Abstract
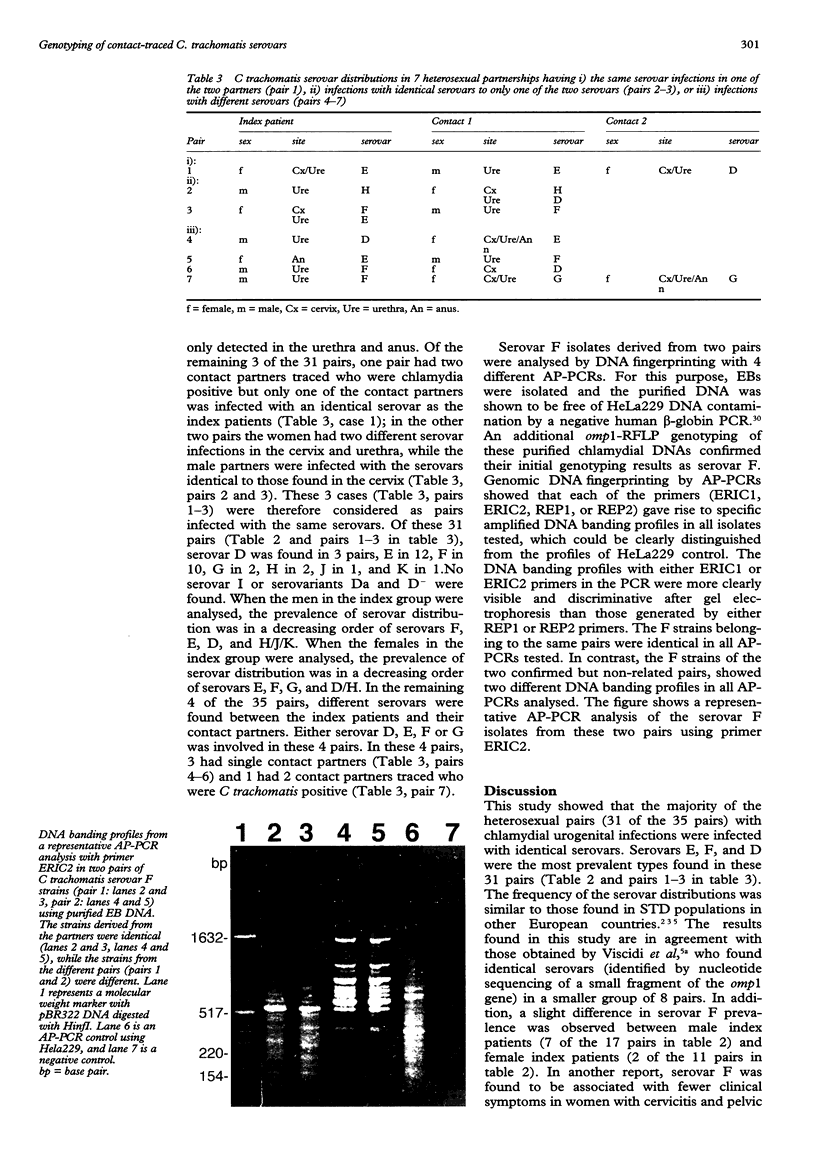
OBJECTIVE--To investigate C trachomatis serovars in contact-traced heterosexual partners. METHODS--Urogenital Chlamydia trachomatis isolates (n = 112) derived from 35 heterosexual patients (index patients) and their 37 chlamydia positive partners (contact patients) were differentiated into serovars by genotyping with restriction fragment length polymorphism (RFLP) analysis of the PCR amplified omp1 gene. In order to investigate whether different strains within the frequently prevalent serovar F were transmitted, two pairs of serovar F (n = 4) were further analysed by genomic DNA fingerprinting with arbitrary primer PCRs (AP-PCRs). RESULTS--Identical C trachomatis serovars were found in 31 of the 35 pairs, serovars E, F, D, and G being most prevalent. In the remaining four pairs different serovars (either D, E, F or G) were found between the index and the contact patients. By AP-PCR analysis the strains of serovar F were found to be identical between the index and the contact patients, but were different between the two pairs in all AP-PCRs used. CONCLUSION--A majority of heterosexual partners, once traced positive for C trachomatis infections, are infected with identical serovars. Identical strains of serovar F found in partners as found by DNA fingerprinting confirms the sexual transmission of C trachomatis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caetano-Anollés G., Bassam B. J., Gresshoff P. M. Primer-template interactions during DNA amplification fingerprinting with single arbitrary oligonucleotides. Mol Gen Genet. 1992 Nov;235(2-3):157–165. doi: 10.1007/BF00279356. [DOI] [PubMed] [Google Scholar]
- Dean D., Schachter J., Dawson C. R., Stephens R. S. Comparison of the major outer membrane protein variant sequence regions of B/Ba isolates: a molecular epidemiologic approach to Chlamydia trachomatis infections. J Infect Dis. 1992 Aug;166(2):383–392. doi: 10.1093/infdis/166.2.383. [DOI] [PubMed] [Google Scholar]
- Frost E. H., Deslandes S., Bourgaux-Ramoisy D. Sensitive detection and typing of Chlamydia trachomatis using nested polymerase chain reaction. Genitourin Med. 1993 Aug;69(4):290–294. doi: 10.1136/sti.69.4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost E. H., Deslandes S., Veilleux S., Bourgaux-Ramoisy D. Typing Chlamydia trachomatis by detection of restriction fragment length polymorphism in the gene encoding the major outer membrane protein. J Infect Dis. 1991 May;163(5):1103–1107. doi: 10.1093/infdis/163.5.1103. [DOI] [PubMed] [Google Scholar]
- Gaydos C. A., Bobo L., Welsh L., Hook E. W., 3rd, Viscidi R., Quinn T. C. Gene typing of Chlamydia trachomatis by polymerase chain reaction and restriction endonuclease digestion. Sex Transm Dis. 1992 Nov-Dec;19(6):303–308. [PubMed] [Google Scholar]
- Kuo C. C., Wang S. P., Holmes K. K., Grayston J. T. Immunotypes of Chlamydia trachomatis isolates in Seattle, Washington. Infect Immun. 1983 Aug;41(2):865–868. doi: 10.1128/iai.41.2.865-868.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe M. F., Suchland R. J., Stamm W. E. Nucleotide sequence of the variable domains within the major outer membrane protein gene from serovariants of Chlamydia trachomatis. Infect Immun. 1993 Jan;61(1):213–219. doi: 10.1128/iai.61.1.213-219.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan J., Ossewaarde J. M., Walboomers J. M., Meijer C. J., van den Brule A. J. Improved PCR sensitivity for direct genotyping of Chlamydia trachomatis serovars by using a nested PCR. J Clin Microbiol. 1994 Feb;32(2):528–530. doi: 10.1128/jcm.32.2.528-530.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan J., Walboomers J. M., Roosendaal R., van Doornum G. J., MacLaren D. M., Meijer C. J., van den Brule A. J. Direct detection and genotyping of Chlamydia trachomatis in cervical scrapes by using polymerase chain reaction and restriction fragment length polymorphism analysis. J Clin Microbiol. 1993 May;31(5):1060–1065. doi: 10.1128/jcm.31.5.1060-1065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan J., van den Brule A. J., Hemrika D. J., Risse E. K., Walboomers J. M., Schipper M. E., Meijer C. J. Chlamydia trachomatis and ectopic pregnancy: retrospective analysis of salpingectomy specimens, endometrial biopsies, and cervical smears. J Clin Pathol. 1995 Sep;48(9):815–819. doi: 10.1136/jcp.48.9.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossewaarde J. M., Manten J. W., Hooft H. J., Hekker A. C. An enzyme immunoassay to detect specific antibodies to protein and lipopolysaccharide antigens of Chlamydia trachomatis. J Immunol Methods. 1989 Oct 24;123(2):293–298. doi: 10.1016/0022-1759(89)90233-0. [DOI] [PubMed] [Google Scholar]
- Poole E., Lamont I. Chlamydia trachomatis serovar differentiation by direct sequence analysis of the variable segment 4 region of the major outer membrane protein gene. Infect Immun. 1992 Mar;60(3):1089–1094. doi: 10.1128/iai.60.3.1089-1094.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez P., de Barbeyrac B., Persson K., Dutilh B., Bebear C. Evaluation of molecular typing for epidemiological study of Chlamydia trachomatis genital infections. J Clin Microbiol. 1993 Aug;31(8):2238–2240. doi: 10.1128/jcm.31.8.2238-2240.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito J., Takada M. [Detection of Chlamydia trachomatis and the serovar determination using PCR and dot-blot hybridization]. Nihon Rinsho. 1992 Jul;50 (Suppl):412–417. [PubMed] [Google Scholar]
- Sayada C., Denamur E., Xerri B., Orfila J., Catalan F., Elion J. Epidémiologie de Chlamydia trachomatis par l'analyse du gène de la protéine majeure de membrane externe. Pathol Biol (Paris) 1992 May;40(5):583–589. [PubMed] [Google Scholar]
- Scieux C., Grimont F., Regnault B., Bianchi A., Kowalski S., Grimont P. A. Molecular typing of Chlamydia trachomatis by random amplification of polymorphic DNA. Res Microbiol. 1993 Jun;144(5):395–404. doi: 10.1016/0923-2508(93)90197-a. [DOI] [PubMed] [Google Scholar]
- Suchland R. J., Stamm W. E. Simplified microtiter cell culture method for rapid immunotyping of Chlamydia trachomatis. J Clin Microbiol. 1991 Jul;29(7):1333–1338. doi: 10.1128/jcm.29.7.1333-1338.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versalovic J., Koeuth T., Lupski J. R. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991 Dec 25;19(24):6823–6831. doi: 10.1093/nar/19.24.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscidi R. P., Bobo L., Hook E. W., 3rd, Quinn T. C. Transmission of Chlamydia trachomatis among sex partners assessed by polymerase chain reaction. J Infect Dis. 1993 Aug;168(2):488–492. doi: 10.1093/infdis/168.2.488. [DOI] [PubMed] [Google Scholar]
- Wagenvoort J. H., Suchland R. J., Stamm W. E. Serovar distribution of urogenital Chlamydia trachomatis strains in The Netherlands. Genitourin Med. 1988 Jun;64(3):159–161. doi: 10.1136/sti.64.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. P., Grayston J. T. Serotyping of Chlamydia trachomatis by indirect fluorescent-antibody staining of inclusions in cell culture with monoclonal antibodies. J Clin Microbiol. 1991 Jul;29(7):1295–1298. doi: 10.1128/jcm.29.7.1295-1298.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. P., Grayston J. T. Three new serovars of Chlamydia trachomatis: Da, Ia, and L2a. J Infect Dis. 1991 Feb;163(2):403–405. doi: 10.1093/infdis/163.2.403. [DOI] [PubMed] [Google Scholar]
- Welsh J., McClelland M. Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res. 1990 Dec 25;18(24):7213–7218. doi: 10.1093/nar/18.24.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workowski K. A., Stevens C. E., Suchland R. J., Holmes K. K., Eschenbach D. A., Pettinger M. B., Stamm W. E. Clinical manifestations of genital infection due to Chlamydia trachomatis in women: differences related to serovar. Clin Infect Dis. 1994 Oct;19(4):756–760. doi: 10.1093/clinids/19.4.756. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Zhang Y. X., Watkins N. G., Caldwell H. D. Nucleotide and deduced amino acid sequences for the four variable domains of the major outer membrane proteins of the 15 Chlamydia trachomatis serovars. Infect Immun. 1989 Apr;57(4):1040–1049. doi: 10.1128/iai.57.4.1040-1049.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hoek J. A., van Haastrecht H. J., Fennema J. S., Kint J. A., van Doornum G. J., Coutinho R. A. Vórkomen en risicofactoren van infectie met Chlamydia trachomatis bij bezoekers van een geslachtsziektenpolikliniek in Amsterdam. Ned Tijdschr Geneeskd. 1989 Dec 2;133(48):2392–2396. [PubMed] [Google Scholar]




