Abstract
1. The authors have studied the effect of caffeine in subthreshold concentration (0.5 mmol l-1 at 2-4 °C) on the contraction threshold, on intramembrane charge movement and calcium transients in voltage-clamped frog skeletal muscle fibres.
2. The single-gap technique (Kovács & Schneider, 1978) was used for the voltage clamping of terminated segments of cut fibres. Ionic conductances were minimized by using caesium glutamate at the open end pool and tetraethylammonium sulphate and tetrodotoxin at the closed end pool.
3. Myoplasmic calcium transients evoked by depolarizing pulses were recorded by measuring the changes in absorbance of the fibres at 720 nm after the intracellular application of Antipyrylazo III dye.
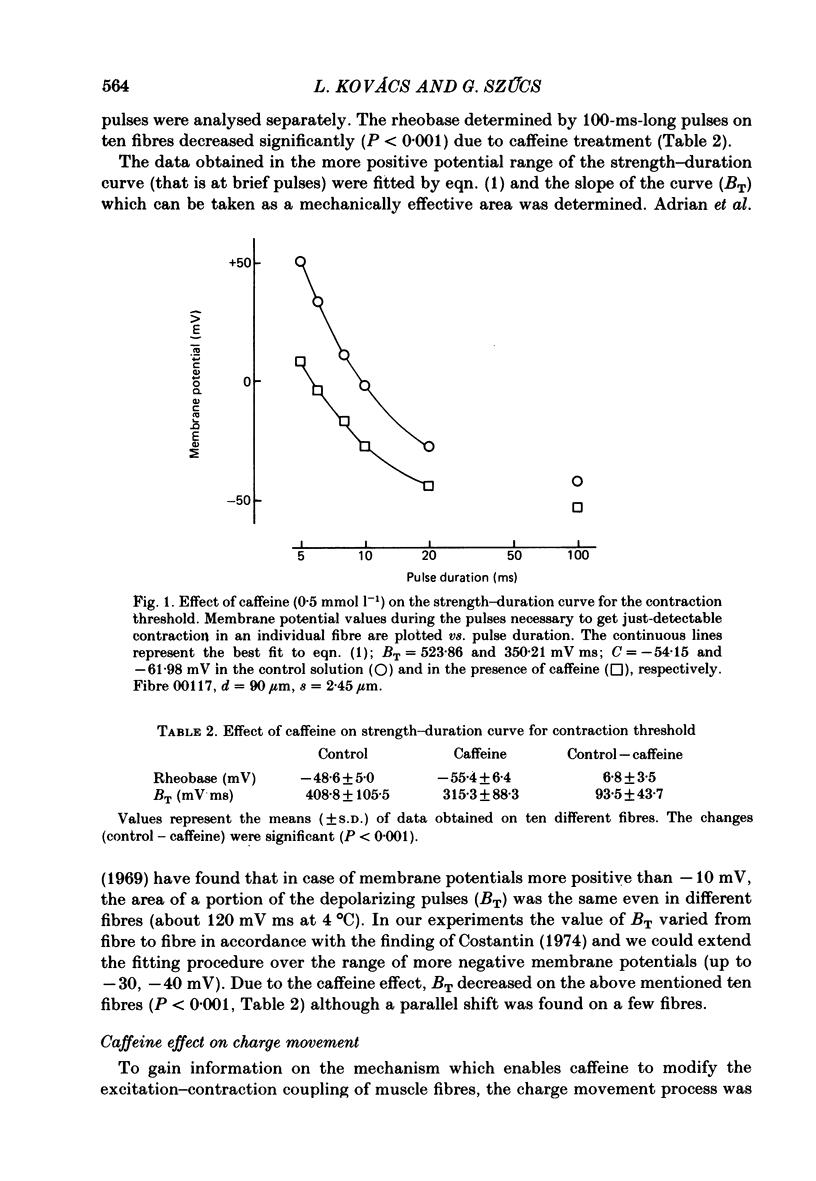
4. The strength—duration curve for contraction threshold was shifted towards more negative membrane potentials in the presence of caffeine. Shift was more definite at shorter pulse durations than at the rheobase.
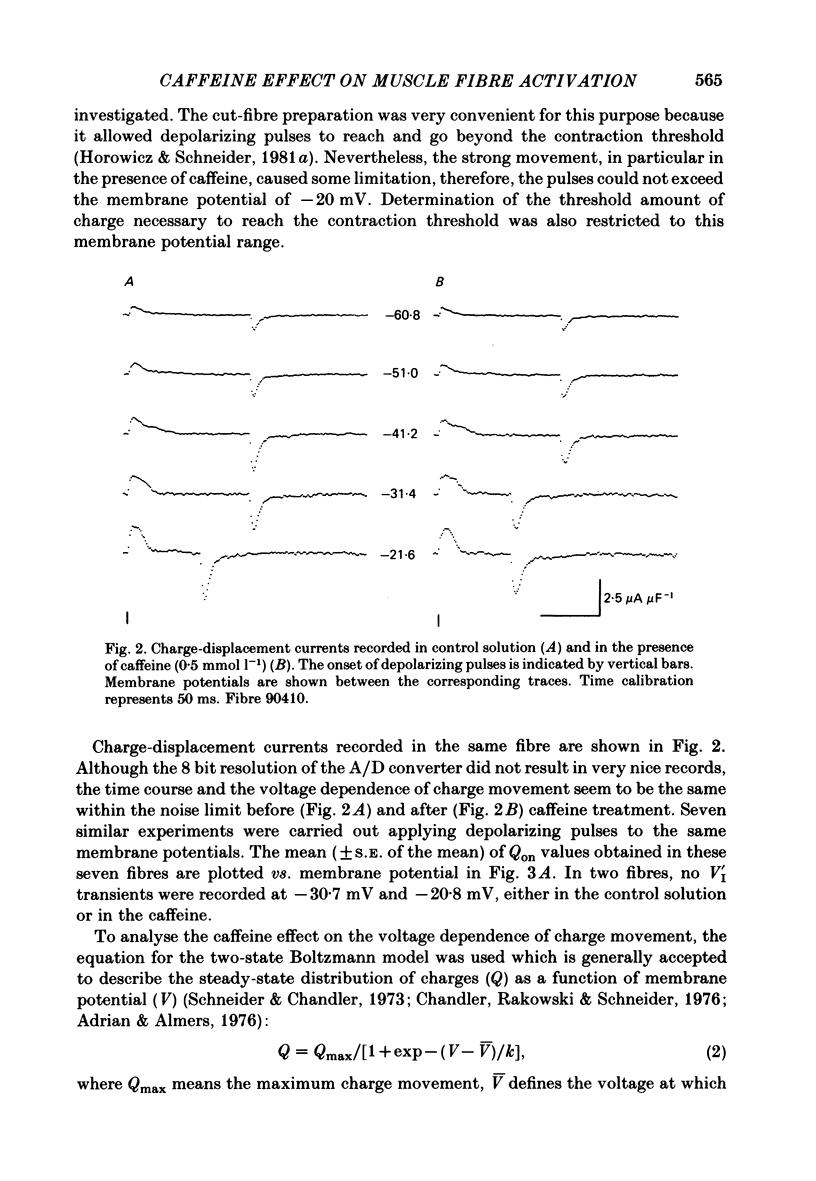
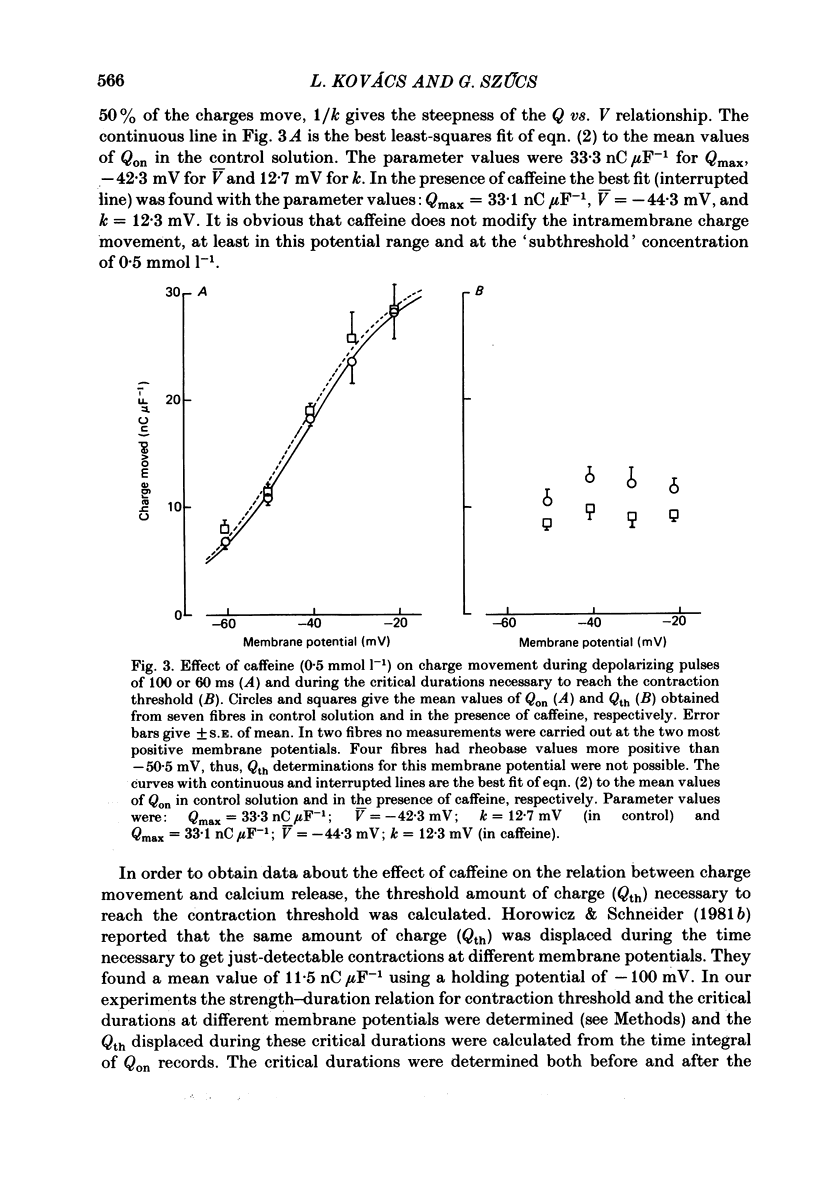
5. The total amount of charge moving during the depolarizing pulses at different membrane potentials was not changed by caffeine treatment, whereas the threshold amounts of charge moved during the critical periods of the contraction threshold decreased at different voltages (by about 23%).
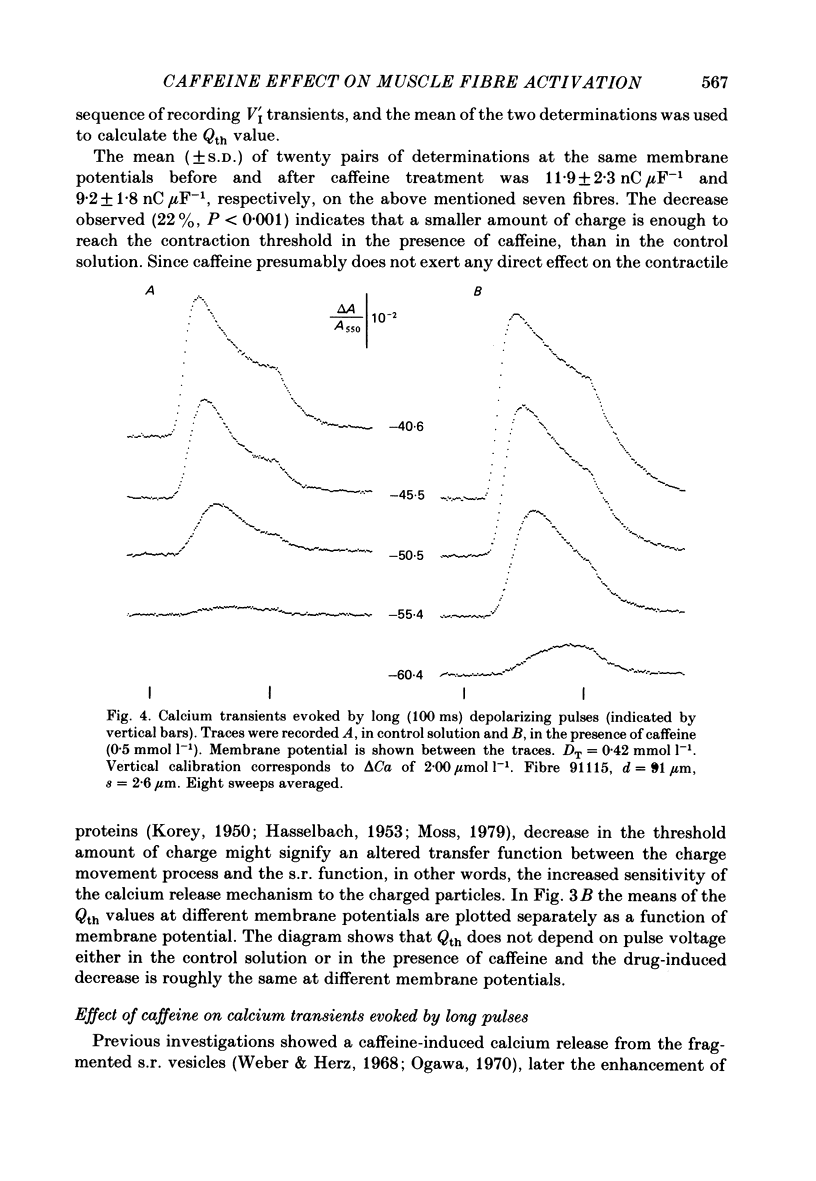
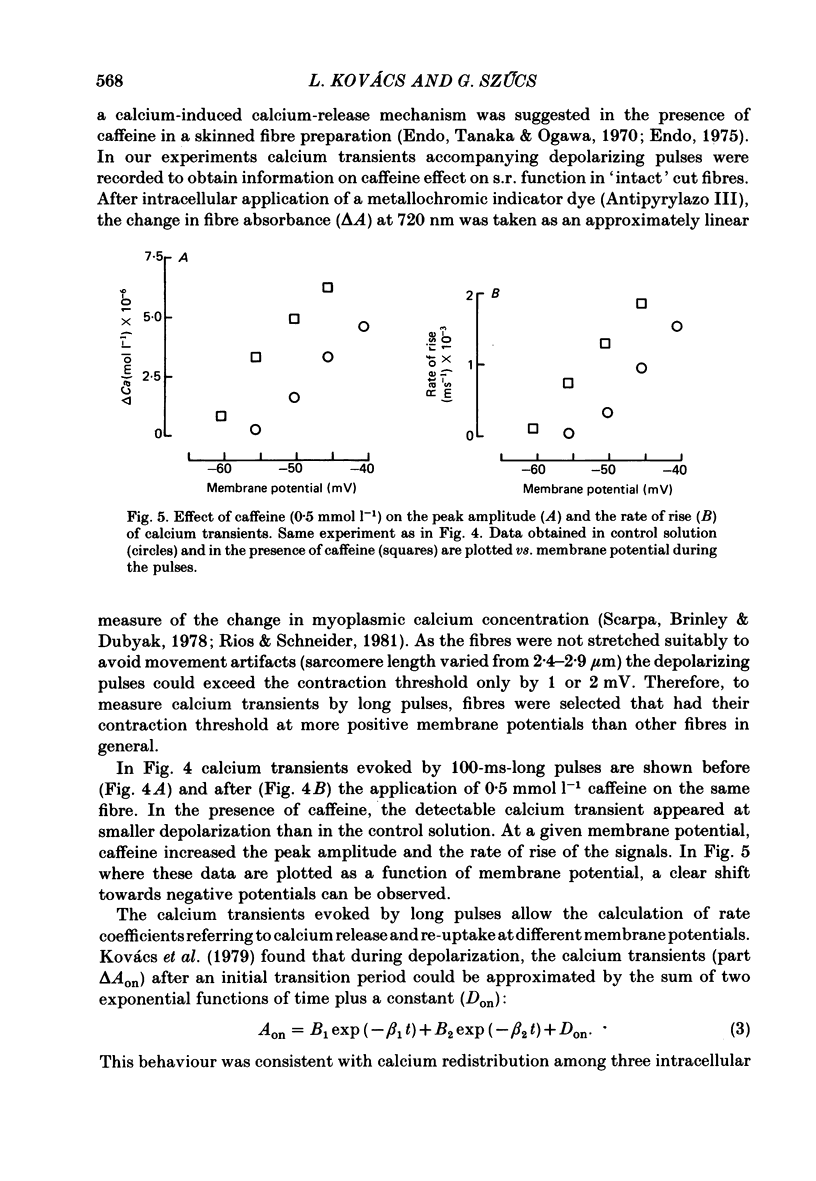
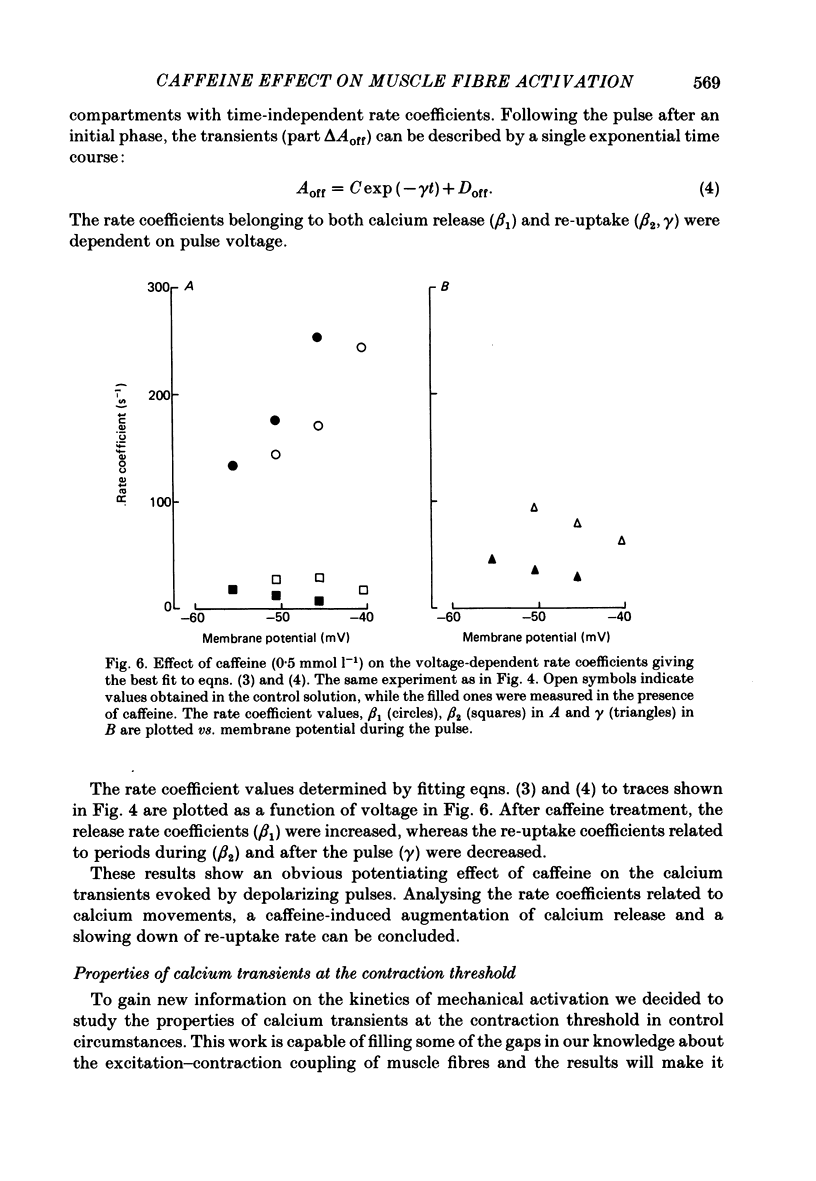
6. In the presence of caffeine, calcium transients accompanying long (100 ms) depolarizing pulses showed increased voltage-dependent peak amplitudes, rising phases and rate coefficients referring to calcium release, but a decreased voltage-dependent re-uptake rate either during or after the pulse.
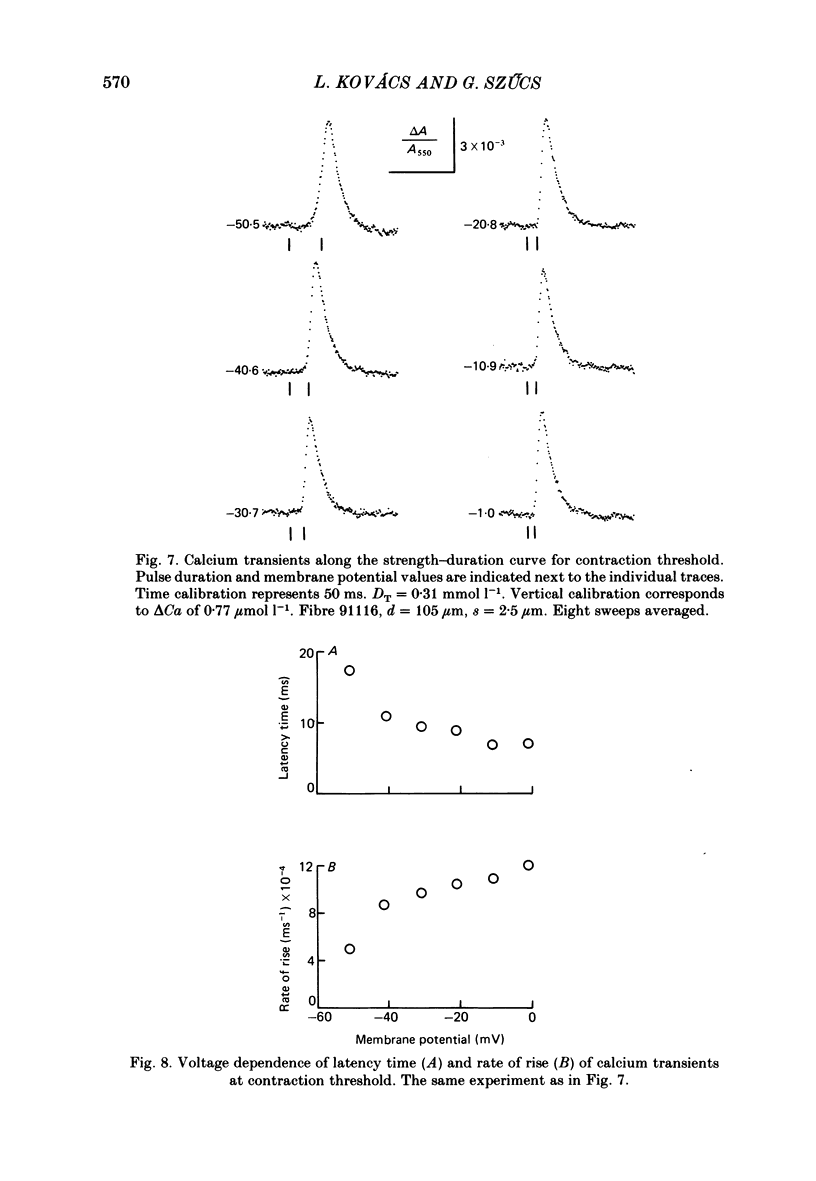
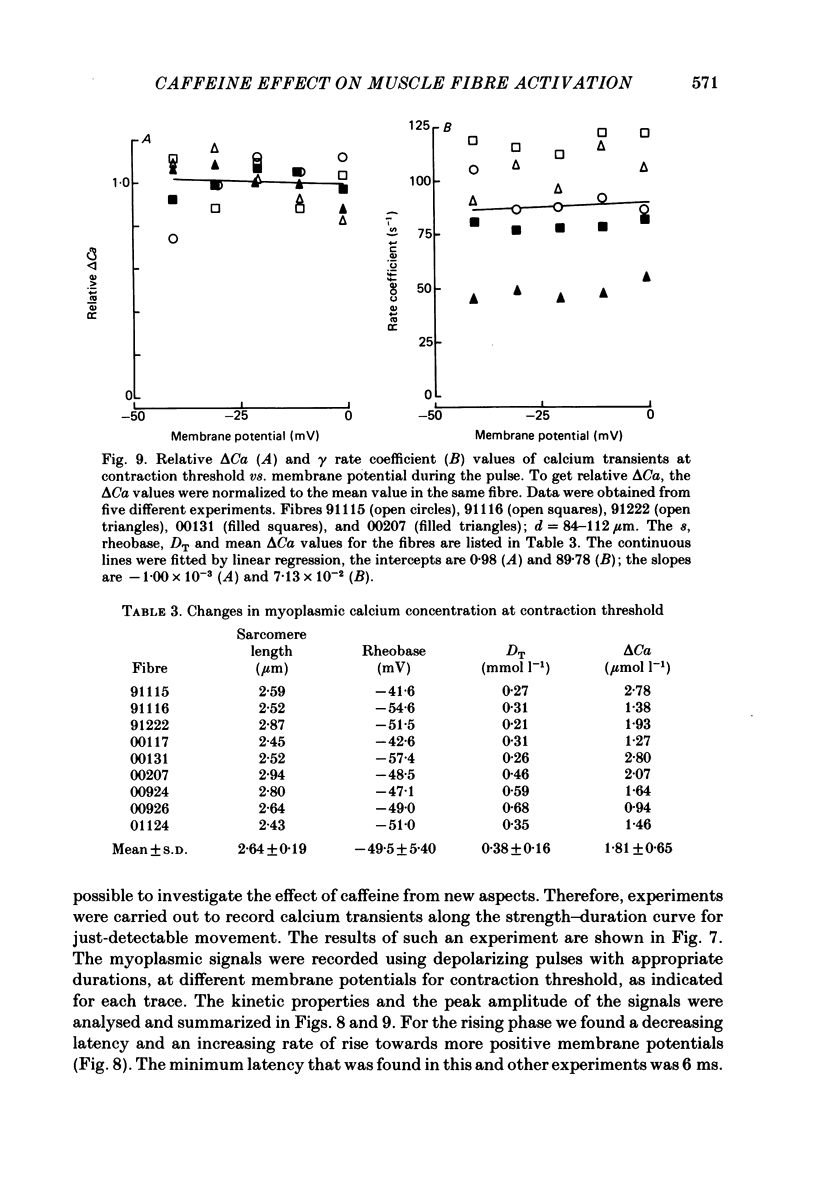
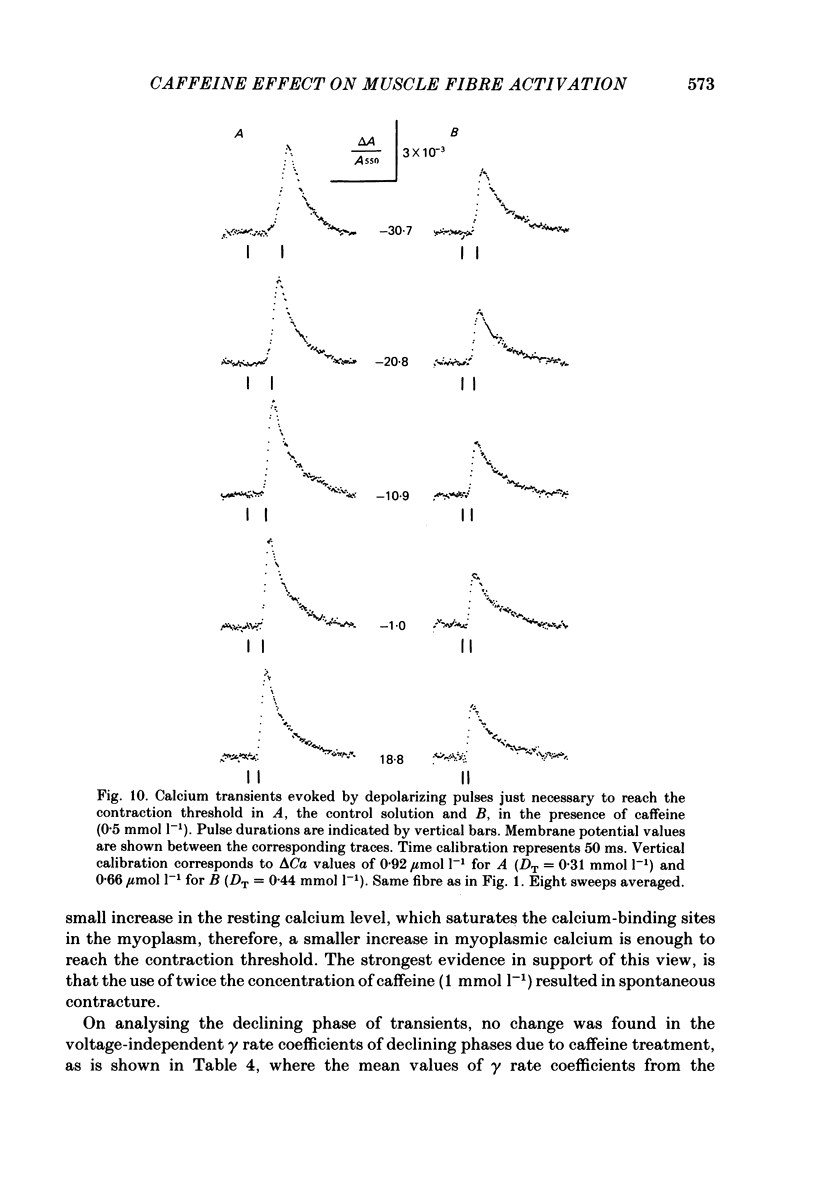
7. Calcium transients evoked by depolarizing pulses along the strength—duration curve for contraction threshold gave the same peak amplitudes (ranging from 0.9 to 2.8 μmol l-1 free myoplasmic calcium on different fibres), but membrane-potential-dependent latency times and rising phases. The rate coefficients for declining phase did not depend on the preceding pulse voltage.
8. On applying caffeine, the calcium transients related to the contraction threshold also had equal but smaller peak amplitudes, shorter latency times and the same magnitude of voltage-independent rate coefficients for the declining phase as in the control solution.
9. The twitch potentiating effect of caffeine can be explained by its facilitating calcium release from the sarcoplasmic reticulum, while the re-uptake rate is not modified. The apparent inhibition of re-uptake can be related to the enhanced release of calcium due to caffeine effect. Due to the sensitizing effect of caffeine on the sarcoplasmic reticulum membrane, smaller amounts of charge are needed to reach the contraction threshold than without caffeine.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELSSON J., THESLEFF S. Activation of the contractile mechanism in striated muscle. Acta Physiol Scand. 1958 Oct 28;44(1):55–66. doi: 10.1111/j.1748-1716.1958.tb01608.x. [DOI] [PubMed] [Google Scholar]
- Adrian R. H., Almers W. Charge movement in the membrane of striated muscle. J Physiol. 1976 Jan;254(2):339–360. doi: 10.1113/jphysiol.1976.sp011235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. The kinetics of mechanical activation in frog muscle. J Physiol. 1969 Sep;204(1):207–230. doi: 10.1113/jphysiol.1969.sp008909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C., Gottschalk G., Lüttgau H. C. The control of contraction activation by the membrane potential. Experientia. 1981 Jun;37(6):580–581. doi: 10.1007/BF01990061. [DOI] [PubMed] [Google Scholar]
- Chandler W. K., Rakowski R. F., Schneider M. F. A non-linear voltage dependent charge movement in frog skeletal muscle. J Physiol. 1976 Jan;254(2):245–283. doi: 10.1113/jphysiol.1976.sp011232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantin L. L. Contractile activation in frog skeletal muscle. J Gen Physiol. 1974 Jun;63(6):657–674. doi: 10.1085/jgp.63.6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Endo M., Tanaka M., Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 1970 Oct 3;228(5266):34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- Horowicz P., Schneider M. F. Membrane charge moved at contraction thresholds in skeletal muscle fibres. J Physiol. 1981 May;314:595–633. doi: 10.1113/jphysiol.1981.sp013726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowicz P., Schneider M. F. Membrane charge movement in contracting and non-contracting skeletal muscle fibres. J Physiol. 1981 May;314:565–593. doi: 10.1113/jphysiol.1981.sp013725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOREY S. Some factors influencing the contractility of a non-conducting fiber preparation. Biochim Biophys Acta. 1950 Jan;4(1-3):58–67. doi: 10.1016/0006-3002(50)90009-6. [DOI] [PubMed] [Google Scholar]
- Kovács L., Ríos E., Schneider M. F. Calcium transients and intramembrane charge movement in skeletal muscle fibres. Nature. 1979 May 31;279(5712):391–396. doi: 10.1038/279391a0. [DOI] [PubMed] [Google Scholar]
- Kovács L., Schneider M. F. Contractile activation by voltage clamp depolarization of cut skeletal muscle fibres. J Physiol. 1978 Apr;277:483–506. doi: 10.1113/jphysiol.1978.sp012286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács L., Schneider M. F. Increased optical transparency associated with excitation--contraction coupling in voltage-clamped cut skeletal muscle fibres. Nature. 1977 Feb 10;265(5594):556–560. doi: 10.1038/265556a0. [DOI] [PubMed] [Google Scholar]
- Kovács L., Schümperli R. A., Szücs G. Comparison of birefringence signals and calcium transients in voltage-clamped cut skeletal muscle fibres of the frog. J Physiol. 1983 Aug;341:579–593. doi: 10.1113/jphysiol.1983.sp014825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumbaraci N. M., Nastuk W. L. Action of caffeine in excitation-contraction coupling of frog skeletal muscle fibres. J Physiol. 1982 Apr;325:195–211. doi: 10.1113/jphysiol.1982.sp014145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttgau H. C., Oetliker H. The action of caffeine on the activation of the contractile mechanism in straited muscle fibres. J Physiol. 1968 Jan;194(1):51–74. doi: 10.1113/jphysiol.1968.sp008394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R. L. Sarcomere length-tension relations of frog skinned muscle fibres during calcium activation at short lengths. J Physiol. 1979 Jul;292:177–192. doi: 10.1113/jphysiol.1979.sp012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y. Some properties of fragmented frog sarcoplasmic reticulum with particular reference to its response to caffeine. J Biochem. 1970 May;67(5):667–683. doi: 10.1093/oxfordjournals.jbchem.a129295. [DOI] [PubMed] [Google Scholar]
- Ríos E., Schneider M. F. Stoichiometry of the reactions of calcium with the metallochromic indicator dyes antipyrylazo III and arsenazo III. Biophys J. 1981 Dec;36(3):607–621. doi: 10.1016/S0006-3495(81)84755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANDOW A., TAYLOR S. R., ISAASON A., SEGUIN J. J. ELECTROCHEMICAL COUPLING IN POTENTIATION OF MUSCULAR CONTRACTION. Science. 1964 Feb 7;143(3606):577–579. doi: 10.1126/science.143.3606.577. [DOI] [PubMed] [Google Scholar]
- Scarpa A., Brinley F. J., Jr, Dubyak G. Antipyrylazo III, a "middle range" Ca2+ metallochromic indicator. Biochemistry. 1978 Apr 18;17(8):1378–1386. doi: 10.1021/bi00601a004. [DOI] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973 Mar 23;242(5395):244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Schneider M. F. Membrane charge movement and depolarization-contraction coupling. Annu Rev Physiol. 1981;43:507–517. doi: 10.1146/annurev.ph.43.030181.002451. [DOI] [PubMed] [Google Scholar]
- Weber A., Herz R. The relationship between caffeine contracture of intact muscle and the effect of caffeine on reticulum. J Gen Physiol. 1968 Nov;52(5):750–759. doi: 10.1085/jgp.52.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A. The mechanism of the action of caffeine on sarcoplasmic reticulum. J Gen Physiol. 1968 Nov;52(5):760–772. doi: 10.1085/jgp.52.5.760. [DOI] [PMC free article] [PubMed] [Google Scholar]


