eye (Lond) 2016;30(12):1558–1567. doi: 10.1038/eye.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuang T.M., Tsai S.Y., Liu C.J., Lee S.M., Chou P. Association between dry eye and depressive symptoms in an elderly chinese population in Taiwan: the shihpai eye study. Eye (Lond) 2021;35(10):2826–2833. doi: 10.1038/s41433-020-01329-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitazawa M., Sakamoto C., Yoshimura M., et al. The relationship of dry eye disease with depression and anxiety: a naturalistic observational study. Transl Vis Sci Technol. 2018;7(6):35. doi: 10.1167/tvst.7.6.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maes M., Bosmans E., De Jongh R., Kenis G., Vandoolaeghe E., Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9(11):853–858. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- 35.Galor A., Feuer W., Lee D.J., et al. Prevalence and risk factors of dry eye syndrome in a United States veterans affairs population. Am J Ophthalmol. 2011;152(3):377–84 e2. doi: 10.1016/j.ajo.2011.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang T.J., Wang I.J., Hu C.C., Lin H.C. Comorbidities of dry eye disease: a nationwide population-based study. Acta Ophthalmol. 2012;90(7):663–668. doi: 10.1111/j.1755-3768.2010.01993.x. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Z., Lee P.H., Chaffin M.D., et al. A genome-wide cross-trait analysis from UK biobank highlights the shared genetic architecture of asthma and allergic diseases. Nat Genet. 2018;50(6):857–864. doi: 10.1038/s41588-018-0121-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caspi A., Sugden K., Moffitt T.E., et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 39.Han X., Shen Q., Hou C., et al. Disease clusters subsequent to anxiety and stress-related disorders and their genetic determinants. Nat Commun. 2024;15(1):1209. doi: 10.1038/s41467-024-45445-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang K.J., Wu H.Y., Yarmishyn A.A., et al. Genetics behind cerebral disease with Ocular comorbidity: finding Parallels between the brain and eye Molecular pathology. Int J Mol Sci. 2022;23(17) doi: 10.3390/ijms23179707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao B, Li Y, Fan Z, et al., Eye-brain connections revealed by multimodal retinal and brain imaging genetics in the UK Biobank, medRxiv (2023). doi: 10.1101/2023.02.16.23286035. [DOI] [PMC free article] [PubMed]
- 42.Vehof J., Wang B., Kozareva D., Hysi P.G., Snieder H., Hammond C.J. The heritability of dry eye disease in a female twin cohort. Invest Ophthalmol Vis Sci. 2014;55(11):7278–7283. doi: 10.1167/iovs.14-15200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sullivan P.F., Neale M.C., Kendler K.S. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157(10):1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- 44.Yamaguchi T. Inflammatory response in dry eye. Invest Ophthalmol Vis Sci. 2018;59(14) doi: 10.1167/iovs.17-23651. [DOI] [PubMed] [Google Scholar]
- 45.Milaneschi Y., Corsi A.M., Penninx B.W., Bandinelli S., Guralnik J.M., Ferrucci L. Interleukin-1 receptor antagonist and incident depressive symptoms over 6 years in older persons: the InCHIANTI study. Biol Psychiatry. 2009;65(11):973–978. doi: 10.1016/j.biopsych.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amparo F., Dastjerdi M.H., Okanobo A., et al. Topical interleukin 1 receptor antagonist for treatment of dry eye disease: a randomized clinical trial. JAMA Ophthalmol. 2013;131(6):715–723. doi: 10.1001/jamaophthalmol.2013.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baune B.T., Dannlowski U., Domschke K., et al. The interleukin 1 beta (IL1B) gene is associated with failure to achieve remission and impaired emotion processing in major depression. Biol Psychiatry. 2010;67(6):543–549. doi: 10.1016/j.biopsych.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 48.Syed S.A., Beurel E., Loewenstein D.A., et al. Defective inflammatory pathways in never-treated depressed patients are associated with poor treatment response. Neuron. 2018;99(5):914–24 e3. doi: 10.1016/j.neuron.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kappelmann N., Lewis G., Dantzer R., Jones P.B., Khandaker G.M. Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol Psychiatry. 2018;23(2):335–343. doi: 10.1038/mp.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith G.D., Ebrahim S. 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32(1):1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- 51.Boehm F.J., Zhou X. Statistical methods for mendelian randomization in genome-wide association studies: a review. Comput Struct Biotechnol J. 2022;20:2338–2351. doi: 10.1016/j.csbj.2022.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cao Y., Lu H., Xu W., Zhong M. Gut microbiota and sjogren's syndrome: a two-sample mendelian randomization study. Front Immunol. 2023;14:1187906. doi: 10.3389/fimmu.2023.1187906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kappelmann N., Arloth J., Georgakis M.K., et al. Dissecting the association between inflammation, metabolic dysregulation, and specific depressive symptoms: a genetic Correlation and 2-sample mendelian randomization study. JAMA Psychiat. 2021;78(2):161–170. doi: 10.1001/jamapsychiatry.2020.3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choi K.W., Chen C.Y., Stein M.B., et al. Assessment of bidirectional relationships between physical activity and depression among adults: a 2-sample mendelian randomization study. JAMA Psychiat. 2019;76(4):399–408. doi: 10.1001/jamapsychiatry.2018.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.GA Wells BS, D O'Connell, J Peterson, V Welch, M Losos, P Tugwell. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [cited November 30 2023]. Available from: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 56.Labbe A., Wang Y.X., Jie Y., Baudouin C., Jonas J.B., Xu L. Dry eye disease, dry eye symptoms and depression: the Beijing eye study. Br J Ophthalmol. 2013;97(11):1399–1403. doi: 10.1136/bjophthalmol-2013-303838. [DOI] [PubMed] [Google Scholar]
- 57.Liang C.Y., Cheang W.M., Wang C.Y., et al. The association of dry eye syndrome and psychiatric disorders: a nationwide population-based cohort study. BMC Ophthalmol. 2020;20(1):123. doi: 10.1186/s12886-020-01395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vehof J., Snieder H., Jansonius N., Hammond C.J. Prevalence and risk factors of dry eye in 79,866 participants of the population-based lifelines cohort study in the Netherlands. Ocul Surf. 2021;19:83–93. doi: 10.1016/j.jtos.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 59.Galor A., Feuer W., Lee D.J., et al. Depression, post-traumatic stress disorder, and dry eye syndrome: a study utilizing the national United States veterans affairs administrative database. Am J Ophthalmol. 2012;154(2):340–6 e2. doi: 10.1016/j.ajo.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 60.Viet Vu C.H., Uchino M., Kawashima M., et al. Lack of social support and social trust as potential risk factors for dry eye disease: JPHC-NEXT study. Ocul Surf. 2019;17(2):278–284. doi: 10.1016/j.jtos.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 61.Kim K.W., Han S.B., Han E.R., et al. Association between depression and dry eye disease in an elderly population. Invest Ophthalmol Vis Sci. 2011;52(11):7954–7958. doi: 10.1167/iovs.11-8050. [DOI] [PubMed] [Google Scholar]
- 62.Na K.S., Han K., Park Y.G., Na C., Joo C.K. Depression, stress, quality of life, and dry eye disease in korean women: a population-based study. Cornea. 2015;34(7):733–738. doi: 10.1097/ICO.0000000000000464. [DOI] [PubMed] [Google Scholar]
- 63.Li M., Gong L., Sun X., Chapin W.J. Anxiety and depression in patients with dry eye syndrome. Curr Eye Res. 2011;36(1):1–7. doi: 10.3109/02713683.2010.519850. [DOI] [PubMed] [Google Scholar]
- 64.van der Vaart R., Weaver M.A., Lefebvre C., Davis R.M. The association between dry eye disease and depression and anxiety in a large population-based study. Am J Ophthalmol. 2015;159(3):470–474. doi: 10.1016/j.ajo.2014.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nam S.M., Peterson T.A., Butte A.J., Seo K.Y., Han H.W. Explanatory model of dry eye disease using health and nutrition examinations: machine Learning and network-based factor analysis from a National Survey. JMIR Med Inform. 2020;8(2):e16153. doi: 10.2196/16153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Um S.B., Yeom H., Kim N.H., Kim H.C., Lee H.K., Suh I. Association between dry eye symptoms and suicidal ideation in a korean adult population. PLoS One. 2018;13(6):e0199131. doi: 10.1371/journal.pone.0199131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Inomata T., Iwagami M., Nakamura M., et al. Characteristics and risk factors associated with diagnosed and undiagnosed symptomatic dry eye using a Smartphone application. JAMA Ophthalmol. 2020;138(1):58–68. doi: 10.1001/jamaophthalmol.2019.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Inomata T., Iwagami M., Nakamura M., et al. Association between dry eye and depressive symptoms: Large-scale crowdsourced research using the DryEyeRhythm iPhone application. Ocul Surf. 2020;18(2):312–319. doi: 10.1016/j.jtos.2020.02.007. [DOI] [PubMed] [Google Scholar]
- 69.Kaiser T., Janssen B., Schrader S., Geerling G. Depressive symptoms, resilience, and personality traits in dry eye disease. Graefes Arch Clin Exp Ophthalmol. 2019;257(3):591–599. doi: 10.1007/s00417-019-04241-1. [DOI] [PubMed] [Google Scholar]
- 70.Yilmaz U., Gokler M.E., Unsal A. Dry eye disease and depression-anxiety-stress: a hospital-based case control study in Turkey. Pak J Med Sci. 2015;31(3):626–631. doi: 10.12669/pjms.313.7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu M., Liu X., Han J., Shao T., Wang Y. Association between sleep quality, mood status, and Ocular Surface Characteristics in patients with dry eye disease. Cornea. 2019;38(3):311–317. doi: 10.1097/ICO.0000000000001854. [DOI] [PubMed] [Google Scholar]
- 72.An Y., Kim H. Sleep disorders, mental health, and dry eye disease in South Korea. Sci Rep. 2022;12(1):11046. doi: 10.1038/s41598-022-14167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Y., Lin T., Jiang A., Zhao N., Gong L. Vision-related quality of life and psychological status in chinese women with sjogren's syndrome dry eye: a case-control study. BMC Womens Health. 2016;16(1):75. doi: 10.1186/s12905-016-0353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ismayilov A.S., Celikel G. Effects of tricyclic antidepressants, selective serotonin reuptake inhibitors, and selective serotonin-norepinephrine reuptake inhibitors on the ocular surface. Arq Bras Oftalmol. 2022;86(5):e20230068. doi: 10.5935/0004-2749.20230068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fernandez C.A., Galor A., Arheart K.L., et al. Dry eye syndrome, posttraumatic stress disorder, and depression in an older male veteran population. Invest Ophthalmol Vis Sci. 2013;54(5):3666–3672. doi: 10.1167/iovs.13-11635. [DOI] [PubMed] [Google Scholar]
- 76.Isik-Ulusoy S., Ulusoy M.O. Influence of different antidepressants on Ocular Surface in patients with major depressive Disorder. J Clin Psychopharmacol. 2021;41(1):49–52. doi: 10.1097/JCP.0000000000001325. [DOI] [PubMed] [Google Scholar]
- 77.Kawashima M., Yamada M., Shigeyasu C., et al. Association of Systemic Comorbidities with dry eye disease. J Clin Med. 2020;9(7) doi: 10.3390/jcm9072040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mrugacz M., Ostrowska L., Bryl A., Szulc A., Zelazowska-Rutkowska B., Mrugacz G. Pro-inflammatory cytokines associated with clinical severity of dry eye disease of patients with depression. Adv Med Sci. 2017;62(2):338–344. doi: 10.1016/j.advms.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 79.Tiskaoglu N.S., Yazici A., Karlidere T., et al. Dry eye disease in patients with newly diagnosed depressive Disorder. Curr Eye Res. 2017;42(5):672–676. doi: 10.1080/02713683.2016.1236966. [DOI] [PubMed] [Google Scholar]
- 80.Lee S., Fuchsberger C., Kim S., Scott L. An efficient resampling method for calibrating single and gene-based rare variant association analysis in case-control studies. Biostatistics. 2016;17(1):1–15. doi: 10.1093/biostatistics/kxv033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ma C., Blackwell T., Boehnke M., Scott L.J. Go TDi, recommended joint and meta-analysis strategies for case-control association testing of single low-count variants. Genet Epidemiol. 2013;37(6):539–550. doi: 10.1002/gepi.21742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Deng Y., Pan W. Improved use of small reference panels for conditional and joint analysis with GWAS Summary statistics. Genetics. 2018;209(2):401–408. doi: 10.1534/genetics.118.300813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang J., Ferreira T., Morris A.P., et al. Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nat Genet. 2012;44(4):369–375. doi: 10.1038/ng.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bulik-Sullivan B.K., Loh P.R., Finucane H.K., et al. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47(3):291–295. doi: 10.1038/ng.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bulik-Sullivan B., Finucane H.K., Anttila V., et al. An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015;47(11):1236–1241. doi: 10.1038/ng.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen E.Y., Tan C.M., Kou Y., et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinf. 2013;14:128. doi: 10.1186/1471-2105-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kuleshov M.V., Jones M.R., Rouillard A.D., et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44(W1):W90–W97. doi: 10.1093/nar/gkw377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xie Z., Bailey A., Kuleshov M.V., et al. Gene set knowledge discovery with enrichr. Curr Protoc. 2021;1(3):e90. doi: 10.1002/cpz1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tasaki S., Suzuki K., Nishikawa A., et al. Multiomic disease signatures converge to cytotoxic CD8 T cells in primary sjogren's syndrome. Ann Rheum Dis. 2017;76(8):1458–1466. doi: 10.1136/annrheumdis-2016-210788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Peng Y., Luo X., Chen Y., et al. LncRNA and mRNA expression profile of peripheral blood mononuclear cells in primary sjogren's syndrome patients. Sci Rep. 2020;10(1):19629. doi: 10.1038/s41598-020-76701-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen X., Cheng Q., Du Y., Liu L., Wu H. Differential long non-coding RNA expression profile and function analysis in primary sjogren's syndrome. BMC Immunol. 2021;22(1):47. doi: 10.1186/s12865-021-00439-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lopes A.P., Hillen M.R., Hinrichs A.C., et al. Deciphering the role of cDC2s in sjogren's syndrome: transcriptomic profile links altered antigen processes with IFN signature and autoimmunity. Ann Rheum Dis. 2023;82(3):374–383. doi: 10.1136/ard-2022-222728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lopes A.P., Bekker C.P.J., Hillen M.R., et al. The transcriptomic profile of monocytes from patients with sjogren's syndrome is associated with inflammatory Parameters and is mimicked by circulating mediators. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.701656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peng Y., Wu X., Zhang S., et al. The potential roles of type I interferon activated neutrophils and neutrophil extracellular traps (NETs) in the pathogenesis of primary sjogren's syndrome. Arthritis Res Ther. 2022;24(1):170. doi: 10.1186/s13075-022-02860-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hillen M.R., Pandit A., Blokland S.L.M., et al. Plasmacytoid DCs from patients with sjogren's syndrome are transcriptionally primed for enhanced pro-inflammatory cytokine production. Front Immunol. 2019;10:2096. doi: 10.3389/fimmu.2019.02096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.de Paiva C.S., Trujillo-Vargas C.M., Schaefer L., Yu Z., Britton R.A., Pflugfelder S.C. Differentially expressed gene pathways in the conjunctiva of sjogren syndrome keratoconjunctivitis sicca. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.702755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Argueso P.H.S. To analyze expression in the conjunctival epithelium of patients with severe dry eye disease. Gene Expression Omnibus. 2011 [Google Scholar]
- 98.Alam J., Yazdanpanah G., Ratnapriya R., et al. IL-17 producing lymphocytes cause dry eye and corneal disease with aging in RXRalpha mutant mouse. Front Med (Lausanne) 2022;9 doi: 10.3389/fmed.2022.849990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Efraim Y., Chen F.Y.T., Cheong K.N., Gaylord E.A., McNamara N.A., Knox S.M. A synthetic tear protein resolves dry eye through promoting corneal nerve regeneration. Cell Rep. 2022;40(9) doi: 10.1016/j.celrep.2022.111307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu Y., Di G., Hu S., et al. Expression profiles of CircRNA and mRNA in lacrimal glands of AQP5(-/-) mice with Primary dry eye. Front Physiol. 2020;11:1010. doi: 10.3389/fphys.2020.01010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Master A., Kontzias A., Huang L., et al. The transcriptome of rabbit conjunctiva in dry eye disease: Large-scale changes and similarity to the human dry eye. PLoS One. 2021;16(7):e0254036. doi: 10.1371/journal.pone.0254036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou T., Li M., Xiao Z., et al. Chronic stress-induced gene changes in vitro and in vivo: potential Biomarkers associated with depression and cancer based on circRNA- and lncRNA-associated ceRNA networks. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.744251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Datta D., Enwright J.F., Arion D., et al. Mapping phosphodiesterase 4D (PDE4D) in macaque dorsolateral prefrontal cortex: Postsynaptic Compartmentalization in layer III pyramidal cell circuits. Front Neuroanat. 2020;14 doi: 10.3389/fnana.2020.578483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yi Z., Li Z., Yu S., et al. Blood-based gene expression profiles models for classification of subsyndromal symptomatic depression and major depressive disorder. PLoS One. 2012;7(2):e31283. doi: 10.1371/journal.pone.0031283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schiweck C., Claes S., Van Oudenhove L., et al. Childhood trauma, suicide risk and inflammatory phenotypes of depression: insights from monocyte gene expression. Transl Psychiatry. 2020;10(1):296. doi: 10.1038/s41398-020-00979-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pantazatos S.P., Huang Y.Y., Rosoklija G.B., Dwork A.J., Arango V., Mann J.J. Whole-transcriptome brain expression and exon-usage profiling in major depression and suicide: evidence for altered glial, endothelial and ATPase activity. Mol Psychiatry. 2017;22(5):760–773. doi: 10.1038/mp.2016.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fulton SL, Bendl J, Gameiro-Ros I, et al., ZBTB7A regulates MDD-specific chromatin signatures and astrocyte-mediated stress vulnerability in orbitofrontal cortex, bioRxiv (2023). doi: 10.1101/2023.05.04.539425.
- 108.van der Zee Y.Y., Eijssen L.M.T., Mews P., et al. Blood miR-144-3p: a novel diagnostic and therapeutic tool for depression. Mol Psychiatry. 2022;27(11):4536–4549. doi: 10.1038/s41380-022-01712-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Westfall S., Caracci F., Zhao D., et al. Microbiota metabolites modulate the T helper 17 to regulatory T cell (Th17/Treg) imbalance promoting resilience to stress-induced anxiety- and depressive-like behaviors. Brain Behav Immun. 2021;91:350–368. doi: 10.1016/j.bbi.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shimada K., Nohara M., Yasuoka A., et al. Mouse model of weak depression exhibiting suppressed cAMP signaling in the amygdala. Lower Lipid Catabolism in Liver, and Correlated Gut Microbiota, Front Behav Neurosci. 2022;16 doi: 10.3389/fnbeh.2022.841450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang Y., Chen Z.P., Hu H., et al. Sperm microRNAs confer depression susceptibility to offspring. Sci Adv. 2021;7(7) doi: 10.1126/sciadv.abd7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Khandelwal N., Dey S.K., Chakravarty S., Kumar A. miR-30 family miRNAs mediate the effect of chronic social defeat stress on hippocampal neurogenesis in mouse depression model. Front Mol Neurosci. 2019;12:188. doi: 10.3389/fnmol.2019.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Herve M., Bergon A., Le Guisquet A.M., et al. Translational identification of transcriptional signatures of major depression and antidepressant response. Front Mol Neurosci. 2017;10:248. doi: 10.3389/fnmol.2017.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Czibere L., Baur L.A., Wittmann A., et al. Profiling trait anxiety: transcriptome analysis reveals cathepsin B (ctsb) as a novel candidate gene for emotionality in mice. PLoS One. 2011;6(8):e23604. doi: 10.1371/journal.pone.0023604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang Y., Jiang H., Meng H., et al. Antidepressant mechanism Research of acupuncture: insights from a genome-wide transcriptome analysis of frontal cortex in rats with chronic restraint stress. Evid Based Complement Alternat Med. 2017;2017:1676808. doi: 10.1155/2017/1676808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang Y., Jiang H., Meng H., et al. Genome-wide transcriptome analysis of hippocampus in rats indicated that TLR/NLR signaling pathway was involved in the pathogenisis of depressive disorder induced by chronic restraint stress. Brain Res Bull. 2017;134:195–204. doi: 10.1016/j.brainresbull.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 117.M B, Effect of Maternal Separation on DOI-induced transcriptome. In: Omnibus GE, editor.; (2013).
- 118.Skrivankova V.W., Richmond R.C., Woolf B.A.R., et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ. 2021;375 doi: 10.1136/bmj.n2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Skrivankova V.W., Richmond R.C., Woolf B.A.R., et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: the STROBE-MR statement. JAMA. 2021;326(16):1614–1621. doi: 10.1001/jama.2021.18236. [DOI] [PubMed] [Google Scholar]
- 120.Paaby A.B., Rockman M.V. The many faces of pleiotropy. Trends Genet. 2013;29(2):66–73. doi: 10.1016/j.tig.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Verbanck M., Chen C.Y., Neale B., Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–698. doi: 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Burgess S., Butterworth A., Thompson S.G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37(7):658–665. doi: 10.1002/gepi.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bowden J., Davey Smith G., Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. 2015;44(2):512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hartwig F.P., Davey Smith G., Bowden J. Robust inference in summary data mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46(6):1985–1998. doi: 10.1093/ije/dyx102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bowden J., Davey Smith G., Haycock P.C., Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304–314. doi: 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sproviero W., Winchester L., Newby D., et al. High blood pressure and risk of dementia: a two-sample mendelian randomization study in the UK biobank. Biol Psychiatry. 2021;89(8):817–824. doi: 10.1016/j.biopsych.2020.12.015. [DOI] [PubMed] [Google Scholar]
- 127.McMonnies C.W. Dry eye disease immune responses and topical therapy. Eye Vis (Lond) 2019;6:12. doi: 10.1186/s40662-019-0137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Drevets W.C., Wittenberg G.M., Bullmore E.T., Manji H.K. Immune targets for therapeutic development in depression: towards precision medicine. Nat Rev Drug Discov. 2022;21(3):224–244. doi: 10.1038/s41573-021-00368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yu K., Bunya V., Maguire M., et al. Systemic conditions associated with severity of dry eye signs and symptoms in the dry eye assessment and management study. Ophthalmology. 2021;128(10):1384–1392. doi: 10.1016/j.ophtha.2021.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gipson I.K., Hori Y., Argueso P. Character of ocular surface mucins and their alteration in dry eye disease. Ocul Surf. 2004;2(2):131–148. doi: 10.1016/s1542-0124(12)70149-0. [DOI] [PubMed] [Google Scholar]
- 131.Duan H., Yang T., Zhou Y., et al. Comparison of mucin levels at the ocular surface of visual display terminal users with and without dry eye disease. BMC Ophthalmol. 2023;23(1):189. doi: 10.1186/s12886-023-02931-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Felder M., Kapur A., Gonzalez-Bosquet J., et al. MUC16 (CA125): tumor biomarker to cancer therapy, a work in progress. Mol Cancer. 2014;13:129. doi: 10.1186/1476-4598-13-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Su J.P., Liu H.F., Zhang H.L., He Y.J., Nie Y. Effects of different degrees of depression on inflammatory response and immune function in patients with ovarian cancer. J Biol Regul Homeost Agents. 2018;32(5):1225–1230. [PubMed] [Google Scholar]
- 134.Yi P.C., Qin Y.H., Zheng C.M., Ren K.M., Huang L., Chen W. Tumor markers and depression scores are predictive of non-suicidal self-injury behaviors among adolescents with depressive disorder: a retrospective study. Front Neurosci. 2022;16 doi: 10.3389/fnins.2022.953842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Belaish S., Israel-Elgali I., Shapira G., et al. Genome wide analysis implicates upregulation of proteasome pathway in major depressive disorder. Transl Psychiatry. 2021;11(1):409. doi: 10.1038/s41398-021-01529-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xu L., Yang K., Zhu M., et al. Trio-based exome sequencing broaden the genetic spectrum in keratoconus. Exp Eye Res. 2023;226 doi: 10.1016/j.exer.2022.109342. [DOI] [PubMed] [Google Scholar]
- 137.Shah R.L., Guggenheim J.A., Eye U.K.B., Vision C. Genome-wide association studies for corneal and refractive astigmatism in UK biobank demonstrate a shared role for myopia susceptibility loci. Hum Genet. 2018;137(11–12):881–896. doi: 10.1007/s00439-018-1942-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Karpin J, Rodriguez TG, Traboulsi C, Rai V, Gibbons RD, Rubin DT, Assessment of Comorbid Depression and Anxiety in Inflammatory Bowel Disease Using Adaptive Testing Technology, Crohns Colitis 360 (2021). 3(1). doi: 10.1093/crocol/otaa095. [DOI] [PMC free article] [PubMed]
- 139.Young T.R., Bourke M., Zhou X., et al. Ten-m2 is required for the generation of binocular visual circuits. J Neurosci. 2013;33(30):12490–12509. doi: 10.1523/JNEUROSCI.4708-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ichinose S., Susuki Y., Hosoi N., et al. Interaction between Teneurin-2 and microtubules via EB proteins provides a platform for GABAA receptor exocytosis. Elife. 2023:12. doi: 10.7554/eLife.83276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ulhaq Z.S. The association between genetic polymorphisms in estrogen receptor genes and the risk of Ocular disease: a meta-analysis. Turk J Ophthalmol. 2020;50(4):216–220. doi: 10.4274/tjo.galenos.2020.91298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pinsonneault J.K., Sullivan D., Sadee W., Soares C.N., Hampson E., Steiner M. Association study of the estrogen receptor gene ESR1 with postpartum depression–a pilot study. Arch Womens Ment Health. 2013;16(6):499–509. doi: 10.1007/s00737-013-0373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sharif R., Bak-Nielsen S., Hjortdal J., Karamichos D. Pathogenesis of keratoconus: the intriguing therapeutic potential of prolactin-inducible protein. Prog Retin Eye Res. 2018;67:150–167. doi: 10.1016/j.preteyeres.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schaumberg D.A., Buring J.E., Sullivan D.A., Dana M.R. Hormone replacement therapy and dry eye syndrome. JAMA. 2001;286(17):2114–2119. doi: 10.1001/jama.286.17.2114. [DOI] [PubMed] [Google Scholar]
- 145.Turaka K., Nottage J.M., Hammersmith K.M., Nagra P.K., Rapuano C.J. Dry eye syndrome in aromatase inhibitor users. Clin Exp Ophthalmol. 2013;41(3):239–243. doi: 10.1111/j.1442-9071.2012.02865.x. [DOI] [PubMed] [Google Scholar]
- 146.Hutchinson C.V., Walker J.A., Davidson C. Oestrogen, ocular function and low-level vision: a review. J Endocrinol. 2014;223(2):R9–R. doi: 10.1530/JOE-14-0349. [DOI] [PubMed] [Google Scholar]
- 147.Sollis E., Mosaku A., Abid A., et al. The NHGRI-EBI GWAS catalog: knowledgebase and deposition resource. Nucleic Acids Res. 2023;51(D1):D977–D985. doi: 10.1093/nar/gkac1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stevenson W., Chauhan S.K., Dana R. Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol. 2012;130(1):90–100. doi: 10.1001/archophthalmol.2011.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mac Giollabhui N., Ng T.H., Ellman L.M., Alloy L.B. The longitudinal associations of inflammatory biomarkers and depression revisited: systematic review, meta-analysis, and meta-regression. Mol Psychiatry. 2021;26(7):3302–3314. doi: 10.1038/s41380-020-00867-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Roda M., Corazza I., Bacchi Reggiani M.L., et al. Dry eye disease and Tear cytokine levels-a meta-analysis. Int J Mol Sci. 2020;21(9) doi: 10.3390/ijms21093111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kohler C.A., Freitas T.H., Stubbs B., et al. Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive Disorder: systematic review and meta-analysis. Mol Neurobiol. 2018;55(5):4195–4206. doi: 10.1007/s12035-017-0632-1. [DOI] [PubMed] [Google Scholar]
- 152.Chen Y.T., Nikulina K., Lazarev S., et al. Interleukin-1 as a phenotypic immunomodulator in keratinizing squamous metaplasia of the ocular surface in sjogren's syndrome. Am J Pathol. 2010;177(3):1333–1343. doi: 10.2353/ajpath.2010.100227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.De Paiva C.S., Villarreal A.L., Corrales R.M., et al. Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-gamma. Invest Ophthalmol Vis Sci. 2007;48(6):2553–2560. doi: 10.1167/iovs.07-0069. [DOI] [PubMed] [Google Scholar]
- 154.Zhang X., Yin Y., Yue L., Gong L. Selective serotonin reuptake inhibitors aggravate depression-associated dry eye via activating the NF-kappaB pathway. Invest Ophthalmol Vis Sci. 2019;60(1):407–419. doi: 10.1167/iovs.18-25572. [DOI] [PubMed] [Google Scholar]
- 155.Zhao H., Yin Y., Lin T., Wang W., Gong L. Administration of serotonin and norepinephrine reuptake inhibitors tends to have less ocular surface damage in a chronic stress-induced rat model of depression than selective serotonin reuptake inhibitors. Exp Eye Res. 2023;231 doi: 10.1016/j.exer.2023.109486. [DOI] [PubMed] [Google Scholar]
- 156.Bitar M.S., Olson D.J., Li M., Davis R.M. The Correlation between dry eyes, anxiety and depression: the sicca, anxiety and depression study. Cornea. 2019;38(6):684–689. doi: 10.1097/ICO.0000000000001932. [DOI] [PubMed] [Google Scholar]
- 157.Xu Y., Phu J., Aung H.L., et al. Frequency of coexistent eye diseases and cognitive impairment or dementia: a systematic review and meta-analysis. Eye. 2023;1–9 doi: 10.1038/s41433-023-02481-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sivak J.M. The aging eye: common degenerative mechanisms between the Alzheimer's brain and retinal disease. Invest Ophthalmol Vis Sci. 2013;54(1):871–880. doi: 10.1167/iovs.12-10827. [DOI] [PubMed] [Google Scholar]
- 159.Chang K.-J., Wu H.-Y., Yarmishyn A.A., et al. Genetics behind cerebral disease with Ocular comorbidity: finding Parallels between the brain and eye Molecular pathology. Int J Mol Sci. 2022;23(17):9707. doi: 10.3390/ijms23179707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wareham L.K., Liddelow S.A., Temple S., et al. Solving neurodegeneration: common mechanisms and strategies for new treatments. Mol Neurodegener. 2022;17(1):23. doi: 10.1186/s13024-022-00524-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Belleau E.L., Treadway M.T., Pizzagalli D.A. The impact of stress and major depressive disorder on hippocampal and medial prefrontal cortex morphology. Biol Psychiatry. 2019;85(6):443–453. doi: 10.1016/j.biopsych.2018.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hennessy E.J., Parker A.E. O'neill LA, Targeting toll-like receptors: emerging therapeutics? Nat Rev Drug Discov. 2010;9(4):293–307. doi: 10.1038/nrd3203. [DOI] [PubMed] [Google Scholar]
- 163.Saikh K.U. MyD88 and beyond: a perspective on MyD88-targeted therapeutic approach for modulation of host immunity. Immunol Res. 2021;69:117–128. doi: 10.1007/s12026-021-09188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.