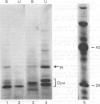
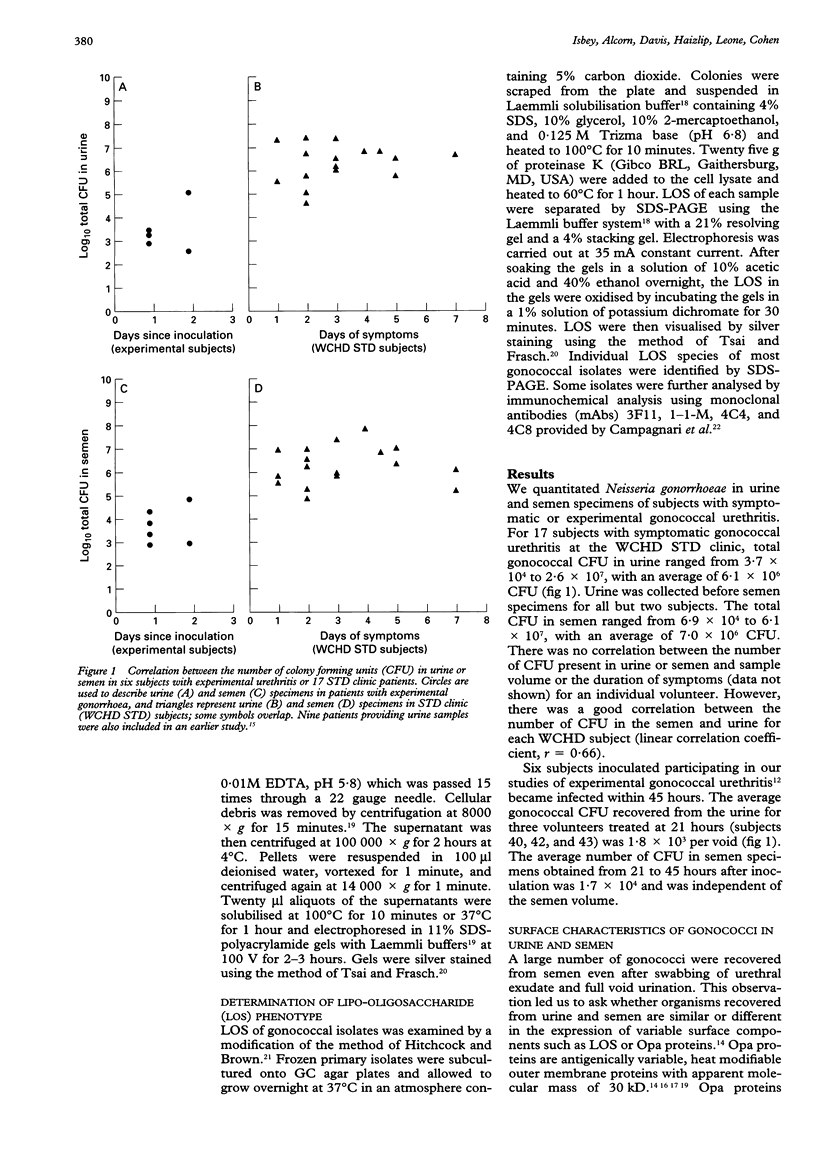
Abstract
OBJECTIVE: To determine the number of Neisseria gonorrhoeae organisms in urine and semen in men with gonococcal urethritis, and to compare selected phenotypic characteristics of organisms harvested from the urethra and semen. DESIGN: Samples from two groups of subjects were examined. Patients with symptomatic urethritis receiving treatment at an STD clinic, as well as six subjects with experimental urethritis. Semen and urine specimens were obtained after the urethral exudate was sampled. RESULTS: Using quantitative cultures, we found an average of 6 x 10(6) gonococci in urine or semen of 17 men with symptomatic urethritis seeking treatment at an STD clinic, and 2 x 10(4) gonococci in secretions of six male subjects with early experimental infection. Gonococcal outer membrane opacity (Opa) proteins and lipo-oligosaccharide (LOS) recovered from urine and semen of these subjects were very similar. CONCLUSIONS: Men with symptomatic gonorrhoea excrete a large number of gonococci in semen which is not affected by the duration of symptoms. The similar phenotype of organisms in urine and semen suggests the bacteria come from the same compartment. These data help to explain the efficiency of gonococcal transmission from men to their partners, and identify an appropriate target for a preventative vaccine or immunotherapy designed to reduce the inoculum in infected patients.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black W. J., Schwalbe R. S., Nachamkin I., Cannon J. G. Characterization of Neisseria gonorrhoeae protein II phase variation by use of monoclonal antibodies. Infect Immun. 1984 Aug;45(2):453–457. doi: 10.1128/iai.45.2.453-457.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagnari A. A., Spinola S. M., Lesse A. J., Kwaik Y. A., Mandrell R. E., Apicella M. A. Lipooligosaccharide epitopes shared among gram-negative non-enteric mucosal pathogens. Microb Pathog. 1990 May;8(5):353–362. doi: 10.1016/0882-4010(90)90094-7. [DOI] [PubMed] [Google Scholar]
- Cohen M. S., Cannon J. G., Jerse A. E., Charniga L. M., Isbey S. F., Whicker L. G. Human experimentation with Neisseria gonorrhoeae: rationale, methods, and implications for the biology of infection and vaccine development. J Infect Dis. 1994 Mar;169(3):532–537. doi: 10.1093/infdis/169.3.532. [DOI] [PubMed] [Google Scholar]
- Cohen M. S., Dallabetta G., Laga M., Holmes K. K. A new deal in HIV prevention: lessons from the global approach. Ann Intern Med. 1994 Feb 15;120(4):340–341. doi: 10.7326/0003-4819-120-4-199402150-00014. [DOI] [PubMed] [Google Scholar]
- Cohen M. S., Sparling P. F. Mucosal infection with Neisseria gonorrhoeae. Bacterial adaptation and mucosal defenses. J Clin Invest. 1992 Jun;89(6):1699–1705. doi: 10.1172/JCI115770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenbach D. A., Buchanan T. M., Pollock H. M., Forsyth P. S., Alexander E. R., Lin J. S., Wang S. P., Wentworth B. B., MacCormack W. M., Holmes K. K. Polymicrobial etiology of acute pelvic inflammatory disease. N Engl J Med. 1975 Jul 24;293(4):166–171. doi: 10.1056/NEJM197507242930403. [DOI] [PubMed] [Google Scholar]
- Grosskurth H., Mosha F., Todd J., Mwijarubi E., Klokke A., Senkoro K., Mayaud P., Changalucha J., Nicoll A., ka-Gina G. Impact of improved treatment of sexually transmitted diseases on HIV infection in rural Tanzania: randomised controlled trial. Lancet. 1995 Aug 26;346(8974):530–536. doi: 10.1016/s0140-6736(95)91380-7. [DOI] [PubMed] [Google Scholar]
- Haizlip J., Isbey S. F., Hamilton H. A., Jerse A. E., Leone P. A., Davis R. H., Cohen M. S. Time required for elimination of Neisseria gonorrhoeae from the urogenital tract in men with symptomatic urethritis: comparison of oral and intramuscular single-dose therapy. Sex Transm Dis. 1995 May-Jun;22(3):145–148. doi: 10.1097/00007435-199505000-00002. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K. K., Eschenbach D. A., Knapp J. S. Salpingitis: overview of etiology and epidemiology. Am J Obstet Gynecol. 1980 Dec 1;138(7 Pt 2):893–900. doi: 10.1016/0002-9378(80)91078-9. [DOI] [PubMed] [Google Scholar]
- Holmes K. K., Johnson D. W., Trostle H. J. An estimate of the risk of men acquiring gonorrhea by sexual contact with infected females. Am J Epidemiol. 1970 Feb;91(2):170–174. doi: 10.1093/oxfordjournals.aje.a121125. [DOI] [PubMed] [Google Scholar]
- Hooper R. R., Reynolds G. H., Jones O. G., Zaidi A., Wiesner P. J., Latimer K. P., Lester A., Campbell A. F., Harrison W. O., Karney W. W. Cohort study of venereal disease. I: the risk of gonorrhea transmission from infected women to men. Am J Epidemiol. 1978 Aug;108(2):136–144. doi: 10.1093/oxfordjournals.aje.a112597. [DOI] [PubMed] [Google Scholar]
- Jerse A. E., Cohen M. S., Drown P. M., Whicker L. G., Isbey S. F., Seifert H. S., Cannon J. G. Multiple gonococcal opacity proteins are expressed during experimental urethral infection in the male. J Exp Med. 1994 Mar 1;179(3):911–920. doi: 10.1084/jem.179.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laga M., Manoka A., Kivuvu M., Malele B., Tuliza M., Nzila N., Goeman J., Behets F., Batter V., Alary M. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS. 1993 Jan;7(1):95–102. doi: 10.1097/00002030-199301000-00015. [DOI] [PubMed] [Google Scholar]
- Lowe T. L., Kraus S. J. Quantitation of Neisseria gonorrhoeae from women with gonorrhea. J Infect Dis. 1976 Jun;133(6):621–626. doi: 10.1093/infdis/133.6.621. [DOI] [PubMed] [Google Scholar]
- Royce R. A., Seña A., Cates W., Jr, Cohen M. S. Sexual transmission of HIV. N Engl J Med. 1997 Apr 10;336(15):1072–1078. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- Schneider H., Cross A. S., Kuschner R. A., Taylor D. N., Sadoff J. C., Boslego J. W., Deal C. D. Experimental human gonococcal urethritis: 250 Neisseria gonorrhoeae MS11mkC are infective. J Infect Dis. 1995 Jul;172(1):180–185. doi: 10.1093/infdis/172.1.180. [DOI] [PubMed] [Google Scholar]
- Schwalbe R. S., Sparling P. F., Cannon J. G. Variation of Neisseria gonorrhoeae protein II among isolates from an outbreak caused by a single gonococcal strain. Infect Immun. 1985 Jul;49(1):250–252. doi: 10.1128/iai.49.1.250-252.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Robbins K., Barrera O., Corwin D., Boslego J., Ciak J., Blake M., Koomey J. M. Gonococcal pilin variants in experimental gonorrhea. J Exp Med. 1987 May 1;165(5):1344–1357. doi: 10.1084/jem.165.5.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Webster L. A., Berman S. M., Greenspan J. R. Surveillance for gonorrhea and primary and secondary syphilis among adolescents, United States--1981-1991. MMWR CDC Surveill Summ. 1993 Aug 13;42(3):1–11. [PubMed] [Google Scholar]