Abstract
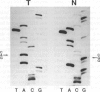
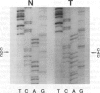
OBJECTIVES: The objectives of this study are to document the status of p53 expression and mutation in cervical cancer at protein, RNA and DNA levels and to relate this to the presence of HPV. MATERIALS AND METHODS: Biopsy specimens from one hundred and three squamous cell carcinoma of the cervix and histologically normal ectocervix were analysed. Fresh tissues were extracted for protein, RNA and DNA and flash frozen tissue cryostat sectioned for immunohistochemical staining. HPV DNA status was determined by PCR using L1 consensus primers and typed for HPV 16 and 18 with E6 specific primers. p53 expression was determined at the protein level by Western blotting on protein extracts and at RNA level by Northern blotting. RESULTS: There was no p53 overexpression or mutation detectable in the protein extracts. Three of 65 (4.6%) of the carcinomas were positive for p53 by immunostaining with the polyclonal antibody CM1. Overexpression at the RNA level was detected in 2 of 32 (6.3%) carcinomas. p53 mutation was screened for by PCR/SSCP (single strand conformation polymorphism) followed by sequencing to define the site of mutation. Two of the cervical cancers (2.0%) showed mutation in p53 in exons 7 or 8. The mutation rate in HPV positive tumours was 1.2% (1/81) and in HPV negative tumours was 5.2% (1/19). CONCLUSION: p53 overexpression or mutation does not seem to play a significant role in cervical carcinomas.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Busby-Earle R. M., Steel C. M., Williams A. R., Cohen B., Bird C. C. p53 mutations in cervical carcinogenesis--low frequency and lack of correlation with human papillomavirus status. Br J Cancer. 1994 Apr;69(4):732–737. doi: 10.1038/bjc.1994.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Collins R. J., Ngan H. Y., Hsu C., Cheung A., King P., Pun T. C., Ma H. K., Srivastava G. Human papillomavirus infection in the cervix of pregnant females in Hong Kong. Cytopathology. 1990;1(3):147–152. doi: 10.1111/j.1365-2303.1990.tb00340.x. [DOI] [PubMed] [Google Scholar]
- Cooper K., Herrington C. S., Evans M. F., Gatter K. C., McGee J. O. p53 antigen in cervical condylomata, intraepithelial neoplasia, and carcinoma: relationship to HPV infection and integration. J Pathol. 1993 Sep;171(1):27–34. doi: 10.1002/path.1711710107. [DOI] [PubMed] [Google Scholar]
- Crook T., Wrede D., Tidy J. A., Mason W. P., Evans D. J., Vousden K. H. Clonal p53 mutation in primary cervical cancer: association with human-papillomavirus-negative tumours. Lancet. 1992 May 2;339(8801):1070–1073. doi: 10.1016/0140-6736(92)90662-m. [DOI] [PubMed] [Google Scholar]
- Crook T., Wrede D., Vousden K. H. p53 point mutation in HPV negative human cervical carcinoma cell lines. Oncogene. 1991 May;6(5):873–875. [PubMed] [Google Scholar]
- Fujita M., Inoue M., Tanizawa O., Iwamoto S., Enomoto T. Alterations of the p53 gene in human primary cervical carcinoma with and without human papillomavirus infection. Cancer Res. 1992 Oct 1;52(19):5323–5328. [PubMed] [Google Scholar]
- Helland A., Holm R., Kristensen G., Kaern J., Karlsen F., Trope C., Nesland J. M., Børresen A. L. Genetic alterations of the TP53 gene, p53 protein expression and HPV infection in primary cervical carcinomas. J Pathol. 1993 Oct;171(2):105–114. doi: 10.1002/path.1711710207. [DOI] [PubMed] [Google Scholar]
- Hsu I. C., Metcalf R. A., Sun T., Welsh J. A., Wang N. J., Harris C. C. Mutational hotspot in the p53 gene in human hepatocellular carcinomas. Nature. 1991 Apr 4;350(6317):427–428. doi: 10.1038/350427a0. [DOI] [PubMed] [Google Scholar]
- Jacquemier J., Molès J. P., Penault-Llorca F., Adélaide J., Torrente M., Viens P., Birnbaum D., Theillet C. p53 immunohistochemical analysis in breast cancer with four monoclonal antibodies: comparison of staining and PCR-SSCP results. Br J Cancer. 1994 May;69(5):846–852. doi: 10.1038/bjc.1994.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastan M. B., Zhan Q., el-Deiry W. S., Carrier F., Jacks T., Walsh W. V., Plunkett B. S., Vogelstein B., Fornace A. J., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992 Nov 13;71(4):587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Kessis T. D., Slebos R. J., Han S. M., Shah K., Bosch X. F., Muñoz N., Hedrick L., Cho K. R. p53 gene mutations and MDM2 amplification are uncommon in primary carcinomas of the uterine cervix. Am J Pathol. 1993 Nov;143(5):1398–1405. [PMC free article] [PubMed] [Google Scholar]
- Lee J.-H., Kang Y.-S., Koh J.-W., Park S.-Y., Kim B.-G., Lee E.-D., Lee K.-H., Park K.-B., Seo Y.-L. p53 gene mutation is rare in human cervical carcinomas with positive HPV sequences. Int J Gynecol Cancer. 1994 Nov;4(6):371–378. doi: 10.1046/j.1525-1438.1994.04060371.x. [DOI] [PubMed] [Google Scholar]
- Midgley C. A., Fisher C. J., Bártek J., Vojtesek B., Lane D., Barnes D. M. Analysis of p53 expression in human tumours: an antibody raised against human p53 expressed in Escherichia coli. J Cell Sci. 1992 Jan;101(Pt 1):183–189. doi: 10.1242/jcs.101.1.183. [DOI] [PubMed] [Google Scholar]
- Miwa K., Miyamoto S., Kato H., Imamura T., Nishida M., Yoshikawa Y., Nagata Y., Wake N. The role of p53 inactivation in human cervical cell carcinoma development. Br J Cancer. 1995 Feb;71(2):219–226. doi: 10.1038/bjc.1995.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok S. C., Lo K. W., Tsao S. W. Direct cycle sequencing of mutated alleles detected by PCR single-strand conformation polymorphism analysis. Biotechniques. 1993 May;14(5):790–794. [PubMed] [Google Scholar]
- Ngan H. Y., Collins R. J., Wong K. Y., Cheung A., Lai C. F., Liu Y. T. Cervical human papilloma virus infection of women attending social hygiene clinics in Hong Kong. Int J Gynaecol Obstet. 1993 Apr;41(1):75–79. doi: 10.1016/0020-7292(93)90157-r. [DOI] [PubMed] [Google Scholar]
- Ngan H. Y., Stanley M., Liu S. S., Ma H. K. HPV and p53 in cervical cancer. Genitourin Med. 1994 Jun;70(3):167–170. doi: 10.1136/sti.70.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto A., Sameshima Y., Yokoyama S., Terashima Y., Sugimura T., Terada M., Yokota J. Frequent allelic losses and mutations of the p53 gene in human ovarian cancer. Cancer Res. 1991 Oct 1;51(19):5171–5176. [PubMed] [Google Scholar]
- Oliner J. D., Pietenpol J. A., Thiagalingam S., Gyuris J., Kinzler K. W., Vogelstein B. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature. 1993 Apr 29;362(6423):857–860. doi: 10.1038/362857a0. [DOI] [PubMed] [Google Scholar]
- Park D. J., Wilczynski S. P., Paquette R. L., Miller C. W., Koeffler H. P. p53 mutations in HPV-negative cervical carcinoma. Oncogene. 1994 Jan;9(1):205–210. [PubMed] [Google Scholar]
- Werness B. A., Levine A. J., Howley P. M. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990 Apr 6;248(4951):76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- Wright T. C., Jr, Richart R. M. Role of human papillomavirus in the pathogenesis of genital tract warts and cancer. Gynecol Oncol. 1990 May;37(2):151–164. doi: 10.1016/0090-8258(90)90327-h. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Human papillomaviruses in the pathogenesis of anogenital cancer. Virology. 1991 Sep;184(1):9–13. doi: 10.1016/0042-6822(91)90816-t. [DOI] [PubMed] [Google Scholar]