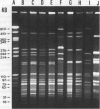
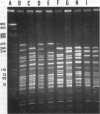
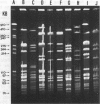
Abstract
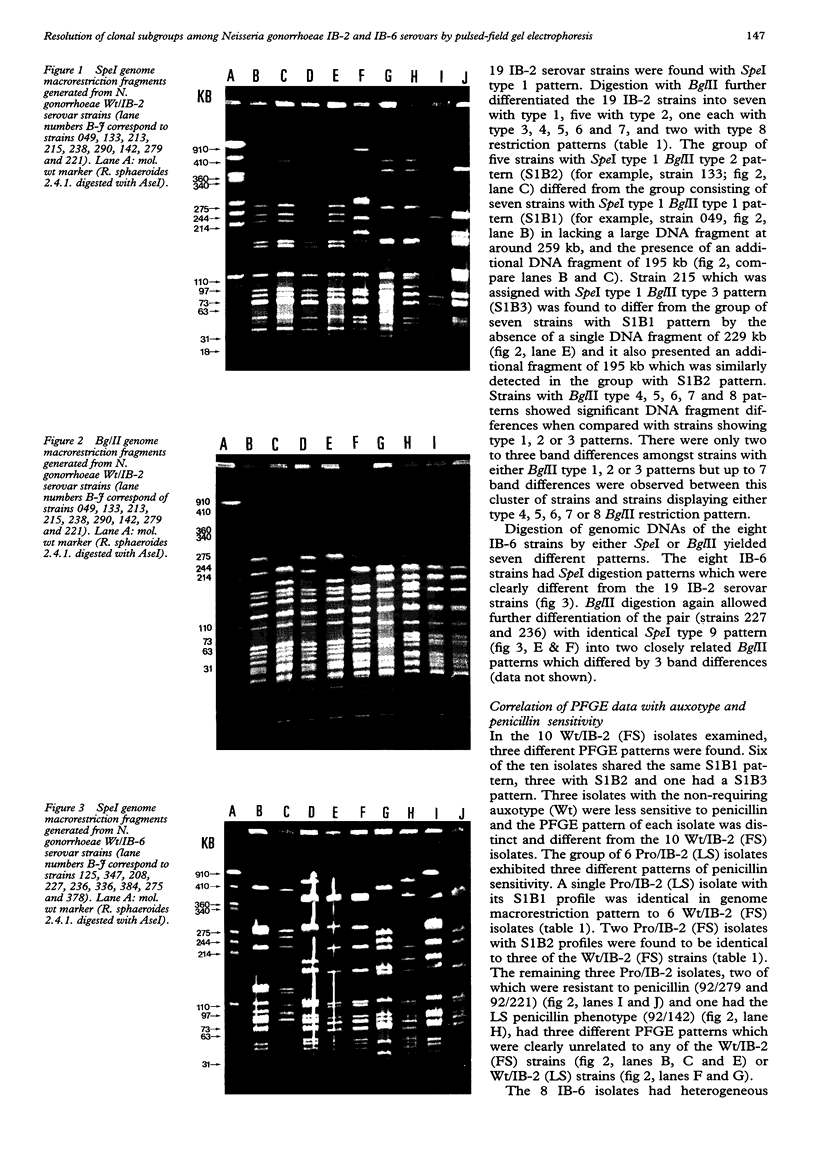
OBJECTIVE--Analysis of macrorestriction patterns by PFGE to resolve the relatedness of clonal subgroups amongst N gonorrhoeae IB-2 and IB-6 serovar strains. MATERIALS AND METHODS--Nineteen IB-2 and eight IB-6 serovar strains that differed in either auxotype or penicillin sensitivity were isolated over a two and a half-year period from patients attending several STD clinics in Sydney. During this period, a major clone, Wt/IB-2 (FS), established on epidemiological grounds, was circulating amongst homosexual males. The genetic relation of this major clone to the other strains present in the community was determined by pulsed-field gel electrophoretic (PFGE) analysis of DNA restriction fragments. Genomic DNA from the 27 isolates were prepared, digested with SpeI and BglII and the restriction patterns were analysed by contour-clamped homogeneous electric field electrophoresis (CHEF) in a CHEF DRIII equipment. RESULTS--Phenotypic characterisation of the 27 isolates by the combined use of auxotype, serological characterisation and penicillin sensitivity indicated the presence of subgroups within each of the two serovars. In the present study, PFGE analysis of SPeI and BglII-generated genomic DNA restriction patterns from six of the ten Wt/IB-2 (FS) correlated well with phenotypic characterisation of this major clone. Four of the ten Wt/IB-2 (FS) were found to be clonally-derived variants of this major clone as minor genome variations (less than 3 DNA fragments) were observed. Distinct clones were represented by three Wt/IB-2 (LS) isolates as the DNA fingerprints generated from these were unrelated to the major clone. Analysis of PFGE patterns of 6 Pro/IB-2 isolates showed that one was genotypically identical to the major clone, two were clonal variants and three had significantly different patterns to indicate that they were genotypically unrelated. Wt/IB-6 isolates had heterogenous PFGE patterns that were clearly unrelated to the Wt/IB-2 serovar strains. Within the IB-6 serovar, there were three isolates with the Wt/IB-6 (FS) phenotype that could be considered as clonal variants whilst the rest were genotypically distinct. CONCLUSIONS--PFGE analysis of macrorestriction patterns generated from SpeI- and BglII-cleavage of genomic DNA has enabled the establishment of clonal origins of strains present in the Sydney community during the period of study. The delineation of strains belonging to major A/S groups by PFGE analysis presents a clearer epidemiological picture than phenotypic characterisation alone.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryan L. E. Two forms of antimicrobial resistance: bacterial persistence and positive function resistance. J Antimicrob Chemother. 1989 Jun;23(6):817–820. doi: 10.1093/jac/23.6.817. [DOI] [PubMed] [Google Scholar]
- Dasi M. A., Nogueira J. M., Camarena J. J., Gil C., García-Verdú R., Barberá J. L., Barberá J. Genomic fingerprinting of penicillinase-producing strains of Neisseria gonorrhoeae in Valencia, Spain. Genitourin Med. 1992 Jun;68(3):170–173. doi: 10.1136/sti.68.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill M. J. Serotyping Neisseria gonorrhoeae: a report of the Fourth International Workshop. Genitourin Med. 1991 Feb;67(1):53–57. doi: 10.1136/sti.67.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp J. S., Tam M. R., Nowinski R. C., Holmes K. K., Sandström E. G. Serological classification of Neisseria gonorrhoeae with use of monoclonal antibodies to gonococcal outer membrane protein I. J Infect Dis. 1984 Jul;150(1):44–48. doi: 10.1093/infdis/150.1.44. [DOI] [PubMed] [Google Scholar]
- Kohl P. K., Ison C. A., Danielsson D., Knapp J. S., Petzoldt D. Current status of serotyping of Neisseria gonorrhoeae. Eur J Epidemiol. 1990 Mar;6(1):91–95. doi: 10.1007/BF00155558. [DOI] [PubMed] [Google Scholar]
- Penicillin sensitivity of gonococci in Australia: development of Australian gonococcal surveillance programme. Members of the Australian Gonococcal Surveillance Programme. Br J Vener Dis. 1984 Aug;60(4):226–230. doi: 10.1136/sti.60.4.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poh C. L., Lau Q. C. Subtyping of Neisseria gonorrhoeae auxotype-serovar groups by pulsed-field gel electrophoresis. J Med Microbiol. 1993 May;38(5):366–370. doi: 10.1099/00222615-38-5-366. [DOI] [PubMed] [Google Scholar]
- Poh C. L., Ocampo J. C., Sng E. H., Bygdeman S. M. Characterisation of PPNG and non-PPNG Neisseria gonorrhoeae isolates from Singapore. Genitourin Med. 1991 Oct;67(5):389–393. doi: 10.1136/sti.67.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowbottom J. H., Tapsall J. W., Plummer D. C., Bodsworth N. J., MacDonald M. A., Chambers I. W., Kaldor J. M. An outbreak of a penicillin-sensitive strain of gonorrhoea in Sydney men. Genitourin Med. 1994 Jun;70(3):196–199. doi: 10.1136/sti.70.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struelens M. J., Schwam V., Deplano A., Baran D. Genome macrorestriction analysis of diversity and variability of Pseudomonas aeruginosa strains infecting cystic fibrosis patients. J Clin Microbiol. 1993 Sep;31(9):2320–2326. doi: 10.1128/jcm.31.9.2320-2326.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanto A., Kaplan S. Physical and genetic mapping of the Rhodobacter sphaeroides 2.4.1 genome: genome size, fragment identification, and gene localization. J Bacteriol. 1989 Nov;171(11):5840–5849. doi: 10.1128/jb.171.11.5840-5849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]