Abstract
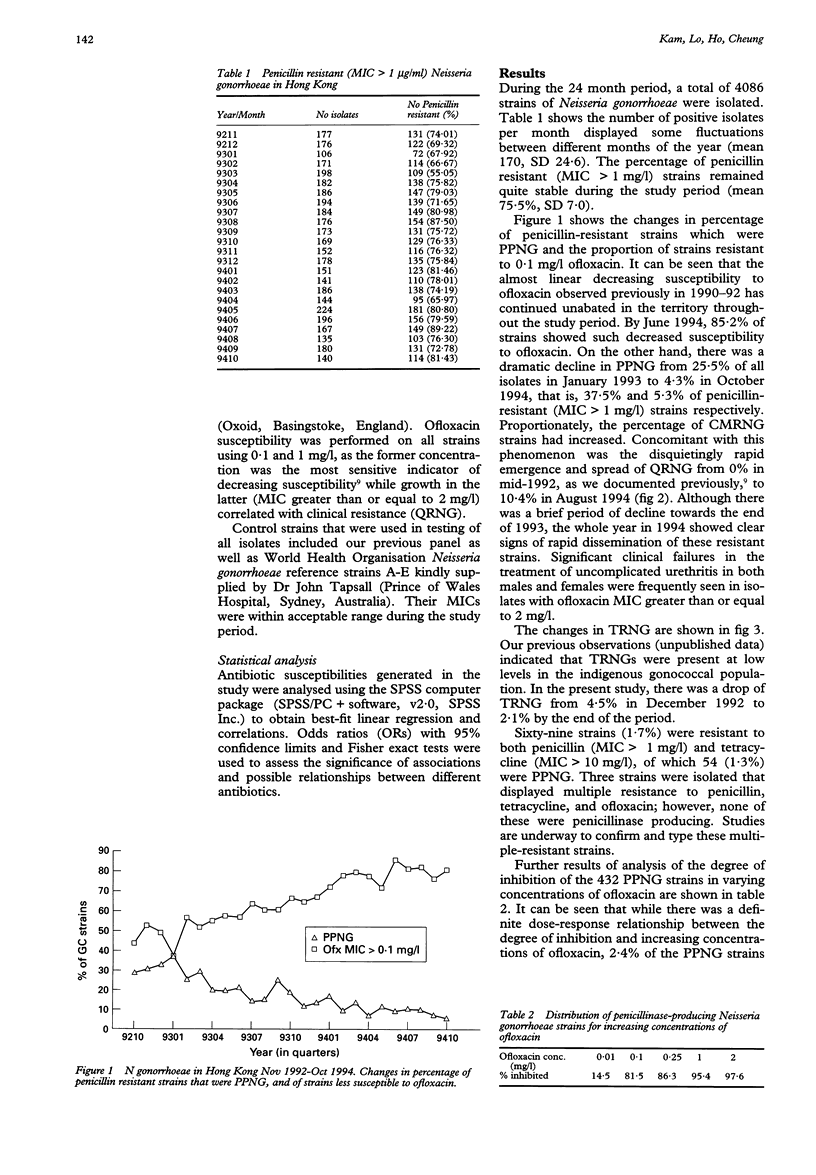
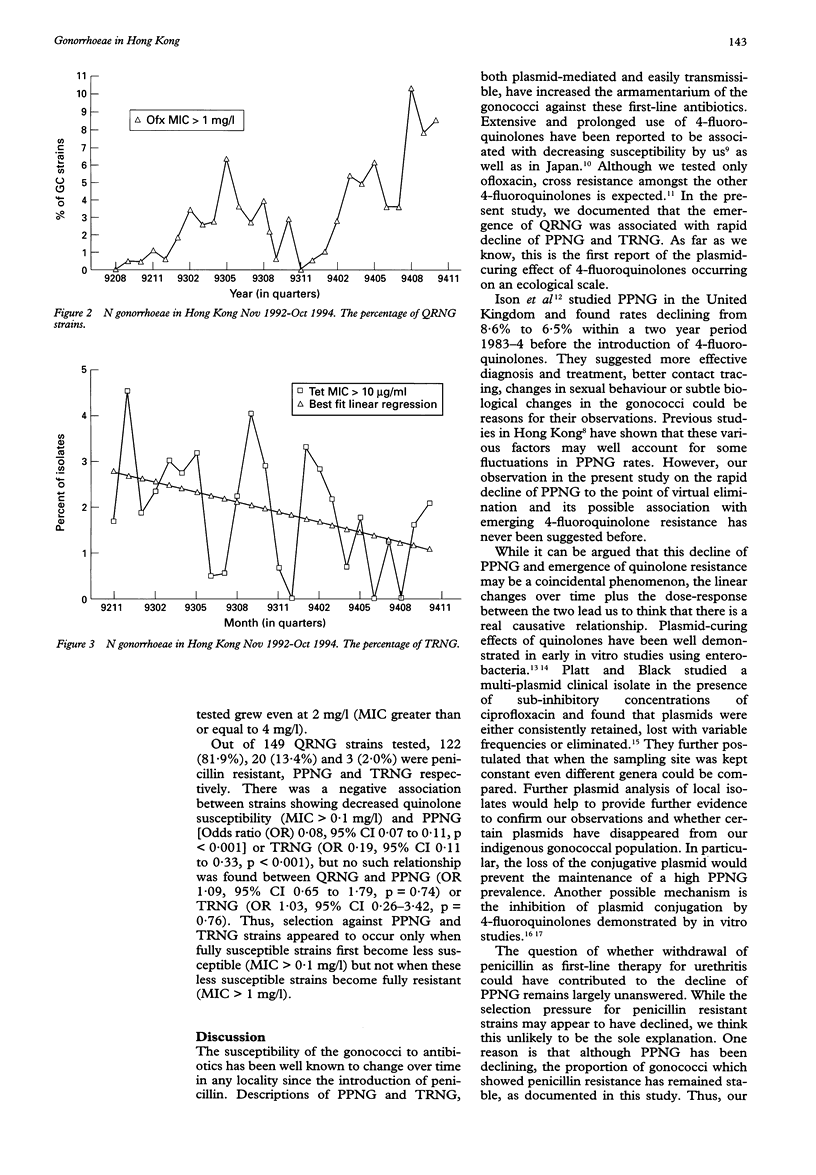
OBJECTIVE--To study the changes in penicillinase-producing (PPNG) and high-level tetracycline resistant (TRNG) Neisseria gonorrhoeae isolated in Hong Kong associated with emerging quinolone resistance (QRNG) over a two year period from November 1992 to October 1994. MATERIALS AND METHODS--Four thousand and eighty-six strains of Neisseria gonorrhoeae isolated, of which 432 were PPNG, were examined for susceptibilities to penicillin and tetracycline by an agar dilution method using the breakpoint minimum inhibitory concentrations (MICs) of 1 and 10 mg/1 respectively. Ofloxacin susceptibility was done using 0.1 and 1 mg/l. Penicillinase production was detected by performing the chromogenic cephalosporin nitrocefin test on all penicillin resistant (MIC > 1 mg/l) strains. RESULTS--Three thousand and eighty (75.4%) and 79 (1.9%) strains were found to be penicillin resistant and TRNG (MIC > 10 mg/l) respectively. Sixty-nine strains (1.7%) were resistant to both, of which 54 (1.3%) were PPNG. Three strains were multiply-resistant to penicillin, tetracycline and ofloxacin; however, none was PPNG. While the percentage of penicillin resistant strains remained stable (mean 75.5%, SD 7.0), TRNG decreased from 4.5% to 2.1%. The most dramatic change was the sharp decline of PPNG from 25.5% in January 1993 to 4.3% in October 1994, concurrent with a linear increase in strains with ofloxacin MIC > 0.1 mg/l. Significant clinical failure was seen in strains having ofloxacin MIC > 1 mg/l (QRNG), which increased drastically from 0.5% to 10.4% during the study period. Selection against PPNG and TRNG strains appeared to occur only when fully quinolone-susceptible strains first become less susceptible (MIC > 0.1 mg/l), but not when these less susceptible strains become fully resistant (MIC > 1 mg/l). CONCLUSION--PPNG is now no longer hyperendemic in Hong Kong. Emergence of QRNG is associated with rapid decline of both PPNG and TRNG. This is the first report of plasmid-curing effect of the 4-fluoroquinolones occurring on an ecological scale.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashford W. A., Golash R. G., Hemming V. G. Penicillinase-producing Neisseria gonorrhoeae. Lancet. 1976 Sep 25;2(7987):657–658. doi: 10.1016/s0140-6736(76)92467-3. [DOI] [PubMed] [Google Scholar]
- Barry A. L., Jones R. N. Cross-resistance among cinoxacin, ciprofloxacin, DJ-6783, enoxacin, nalidixic acid, norfloxacin, and oxolinic acid after in vitro selection of resistant populations. Antimicrob Agents Chemother. 1984 Jun;25(6):775–777. doi: 10.1128/aac.25.6.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman L. G. R-plasmid transfer and its response to nalidixic acid. J Bacteriol. 1977 Jul;131(1):76–81. doi: 10.1128/jb.131.1.76-81.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu M. L., Ho L. J., Lin H. C., Wu Y. C. Epidemiology of penicillin-resistant Neisseria gonorrhoeae isolated in Taiwan, 1960-1990. Clin Infect Dis. 1992 Feb;14(2):450–457. doi: 10.1093/clinids/14.2.450. [DOI] [PubMed] [Google Scholar]
- Crumplin G. C., Smith J. T. The effect of R-factor plasmids on host-cell responses to nalidixic acid I. Increased susceptibility of nalidixic acid-sensitive hosts. J Antimicrob Chemother. 1981 Apr;7(4):379–388. doi: 10.1093/jac/7.4.379. [DOI] [PubMed] [Google Scholar]
- Dillon J. R., Pauzé M., Yeung K. H. Molecular and epidemiological analysis of penicillinase producing strains of Neisseria gonorrhoeae isolated in Canada 1976-84: evolution of new auxotypes and beta lactamase encoding plasmids. Genitourin Med. 1986 Jun;62(3):151–157. doi: 10.1136/sti.62.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escamilla J., Bourgeois A. L., Gardiner C. H., Kilpatrick M. E. Penicillinase-producing Neisseria gonorrhoeae in various seaport cities of Latin America. Sex Transm Dis. 1988 Jul-Sep;15(3):141–143. doi: 10.1097/00007435-198807000-00004. [DOI] [PubMed] [Google Scholar]
- Hook E. W., 3rd, Holmes K. K. Gonococcal infections. Ann Intern Med. 1985 Feb;102(2):229–243. doi: 10.7326/0003-4819-102-2-229. [DOI] [PubMed] [Google Scholar]
- Ison C. A., Gedney J., Harris J. R., Easmon C. S. Penicillinase producing gonococci: a spent force? Genitourin Med. 1986 Oct;62(5):302–307. doi: 10.1136/sti.62.5.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam K. M., Lai C. F., Egglestone S., Chan C. B. Patterns of antibiotic susceptibility of gonococci isolated in Hong Kong, 1987-1990. Sex Transm Dis. 1992 Sep-Oct;19(5):284–287. doi: 10.1097/00007435-199209000-00008. [DOI] [PubMed] [Google Scholar]
- Kam K. M., Lo K. K., Lai C. F., Lee Y. S., Chan C. B. Ofloxacin susceptibilities of 5,667 Neisseria gonorrhoeae strains isolated in Hong Kong. Antimicrob Agents Chemother. 1993 Sep;37(9):2007–2008. doi: 10.1128/aac.37.9.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Inoue S., Shimizu M., Iyobe S., Mitsuhashi S. Inhibition of conjugal transfer of R plasmids by pipemidic acid and related compounds. Antimicrob Agents Chemother. 1976 Nov;10(5):779–785. doi: 10.1128/aac.10.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips I. Beta-lactamase-producing, penicillin-resistant gonococcus. Lancet. 1976 Sep 25;2(7987):656–657. doi: 10.1016/s0140-6736(76)92466-1. [DOI] [PubMed] [Google Scholar]
- Platt D. J., Black A. C. Plasmid ecology and the elimination of plasmids by 4-quinolones. J Antimicrob Chemother. 1987 Jul;20(1):137–138. doi: 10.1093/jac/20.1.137. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Kumazawa J., Matsumoto T., Kobayashi I. High prevalence of Neisseria gonorrhoeae strains with reduced susceptibility to fluoroquinolones in Japan. Genitourin Med. 1994 Apr;70(2):90–93. doi: 10.1136/sti.70.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisser J., Wiedemann B. Elimination of plasmids by new 4-quinolones. Antimicrob Agents Chemother. 1985 Nov;28(5):700–702. doi: 10.1128/aac.28.5.700. [DOI] [PMC free article] [PubMed] [Google Scholar]


