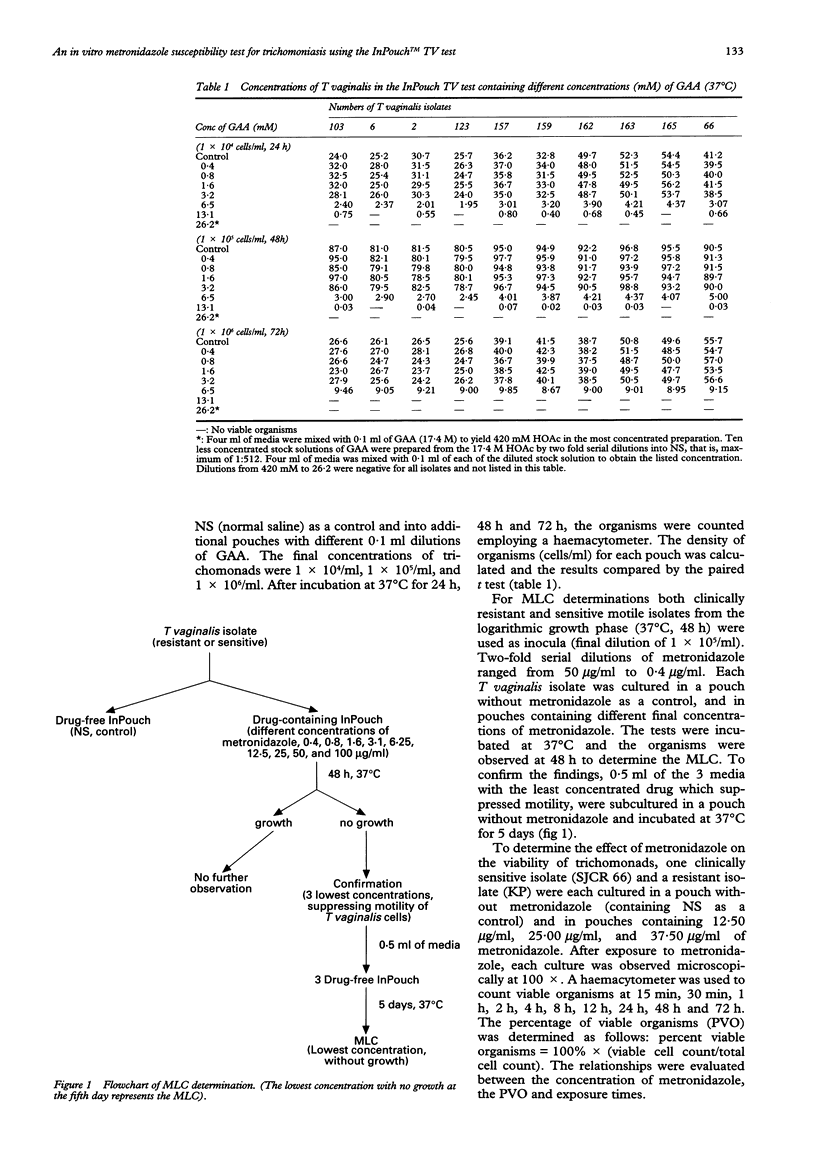
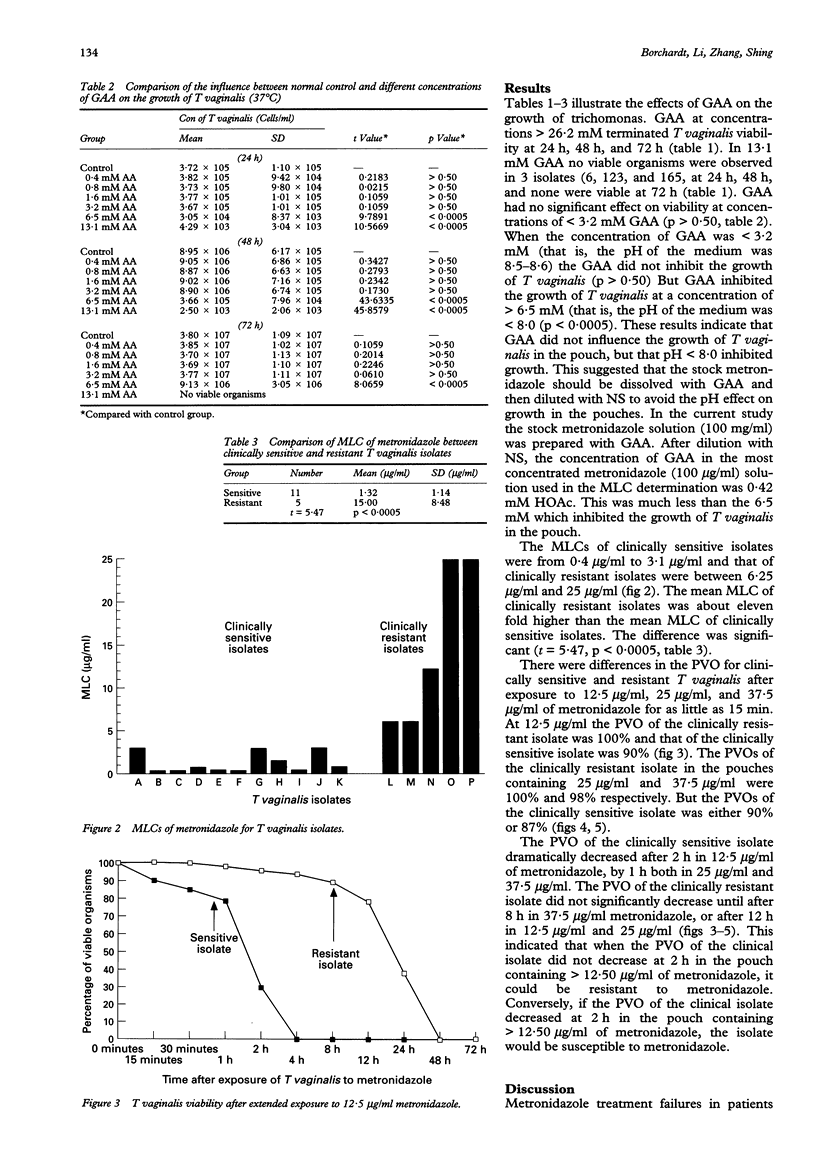
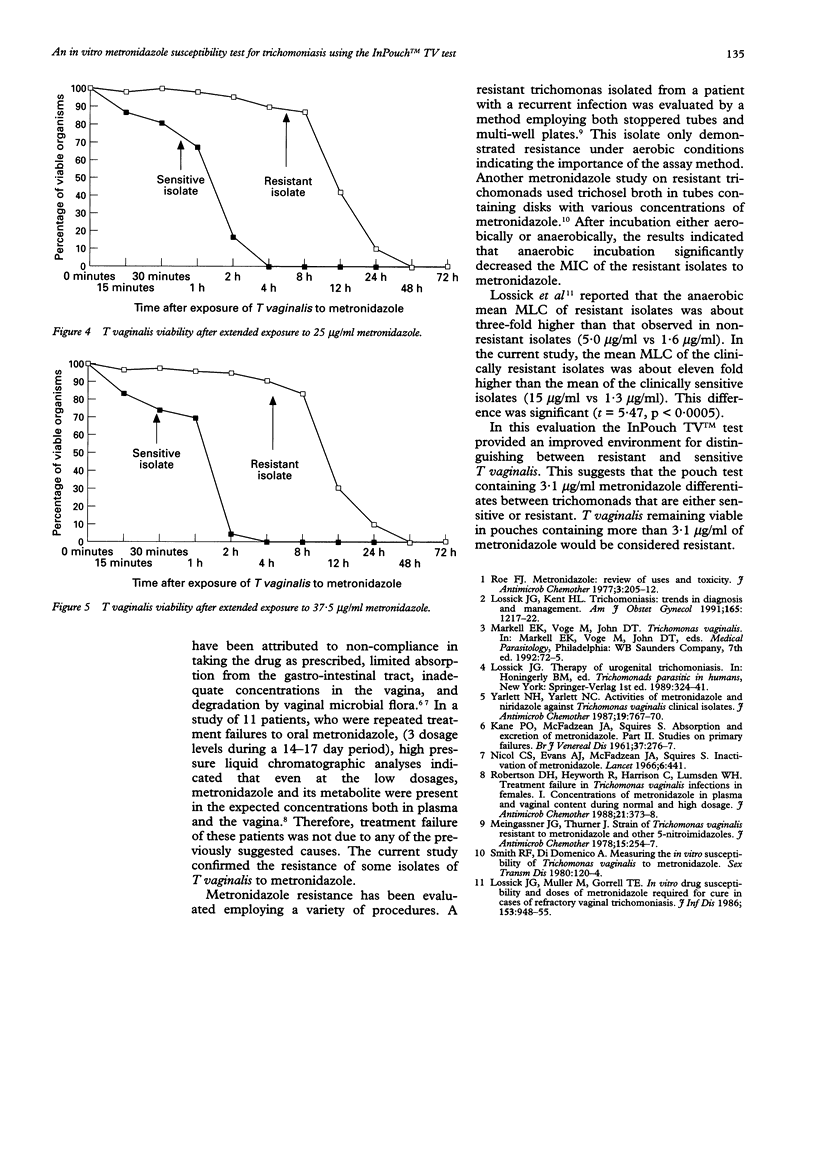
Abstract
OBJECTIVE: An efficient anaerobic culture system, the InPouch TV test, was used to determine the susceptibility of Trichomonas vaginalis to metronidazole. Glacial acetic acid was employed as a solvent for metronidazole. METHODS: T vaginalis isolates were cultured from 16 symptomatic female patients. The 11 who responded to oral metronidazole, 250 mg tid for 7 days, were considered as having sensitive trichomonads; the 5 who remained infected after treatment were considered to have resistant organisms. All isolates were cultured for minimum lethal concentrations (MLC). Metronidazole was added to a series of pouches; two-fold dilution of the most concentrated was 50 micrograms/ml and the least was 0.4 micrograms/ml. The inoculum of viable trichomonads was 1 x 105/ml in each pouch. Pouches were incubated at 37 degrees C for 48 h, examined microscopically for motile trichomonads, and then 0.5 ml was subcultured to drug free pouches. After 5 days incubation at 37 degrees C, each subculture and culture were examined microscopically for viable trichomonads. RESULTS: Eleven isolates of T vaginalis from patients responding to metronidazole treatment had MLC between 0.4 to 3.1 micrograms/ml. The MLC from the 5 treatment failure patients were between 12.5 to 50 micrograms/ml. CONCLUSIONS: For the 16 patients in this study, the MLC values determined with the InPouch TV test differentiated between infection caused by metronidazole sensitive and resistant trichomonads. The mean MLC of clinically resistant isolates was approximately eleven fold higher than the mean MLC of clinically sensitive isolates (15 micrograms/ml vs 1.32 micrograms/ml). There was a significant difference between clinically resistant and sensitive isolates (t = 5.47, p < 0.0005). This study suggests that the InPouch TV test could be used for distinguishing between metronidazole resistant and sensitive isolates.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- KANE P. O., McFADZEAN J. A., SQUIRES S. Absorption and excretion of metronidazole. II. Studies on primary failures. Br J Vener Dis. 1961 Dec;37:276–277. doi: 10.1136/sti.37.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lossick J. G., Kent H. L. Trichomoniasis: trends in diagnosis and management. Am J Obstet Gynecol. 1991 Oct;165(4 Pt 2):1217–1222. doi: 10.1016/s0002-9378(12)90730-9. [DOI] [PubMed] [Google Scholar]
- Lossick J. G., Müller M., Gorrell T. E. In vitro drug susceptibility and doses of metronidazole required for cure in cases of refractory vaginal trichomoniasis. J Infect Dis. 1986 May;153(5):948–955. doi: 10.1093/infdis/153.5.948. [DOI] [PubMed] [Google Scholar]
- Meingassner J. G., Thurner J. Strain of Trichomonas vaginalis resistant to metronidazole and other 5-nitroimidazoles. Antimicrob Agents Chemother. 1979 Feb;15(2):254–257. doi: 10.1128/aac.15.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. H., Heyworth R., Harrison C., Lumsden W. H. Treatment failure in Trichomonas vaginalis infections in females. I. Concentrations of metronidazole in plasma and vaginal content during normal and high dosage. J Antimicrob Chemother. 1988 Mar;21(3):373–378. doi: 10.1093/jac/21.3.373. [DOI] [PubMed] [Google Scholar]
- Roe F. J. Metronidazole: review of uses and toxicity. J Antimicrob Chemother. 1977 May;3(3):205–212. doi: 10.1093/jac/3.3.205. [DOI] [PubMed] [Google Scholar]
- Smith R. F., Di Domenico A. Measuring the in vitro susceptibility of Trichomonas vaginalis to metronidazole: a disk broth method. Sex Transm Dis. 1980 Jul-Sep;7(3):120–124. doi: 10.1097/00007435-198007000-00005. [DOI] [PubMed] [Google Scholar]
- Yarlett N., Hof H., Yarlett N. C. Activities of metronidazole and niridazole against Trichomonas vaginalis clinical isolates. J Antimicrob Chemother. 1987 Jun;19(6):767–770. doi: 10.1093/jac/19.6.767. [DOI] [PubMed] [Google Scholar]


