Abstract
Macroscopic hematuria (MH)-associated acute kidney injury (AKI) is a rare condition that causes acute tubular damage due to severe glomerular bleeding with MH. A 66-year-old Japanese woman with no significant past medical history was referred for severe kidney injury with oliguric MH. Her prior medical checkup results showed no occult blood in her urine. Seven days earlier, she had experienced transient severe acute right lumbar back pain. On admission, her serum urea nitrogen was 147 mg/dL, serum creatinine (sCr) 18.3 mg/dL, urinary red blood cells (RBCs) > 100/hpf, urinary protein 28.8 g/gCr, with no hydronephrosis in either kidney, but two stones were found in the right kidney and right ureteropelvic junction. At the start of her hemodialysis, the patient was treated with high-dose steroids because of suspected rapidly progressive glomerulonephritis. A renal biopsy of the left kidney showed acute tubular injury with massive RBC casts filling the tubular lumen. Glomerulitis was not detected, but electron microscopy revealed diffuse glomerular thin basement membrane (TBM). Despite immediate steroid discontinuation, the patient’s renal function and MH improved, and she was weaned from hemodialysis. The stones resolved 2 months after onset, but microscopic hematuria persisted for 7 months post-onset. The sCr level was fixed at 1.1 mg/dL 20 months post-onset. This is the first report of MH–AKI in a TBM without the risk of MH–AKI development, such as bleeding tendency or iron overload. In this TBM, a colic attack of the renal urinary tract induced glomerular bleeding, and intolerance to hematuria may have caused severe tubular damage.
Keywords: Macroscopic hematuria-associated acute kidney injury, MH–AKI, Thin basement membrane, TBM, Renal urinary tract stone
Introduction
Macroscopic hematuria (MH)-associated acute kidney injury (AKI) (MH–AKI) is a rare condition that has usually been encountered in the setting of glomerular disease with persistent hematuria, such as IgA nephropathy (IgAN) [1–4]. MH–AKI is an acute renal tubular injury resulting from severe glomerular bleeding, with obstruction by red blood cell (RBC) casts and direct cytotoxicity due to heme iron overload as the primary pathology. Heme iron loading causes mitochondrial damage and oxidation and apoptosis of tubular cells, leading to inflammation and fibrosis [1, 3]. The common histopathological features of MH–AKI are quite similar to those of the later-proposed anticoagulant-related nephropathy (ARN) occurring under warfarin/direct oral anticoagulant use [5, 6]. Many cases have been reported in which the use of anticoagulants in patients with IgAN may have been associated with the development of MH–AKI or ARN. Among the reported patients with thin basement membrane (TBM), which is often compared to IgAN in terms of primary glomerular disease causing persistent hematuria, only three cases of MH–AKI were present [7–9], but all of these were associated with anticoagulation [7], thrombocytopenia [8], or iron overload [9], which could be risk factors for MH–AKI.
Here, we present a case of TBM that was diagnosed incidentally at the onset of MH–AKI. The patient had no history of microscopic hematuria and no risk factors for MH–AKI, such as bleeding tendency or iron overload. In this case, a colic attack of the renal urinary tract likely triggered the onset of MH–AKI.
Case report
A 66-year-old Japanese woman with no significant previous medical history was referred to our hospital for severe kidney injury with oliguric MH. Seven days earlier, she had experienced transient severe acute right lumbar back pain, which was followed by a gradual worsening of malaise and loss of appetite. Immediately after the onset of lumbar back pain, she noticed that her urine was low in volume and red or very dark in color. There was no history of lumbar back pain. Details of her previous medical checkup results were not available, but she denied any abnormal renal function or noted occult blood in her urine. She had no medical history and was not taking any oral medications or health supplements. No family history of renal disease was noted. On admission, the patient’s physical findings were as follows: height 153 cm, weight 58 kg, blood pressure 160/80 mmHg, pulse 80/min, regular rhythm, SpO2 98% (room air), no abnormalities in her conscious state, and no abnormal findings in the chest, abdomen, extremities, or skin except for mild facial edema. At admission she showed severe renal impairment with serum creatinine (sCr) at 18.3 mg/dL, serum urea nitrogen at 147 mg/dL, urine protein at 28.8 g/gCr, and urine sediment of RBC > 100/hpf (high-power field). Urinary deformed RBCs were detected, but the percentage was low and not sufficient to confirm glomerular hematuria. The value of fractional urinary excretion of sodium was 63.3%. She also had high C-reactive protein at 6.2 mg/dL, and the hemoglobin concentration value was 11.2 g/dL. Coagulation–fibrinolysis tests showed no prolongation of either the prothrombin time of 12.5 s (%:92%; international normalized ratio: 1.04) or the activated partial thromboplastin time of 28.9 s. The d-dimer level was slightly elevated at 5.4 μg/mL.
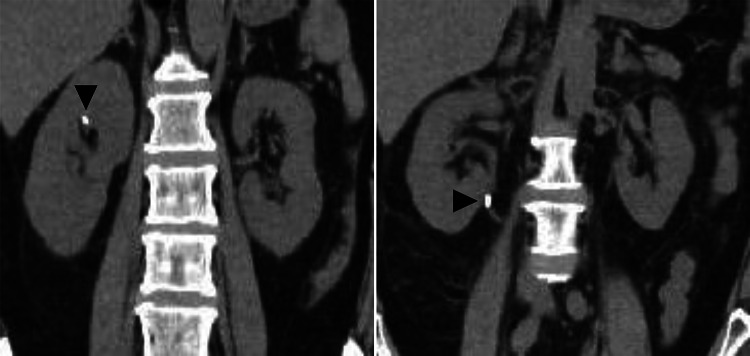
The results of tests for antineutrophil cytoplasmic antibody and antiglomerular basement membrane antibodies were negative; all other autoantibodies were negative, and complement was within normal range. No monoclonal protein was detected in either blood or urine. A summary of the laboratory findings at the time of admission is shown in Fig. 1. Computed tomography (CT) imaging on admission showed no hydronephrosis in either kidney, but two small stones were identified in the right kidney and right ureteropelvic junction (Fig. 2). The kidney sizes were 10.3 × 5.4 cm on the right and 9.9 × 5.6 cm on the left, with no significant enlargement. Intermittent hemodialysis was started on the day of the patient’s admission.
Fig. 1.
Summary of clinical laboratory findings at the time of admission. (reference ranges): ab antibody, ALP alkaline phosphatase (38–113), ALT alanine aminotransferase (5–45), ANA antinuclear antibody (< 40); APTT activated partial thromboplastin time (20–40), ASO anti-streptolysin O (0–160), AST aspartate aminotransferase (8–40), β2MG beta2-microgloburin (serum:0.9–2.5,urine 0–360), C3 complement component 3 (65.0–135.0), C4 complement component 4 (13.0–35.0), CH50 homolytic complement activity (25–48), CPK creatine kinase (40–165), Cr creatinine (0.46–0.8), CRP C-reactive protein (0–0.14), d-dimer (0–1.0), Fe serum iron (50–160), FENa fractional urinary excretion of sodium, FER ferritin (5.0–152.0), GBM glomerular basement membrane (anti-GBM ab < 2.0), IP: inorganic phosphorus (2.5–4.5); IgA immunoglobulin A(110–410), IgG. immunoglobulin G (870–1700); IgG4 (11–121), IgM immunoglobulin M (46–260), INR international normalized ratio, LDH lactate dehydrogenase (124–222), MPO-ANCA myeloperoxidase-anti-neutrophil cytoplasmic antibody (≤ 0.5), NAG N-actyl-β-D-glucosaminidase (0.0–11.5), PR3-ANCA proteinase-3-anti-neutrophil cytoplasmic antibody (≤ 0.5), PT prothrombin time (10.5–13.0), RBC red blood cell, RF rheumatoid factor (0–15), T-chol total cholesterol (130–219), Tf transferrin (200–340), TIBC total iron binding capacity (250–450), WBC white blood cell
Fig. 2.
A CT scan at the patient’s admission revealed two stones (arrowheads) in the right renal pelvis and the right ureteropelvic junction, respectively. There was no evidence of hydronephrosis in either kidney
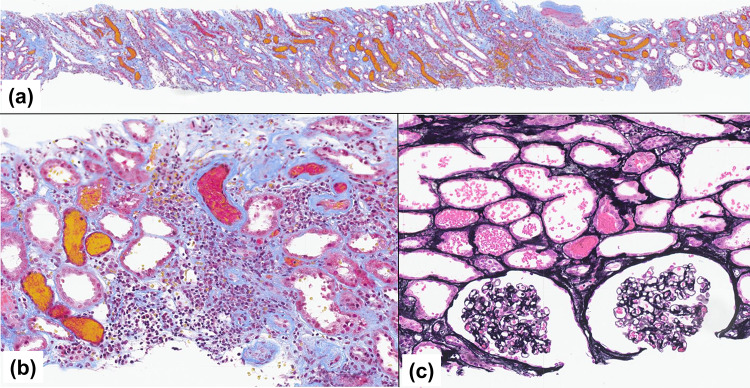
After a renal biopsy was performed for suspected rapidly progressive glomerulonephritis on day 4, the patient was started on intravenous methylprednisolone 1000 mg for 3 consecutive days, followed by oral prednisolone 40 mg/day. In the left renal biopsy, the presence of RBCs or RBC casts filling the distal tubular lumens was prominent throughout all specimens (Fig. 3a). Extensive tubular epithelial cell damage and focal inflammatory cell infiltration were observed (Fig. 3b). There were no significant chronic tubulointerstitial changes such as tubular atrophy or interstitial fibrosis throughout all specimens. No significant lesions were observed in any of the 34 glomeruli (Fig. 3c) except for 2 sclerotic glomeruli. Mild fibrous intimal thickening was seen in small arteries.
Fig. 3.
a Large numbers of red blood cells (RBCs) and RBC casts were widespread in the tubules of both the cortical labyrinth and medullary ray regions (light microscopy, Masson’s trichrome stain, × 40). b Tubular epithelial cell injury with focal inflammatory cell infiltration around flattened tubular epithelial cells was observed against a background of RBCs and RBC casts in the tubular lumens (light microscopy, Masson’s trichrome stain, × 100). c The glomeruli showed no proliferative lesions or crescent formation, and RBCs were prominent in the tubular lumens (light microscopy, periodic acid–methenamine silver stain, × 200)
Immunofluorescent staining on a frozen section showed no significant deposition. An examination by electron microscopy revealed that the lesions were characterized primarily by diffuse severe tubular epithelial cell injury. None of the following were observed: endocapillary or mesangial hypercellularity, expansion of the mesangial matrix, or electron-dense deposition in the glomeruli. Podocyte foot process effacement was observed locally, but was not noticeable. The glomeruli observed had a diffusely thin glomerular basement membrane, and there were scattered leaks of RBCs into Bowman’s cavity (Fig. 4). After admission, the patient’s urine output did not increase even with a furosemide bolus administration at the maximum dose of 120 mg/day, but on day 5 her urine output began to increase with MH. Thereafter, both her sCr level and MH gradually improved with a rapid increase in urine output, and the patient was weaned from hemodialysis after the fourth session on day 7. Prednisolone administration was stopped immediately on day 10.
Fig. 4.

The patient’s glomerular basement membrane was diffusely thin, with scattered RBC leakage into the Bowman’s cavity (transmission electron microscopy, × 1000). The inset at the lower right is × 8000; one scale in the black bar below represents 200 nm. The thickness of the glomerular basement membrane was 150–200 nm
The patient was discharged on day 20. At discharge, her sCr level was 4.1 mg/dL and the urine sediment of RBCs was 50–99/hpf, although her MH and proteinuria had resolved. A follow-up CT scan which the patient underwent incidentally 2 months after this onset showed the disappearance of the stones in the right urinary tract. There was no significant difference in the size of the right or left kidney compared to that at the onset of MH–AKI 2 months earlier. In contrast, microscopic hematuria persisted until 7 months post-onset. The patient’s sCr level was fixed at 1.1–1.2 mg/dL (estimated glomerular filtration rate [eGFR] 35–38 mL/min/1.73m2) at 20 months post-onset. After the disappearance of the patient’s microscopic hematuria, the urinary excretion of uric acid (UA) and calcium were measured in the patient’s spot urine at each of seven visits at 2- to 3-month intervals to determine the risk for stones in the urinary tract. The median and range of urinary UA excretion (mg/gCr) were 522.1 and 441.4–666.7, respectively (cf. ≥ 750 mg/day for hyperuricosuria [10]), and the corresponding values of urinary Ca excretion (mg/gCr/kg) were 2.49 and 1.90–3.75 (cf. ≥ 4 mg/kg/day for hypercalciuria in women [10]). These results did not support the administration of any therapeutic intervention for urinary tract stone prevention in this patient. The patient is now receiving nutritional guidance to minimize intake of acidic foods, purines, and oxalic acid-containing foods, along with salt restriction, and are also advised to drink water (maintaining urine output at 2 L/day) and take citric acid. The clinical course of this case from admission to discharge and after discharge are shown in Figs. 5 and 6, respectively.
Fig. 5.
The clinical course of the present case from admission to discharge is shown. Diuresis began rapidly from day 5 and renal function began to recover. Hemodialysis was discontinued after four sessions. Steroids were promptly discontinued after reviewing the results of the renal biopsy. HD hemodialysis, hpf: high power fields, mPSL methylprednisolone, PSL prednisolone, sCr serum creatinine, UP urinary protein, U-RBC urinary red blood cell sediment
Fig. 6.
The time course of a patient’s renal function and hematuria after discharge is shown. After the 9th month, microscopic hematuria completely disappeared, but chronic renal dysfunction persisted with sCr around 1.1–1.2 mg/dL. hpf high power fields, sCr serum creatinine, U-RBC urinary red blood cell sediment
Discussion
MH–AKI is a rare condition that has been reported in patients with glomerular disease accompanied by persistent hematuria (mostly in patients with IgAN). It was recently reported that hematuria can also be a factor in the development of chronic kidney disease (CKD) or progression to end-stage renal failure in IgAN [11–13]. Overloading of tubular cells with RBCs and heme iron can lead to mitochondrial damage, oxidation, and apoptosis of tubular cells, leading to chronic inflammation and fibrosis, which may contribute to the progression of CKD [1, 3]. In a case series and review of MH–AKI cases in IgAN, the risk factors for incomplete recovery of renal function were described as older age, longer duration of MH, severe tubular necrosis, no previous history of MH, and poor peak/basal eGFR [1, 4].
The renal function of our present patient with MH–AKI recovered considerably over time, but chronic renal dysfunction remained 20 months after the onset of the disease.
TBM is characterized by diffuse thinning of the glomerular basement membrane, and it is an inherited disease in autosomal dominant form with heterozygous mutations in either COL4A3 or COL4A4, which encode α3 or α4 chains that form type IV collagen [14]. TBM often presents with persistent microscopic hematuria, with MH in 5–30% of these cases [14, 15]. Although previously described as benign familial hematuria, some TBM cases are associated with proteinuria and hereditary focal segmental glomerulosclerosis and progress to CKD and end-stage renal disease [14–16]. TBM is estimated to be present in < 1% of the general population [14], but was reported to be present in 5–7 of 76 (approx. 5–9%) kidneys donated for cadaveric kidney transplantation [17]. In a prospective, observational study of 80 patients who underwent a renal biopsy for recurrent gross hematuria or persistent microscopic hematuria, 18 of 42 patients with normal glomeruli on light microscopy had TBM; in this TBM group, all but 1 patient had persistent microscopic hematuria, whereas among the remaining 24 patients without TBM, the MH resolved in all patients and microscopic hematuria resolved in half of them [18]. The prevalence of latent TBM cases without hematuria is unknown. The proportion of TBM patients with a family history of hematuria is 30–50%, and thus the absence of a family history of hematuria does not exclude TBM [15]. Our patient’s TBM was not confirmed by either a family history or a history of hematuria, and the microscopic hematuria disappeared completely, although it took more than 7 months of recovery time.
An interesting study reported by Praga et al. in 1998 revealed that the prevalence of hypercalciuria or hyperuricosuria was as high as 37% in TBM, i.e., higher than in IgAN or healthy controls, and that kidney stones (44%), MH (44%), and colic attacks (27%) were significantly more common among patients TBM with hypercalciuria or hyperuricosuria, respectively (10%, 7%, and 3% in those without hypercalciuria or hyperuricosuria, respectively) [10]. In that report, renal function remained normal in all cases of TBM during 8.8 ± 4.1 years of follow-up. During the recovery phase of MH–AKI in our present patient, the definitions of hypercalciuria and hyperuricosuria [10] were not met, and no therapeutic intervention (such as uric acid synthesis inhibitors for hyperuricosuria or thiazide diuretics for hypercalciuria [10]) was required. Unfortunately, we were unable to evaluate the patient’s urinary UA and Ca excretion at the onset of her MH–AKI, but we cannot rule out the possibility that there was a transient increase in the excretion of these substances during a period of poor food intake and recovery from dehydration. Note that urinary UA is easy to measure, and hyperuricosuria (urinary UA > 750 mg/day) is a better indicator of risk for urinary tract infection than hyperuricemia.
Our patient developed severe MH–AKI that was apparently triggered by a colic attack from renal urinary tract stones. Other conditions that should be ruled out in MH of acute back pain include renal infarction and renal vein thrombosis, but in this case, contrast-enhanced CT could not be performed due to severe renal dysfunction. We preferred to perform a renal biopsy to rule out rapidly progressive glomerulonephritis, but other imaging studies such as MR angiography, renal scintigraphy, and Doppler echography may have been the first choice to exclude these thrombotic setting. Concluding from the clinical course, the pathophysiology of this case could not have been proven without renal biopsy. The primary cause of MH in her case was most likely glomerular bleeding at the onset of AKI, which occurred somewhat later after the colic attack, rather than of lower urinary tract origin. In fact, the histopathologic findings in her case showed that MH–AKI occurred in the renal parenchyma, contralaterally upstream to the stone. In addition, microscopic hematuria persisted for ≥ 5 months after the confirmation of the disappearance of the stones.
Since we could not find any previous reports linking urinary tract stones/obstruction or colic attacks to the development of MH–AKI, we hypothesized that this patient’s unique condition with a fragile glomerular basement membrane was associated with the onset of MH–AKI. We extended our search to physiologic findings regarding urinary tract transit troubles and their impact on glomerular hemodynamics. The mechanism by which glomerular bleeding from kidneys in our patient was induced from a renal colic attack (presumably increased pressure in the right renal pelvis) was suggested by the experimental results obtained in studies of an animal model of unilateral ureteral ligation [19–22]. In the kidney on the side with unilateral ureteral ligation, there was a shift from intrarenal vasodilation early after ligation, followed by vasoconstriction. This is due to a marked change in tubuloglomerular feedback (TGF) activity, which decreases (an increase in glomerular inflow) in the early phase of occlusion and switches to a marked increase (a sharp decrease in glomerular inflow) in the late phase. The dramatic changes in TGF activity induced by these acute ureteral obstructions appear to be related to changes in the net interstitial pressure of the obstructed kidney. That is, the net interstitial pressure increases in the early phase and decreases in the late phase or after resolution of the obstruction. The contralateral kidney appears to respond in the opposite manner, with TGF activity declining considerably during occlusion, contributing to a compensatory increase in the GFR [22].
We speculated that the fragile glomerular basement membrane in our patient’s case may have triggered the glomerular bleeding due to the significant fluctuations in TGF activity, glomerular blood flow, and intraglomerular pressure that occurred in both kidneys after the renal colic attack.
In this case, despite the severity of AKI in the laboratory data, there was no renal enlargement at the onset and no change in kidney size after recovery from AKI. This suggests that the primary mechanism of AKI was not derived from diffuse interstitial edema or severe inflammatory cell infiltration, but from obstruction of the renal tubular lumen by RBCs or RBC casts, or from a hyperacute phase of direct renal tubular cell damage. The mechanisms that led to the rather severe MH–AKI in our patient may be as follows. We suspect that two unique conditions of TBM in this case are involved. First, at the onset of MH–AKI, the environment was conducive to renal stone formation, and the salts/crystals may have synergistically reduced intratubular fluidity and promoted the formation of RBC casts [10]. Second, it is likely that this TBM patient had no history of MH or persistent microscopic hematuria and no tubular cell tolerance to RBCs or iron loading. Considering the relatively high prevalences of IgAN or TBM in the general population, the incidence of MH–AKI is likely to be low [1, 2, 15] even though there are unreported cases.
In addition, the reported cases of MH–AKI in IgAN include a number of cases of ARN caused by the use of anticoagulants. Of the three reported cases of MH–AKI in TBM, two were due to warfarin use [7] and thrombocytopenia [8], and the remaining case was due to severe iron overload caused by frequent RBC transfusions for myelodysplastic syndrome [9]. In MH–AKI associated with IgAN, one of the risks for an incomplete recovery of renal function is the lack of a history of MH [4]. It is widely known that exposing organs to a severe or frequent ischemic environment (known as ischemic preconditioning) allows the organs to acquire ischemic tolerance. Regarding tolerance to oxidative stress, studies of AKI and ischemia–reperfusion kidney animal models have demonstrated that preconditioning with oxidants such as a combination of Fe sucrose + tin protoporphyrin induces antioxidant factors, acquires oxidative stress tolerance, and reduces cellular tissue damage [23]. It is possible that in the present patient, this severe MH–AKI event may have helped prevent recurrent AKI or minimize the exacerbations of AKI/CKD.
Declarations
Conflict of interest
All of the authors declare no competing interest.
Informed consent
Fully informed written consent for the publication of her case was obtained from the patient.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Moreno JA, Martín-Cleary C, Gutiérrez E, Toldos O, Blanco-Colio LM, Praga M, Ortiz A, Egido J. AKI associated with macroscopic glomerular hematuria: clinical and pathophysiologic consequences. Clin J Am Soc Nephrol. 2012;7:175–84. [DOI] [PubMed] [Google Scholar]
- 2.Kveder R, Lindic J, Ales A, Kovac D, Vizjak A, Ferluga D. Acute kidney injury in immunoglobulin A nephropathy: potential role of macroscopic hematuria and acute tubulointerstitial injury. Ther Apher Dial. 2009;13:273–7. [DOI] [PubMed] [Google Scholar]
- 3.Gutiérrez E, Egido J, Rubio-Navarro A, Buendía I, Blanco Colio LM, Toldos O, Manzarbeitia F, de Lorenzo A, Sanchez R, Ortiz A, Praga M, Moreno JA. Oxidative stress, macrophage infiltration and CD163 expression are determinants of long-term renal outcome in macrohematuria-induced acute kidney injury of IgA nephropathy. Nephron Clin Pract. 2012;121(1–2):c42-53. [DOI] [PubMed] [Google Scholar]
- 4.Gutiérrez E, González E, Hernández E, Morales E, Martínez MA, Usera G, Praga M. Factors that determine an incomplete recovery of renal function in macrohematuria-induced acute renal failure of IgA nephropathy. Clin J Am Soc Nephrol. 2007;2:51–7. [DOI] [PubMed] [Google Scholar]
- 5.Brodsky SV, Nadasdy T, Rovin BH, Satoskar AA, Nadasdy GM, Wu HM, Bhatt UY, Hebert LA. Warfarin-related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rate. Kidney Int. 2011;80:181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narasimha Krishna V, Warnock DG, Saxena N, Rizk DV. Oral anticoagulants and risk of nephropathy. Drug Saf. 2015;38:527–33. [DOI] [PubMed] [Google Scholar]
- 7.Abt AB, Carroll LE, Mohler JH. Thin basement membrane disease and acute renal failure secondary to gross hematuria and tubular necrosis. Am J Kidney Dis. 2000;35:533–6. [DOI] [PubMed] [Google Scholar]
- 8.Lim AK, Brown S, Simpson I, Dowling JP. Acute kidney injury due to glomerular haematuria and obstructive erythrocyte casts associated with thrombocytopenia and thin basement membrane disease: a case report. BMC Nephrol. 2015;16:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oda K, Katayama K, Tanoue A, Murata T, Hirota Y, Mizoguchi S, Hirabayashi Y, Ito T, Ishikawa E, Dohi K, Ito M. Acute kidney injury due to thin basement membrane disease mimicking deferasirox nephrotoxicity: a case report. BMC Nephrol. 2018;19:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Praga M, Martínez MA, Andrés A, Alegre R, Vara J, Morales E, Herrero JC, Novo O, Rodicio JL. Association of thin basement membrane nephropathy with hypercalciuria, hyperuricosuria and nephrolithiasis. Kidney Int. 1998;54:915–20. [DOI] [PubMed] [Google Scholar]
- 11.Orlandi PF, Fujii N, Roy J, Chen HY, Lee Hamm L, Sondheimer JH, He J, Fischer MJ, Rincon-Choles H, Krishnan G, Townsend R, Shafi T, Hsu CY, Kusek JW, Daugirdas JT, Feldman HI, CRIC Study Investigators. Hematuria as a risk factor for progression of chronic kidney disease and death: Findings from the chronic renal insufficiency cohort (CRIC) study. BMC Nephrol. 2018. 10.1186/s12882-018-0951-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He P, Wang H, Huang C, He L. Hematuria was a high risk for renal progression and ESRD in immunoglobulin A nephropathy: a systematic review and meta-analysis. Ren Fail. 2021;43:488–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Um YJ, Chang Y, Kim Y, Kwon MJ, Jung HS, Lee KB, Joo KJ, Cho IY, Wild SH, Byrne CD, Ryu S. Risk of CKD following detection of microscopic hematuria: a retrospective cohort study. Am J Kidney Dis. 2023;81:425-433.e1. [DOI] [PubMed] [Google Scholar]
- 14.Savige J, Rana K, Tonna S, Buzza M, Dagher H, Wang YY. Thin basement membrane nephropathy. Kidney Int. 2003;64:1169–78. [DOI] [PubMed] [Google Scholar]
- 15.Uzzo M, Moroni G, Ponticelli C. Thin basement membrane: An underrated cause of end-stage renal disease. Nephron. 2023;147:383–91. [DOI] [PubMed] [Google Scholar]
- 16.Tryggvason K, Patrakka J. Thin basement membrane nephropathy. J Am Soc Nephrol. 2006;17:813–22. [DOI] [PubMed] [Google Scholar]
- 17.Dische FE, Anderson VE, Keane SJ, Taube D, Bewick M, Parsons V. Incidence of thin membrane nephropathy: Morphometric investigation of a population sample. J Clin Pathol. 1990;43:457–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiebosch AT, Frederik PM, van Breda Vriesman PJ, Mooy JM, van Rie H, van de Wiel TW, Wolters J, Zeppenfeldt E. Thin-basement-membrane nephropathy in adults with persistent hematuria. N Engl J Med. 1989;320:14–8. [DOI] [PubMed] [Google Scholar]
- 19.Wahlberg J. The renal response to ureteral obstruction. Scand J Urol Nephrol Suppl. 1983;73:1–30. [PubMed] [Google Scholar]
- 20.Wahlberg J, Stenberg A, Wilson DR, Persson AE. Tubuloglomerular feedback and interstitial pressure in obstructive nephropathy. Kidney Int. 1984;26:294–301. [DOI] [PubMed] [Google Scholar]
- 21.Tanner GA. Tubuloglomerular feedback after nephron or ureteral obstruction. Am J Physiol. 1985;248(5 Pt 2):F688–97. [DOI] [PubMed] [Google Scholar]
- 22.Boberg U, Wahlberg J, Persson AE. Tubuloglomerular feedback response in the contralateral kidney after 24-hour unilateral ureteral obstruction. Ups J Med Sci. 1985;90:193–9. [DOI] [PubMed] [Google Scholar]
- 23.Zager RA. Oxidant-induced preconditioning: a pharmacologic approach for triggering renal ‘self defense.’ Physiol Rep. 2022;10(20): e15507. [DOI] [PMC free article] [PubMed] [Google Scholar]