Abstract
Humans are infected by four recognized species of malaria parasites. The last of these to be recognized and described is Plasmodium ovale. Like the other malaria parasites of primates, this parasite is only transmitted via the bites of infected Anopheles mosquitoes. The prepatent period in the human ranges from 12 to 20 days. Some forms in the liver have delayed development, and relapse may occur after periods of up to 4 years after infection. The developmental cycle in the blood lasts approximately 49 h. An examination of records from induced infections indicated that there were an average of 10.3 fever episodes of ≥101°F and 4.5 fever episodes of ≥104°F. Mean maximum parasite levels were 6,944/μl for sporozoite-induced infections and 7,310/μl for trophozoite-induced infections. Exoerythrocytic stages have been demonstrated in the liver of humans, chimpanzees, and Saimiri monkeys following injection of sporozoites. Many different Anopheles species have been shown to be susceptible to infection with P. ovale, including A. gambiae, A. atroparvus, A. dirus, A. freeborni, A. albimanus, A. quadrimaculatus, A. stephensi, A. maculatus, A. subpictus, and A. farauti. An enzyme-linked immunosorbent assay has been developed to detect mosquitoes infected with P. ovale using a monoclonal antibody directed against the circumsporozoite protein. Plasmodium ovale is primarily distributed throughout sub-Saharan Africa. It has also been reported from numerous islands in the western Pacific. In more recent years, there have been reports of its distribution on the Asian mainland. Whether or not it will become a major public health problem there remains to be seen. The diagnosis of P. ovale is based primarily on the characteristics of the blood stages and its differentiation from P. vivax. The sometimes elliptical shape of the infected erythrocyte is often diagnostic when combined with other, subtler differences in morphology. The advent of molecular techniques, primarily PCR, has made diagnostic confirmation possible. The development of techniques for the long-term frozen preservation of malaria parasites has allowed the development diagnostic reference standards for P. ovale. Infections in chimpanzees are used to provide reference and diagnostic material for serologic and molecular studies because this parasite has not been shown to develop in other nonhuman primates, nor has it adapted to in vitro culture. There is no evidence to suggest that P. ovale is closely related phylogenetically to any other of the primate malaria parasites that have been examined.
INTRODUCTION
Plasmodium ovale was the last of the malaria parasites of humans to be described. The pronounced stippling of the infected erythrocyte and its tertian periodicity led early investigators to consider it a variant form of Plasmodium vivax. In 1900, Craig (32) described a malaria parasite in the blood of American soldiers returning from the Philippines that had peculiar morphological characteristics and a tertian fever pattern. It is possible that he was describing infections with P. ovale. Macfie and Ingram in 1917 (64) described a parasite in the blood of a child in the Gold Coast that may also have been P. ovale. Subsequently, Stephens (90) observed in the blood of an East African patient some erythrocytes that were oval and with fimbriated edges. In 1922, he published a full description of the forms in the blood and named the parasite P. ovale in recognition of the oval shape of some of the infected erythrocytes.
Some investigators were slow to recognize P. ovale as a distinct species (40). However, subsequent detailed studies confirmed the validity of the species (48, 49, 50, 51, 52, 86, 91). Following the establishment of the Donaldson strain of the parasite for use in malaria therapy for the treatment of patients with neurosyphilis, additional detailed studies on the morphology and periodicity of the parasite were made. The Donaldson strain of P. ovale was isolated from a returning serviceman who had acquired the infection in the western Pacific, probably the Philippines (53, 57, 59, 96). Plasmodium ovale is seldom seen except in sub-Saharan Africa and on some islands of the western Pacific. The movement of human populations poses the possibility of its presence and establishment in other tropical regions where susceptible vectors may be present. Reported here is a summary of the biology, morphology, diagnosis, and experimental vectors of the parasite.
LIFE HISTORY
Plasmodium ovale has developmental cycles in the human host and in the vector mosquito. Following introduction of sporozoites via the bite of infected mosquitoes, these forms rapidly invade the liver, where, within a single parenchymal cell, the parasite matures in approximately 9 days. Eventually, many hundreds of merozoites are produced. Upon release, these merozoites invade reticulocytes and initiate the erythrocytic cycle. The development of some of the parasites in the liver cells is delayed or suspended as hypnozoites, occasionally for many months. Following a developmental cycle in the erythrocyte that lasts, on average, 49 h, from 8 to 20 merozoites are released to reinvade other erythrocytes. As with other species of Plasmodium that infect humans, some of the merozoites that invade erythrocytes develop into two forms of gametocytes. The developmental time to maturity of gametocytes is the same as that of the asexual stage, approximately 49 h.
During feeding, mosquitoes take up both microgametocytes and macrogametocytes. Within the gut of the mosquito, exflagellation of the microgametocyte occurs, resulting in the formation of up to eight microgametes. Following fertilization of the macrogamete, a mobile ookinete is formed that penetrates the peritropic membrane surrounding the blood meal and travels to the outer wall of the midgut of the mosquito. There, under the basal membrane, the oocyst develops. After a period of several weeks, depending on the temperature, hundreds of sporozoites are produced within each oocyst. The oocyst ruptures, and sporozoites are released into the hemocoel of the mosquito. Circulation carries the sporozoites to the salivary glands, which the sporozoites invade and where they become concentrated in the acinal cells. During feeding, sporozoites are introduced into the salivary duct and are injected into the venules of the bitten human, initiating the cycle again.
Human Host
Prepatent period.
Humans are the only natural hosts for P. ovale. Much of what is known about this parasite was obtained during malaria therapy of naïve patients over 60 years ago. The prepatent period is the interval between sporozoite inoculation and the first detection of parasites in the peripheral blood. Sinton et al. (88) reported a mean prepatent period of about 15 days, whereas James et al. (52), working with six different strains of the parasite, reported a mean of 13.6 days. The Donaldson strain exhibited prepatent periods of 12 to 20 days, with a mean of 15.3 days; for the Liberian strain, prepatent periods of 13.5 to 15 days have been reported (37, 58). A retrospective examination of induced infections with P. ovale was made by Collins and Jeffery (23). These data were extracted from the records of patients that were given malaria therapy for the treatment of neurosyphilis between 1940 and 1963.
Prior to the introduction of penicillin for the treatment of syphilis, malaria was one of the most effective treatments for the disease (96). The range in prepatent periods following sporozoite injection was 14 to 20 days. A listing of prepatent periods (Table 1) for 30 patients infected via sporozoites with the Donaldson and Liberian strains indicated prepatent periods of 12 to 20 days, with a median of 14.5 days.
TABLE 1.
Prepatent period, maximum parasite count, days with a count of ≥1,000/μl, fevers of ≥101°F, and maximum fevers of ≥104°F for 30 patients infected with Plasmodium ovale via sporozoites
| Patient | Strain | Prepatent period (days) | Parasites/μl
|
Fever
|
|||
|---|---|---|---|---|---|---|---|
| Maximum no. | Days >1,000 | Days >101°F | Maximum fever (°F) | Days >104°F | |||
| G-354 | Donaldson | 14 | 380 | 0 | 6 | 105.4 | 2 |
| G-346 | Donaldson | 12 | 2,220 | 5 | 14 | 106.6 | 5 |
| G-402 | Donaldson | 16 | 2,250 | 7 | 9 | 105.4 | 5 |
| G-355 | Donaldson | 16 | 3,090 | 6 | 18 | 105.8 | 10 |
| G-357 | Donaldson | 14 | 3,360 | 5 | 10 | 106.0 | 6 |
| G-377 | Donaldson | 15 | 3,420 | 6 | 16 | 106.0 | 7 |
| G-355 | Donaldson | 17 | 3,780 | 5 | 1 | 102.0 | 0 |
| S-1135 | Donaldson | 15 | 4,576 | 5 | 8 | 105.4 | 4 |
| S-1080 | Donaldson | 16 | 4,832 | 7 | 9 | 104.6 | 3 |
| G-480 | Donaldson | 13 | 4,848 | 10 | 8 | 105.6 | 3 |
| G-331 | Donaldson | 15 | 5,424 | 12 | 14 | 104.8 | 3 |
| S-1074 | Donaldson | 17 | 6,424 | 10 | 9 | 105.0 | 3 |
| G-467 | Donaldson | 15 | 6,540 | 15 | 22 | 107.0 | 10 |
| S-1089 | Donaldson | 16 | 6,992 | 6 | 9 | 104.6 | 3 |
| G-490 | Liberian | 16 | 7,632 | 12 | 10 | 106.6 | 4 |
| G-460 | Donaldson | 14 | 7,848 | 10 | 12 | 105.4 | 7 |
| G-409 | Donaldson | 17 | 8,946 | 7 | 3 | 102.8 | 0 |
| G-329 | Donaldson | 14 | 8,560 | 12 | 9 | 104.8 | 2 |
| G-472 | Liberian | 14 | 9,810 | 11 | 15 | 105.8 | 7 |
| G-419 | Donaldson | 14 | 10,200 | 11 | 14 | 105.8 | 10 |
| G-344 | Donaldson | 14 | 10,890 | 19 | 14 | 106.0 | 7 |
| G-306 | Donaldson | 14 | 11,960 | 23 | NAa | NA | NA |
| G-488 | Donaldson | 14 | 12,150 | 14 | 9 | 105.0 | 4 |
| G-386 | Donaldson | 15 | 13,080 | 9 | 6 | 105.0 | 2 |
| G-481 | Donaldson | 20 | 14,832 | 9 | 6 | 105.0 | 2 |
| G-340 | Donaldson | 16 | 18,000 | 14 | 11 | 105.0 | 5 |
| G-449 | Donaldson | 14 | 18,180 | 13 | 9 | 104.2 | 2 |
| G-356 | Donaldson | 14 | 18,180 | 11 | 14 | 106.2 | 12 |
| G-487 | Donaldson | 14 | 18,540 | 19 | 10 | 106.0 | 7 |
| G-484 | Donaldson | 14 | 27,600 | 12 | 10 | 106.9 | 6 |
NA, no fever chart available.
Fever.
James et al. (52) reported that 15% of patients had 10 or more febrile paroxysms. With the Donaldson strain, only 10% of patients had over 10 paroxysms with peak temperatures exceeding 103°F (59). Mean maximum fever was 105.2°F. The median interval between peaks in the fever indicated that the periodicity (time for each developmental cycle) was approximately 49 h. A retrospective examination of records from induced infections (23) indicated that 47.1% of the fever episodes were ≥ 104°F. Patients reinfected with P. ovale rarely had fevers ≥ 104°F. An examination of fever episodes for 30 patients infected via sporozoites (Table 1) and 60 patients infected by the inoculation of parasitized erythrocytes (Table 2) indicated maximum fevers ranging from 102.0o to 107.0°F and 103.8o to 107.8°F, respectively. Mean maximum fevers were 103.3o and 105.4°F, respectively. For all patients, there were an average of 10.3 fever episodes of ≥101 and 4.5 fever episodes of ≥104°F.
TABLE 2.
Maximum parasite count, days with a count of ≥1,000/μl, fevers of ≥101°F, and maximum fevers of ≥104°F for 60 patients infected with Plasmodium ovale via trophozoites
| Patient | Strain | Parasites/μl
|
Fever
|
|||
|---|---|---|---|---|---|---|
| Maximum no. | Days ≥1,000 | Days ≥101°F | Maximum fever (°F) | Days ≥104°F | ||
| S-1310 | Donaldson | 1,280 | 10 | 8 | 104.8 | 2 |
| G-478 | Donaldson | 1,284 | 2 | 19 | 105.8 | 12 |
| S-1269 | Donaldson | 1,510 | 3 | 10 | 105.8 | 6 |
| S-1311 | Donaldson | 1,840 | 5 | 6 | 106.0 | 2 |
| G-479 | Donaldson | 2,496 | 13 | 9 | 104.2 | 2 |
| S-1278 | Donaldson | 3,120 | 7 | 6 | 105.4 | 3 |
| S-1271 | Donaldson | 3,300 | 16 | 9 | 105.6 | 5 |
| S-1273 | Donaldson | 3,920 | 8 | 8 | 104.8 | 3 |
| G-309 | Donaldson | 4,032 | 7 | 4 | 104.8 | 1 |
| G-21 | Donaldson | 4,040 | 8 | 11 | 106.4 | 7 |
| S-1328 | Donaldson | 4,200 | 8 | 7 | 106.6 | 5 |
| S-1327 | Donaldson | 4,510 | 5 | 6 | 105.2 | 1 |
| S-1110 | Donaldson | 5,700 | 13 | 12 | 105.4 | 2 |
| S-1092 | Donaldson | 6,133 | 25 | 11 | 104.0 | 2 |
| G-405 | Donaldson | 6,420 | 7 | 13 | 105.4 | 9 |
| S-1264 | Donaldson | 6,591 | 12 | 11 | 104.8 | 4 |
| G-447 | Donaldson | 6,780 | 9 | 5 | 104.6 | 3 |
| G-358 | Donaldson | 6,840 | 9 | 11 | 107.0 | 7 |
| G-291 | Donaldson | 6,960 | 7 | 4 | 105.8 | 2 |
| G-417 | Donaldson | 7,720 | 10 | 9 | 105.0 | 4 |
| G-470 | Liberian | 7,380 | 17 | 15 | 105.2 | 6 |
| G-391 | Donaldson | 7,632 | 7 | 3 | 105.0 | 1 |
| G-399 | Donaldson | 7,704 | 13 | 8 | 105.8 | 2 |
| G-468 | Liberian | 7,776 | 12 | 10 | 105.0 | 3 |
| G-361 | Donaldson | 8,160 | 11 | 12 | 106.0 | 8 |
| G-485 | Liberian | 8,160 | 14 | 17 | 105.6 | 6 |
| G-374 | Donaldson | 8,208 | 11 | 14 | 104.2 | 2 |
| S-1128 | Donaldson | 8,569 | 28 | 14 | 105.4 | 7 |
| G-434 | Donaldson | 9,000 | 10 | 4 | 105.4 | 3 |
| S-1267 | Donaldson | 9,521 | 18 | 17 | 106.4 | 11 |
| G-448 | Donaldson | 9,540 | 15 | 9 | 107.0 | 5 |
| G-442 | Donaldson | 9,680 | 12 | 10 | 105.2 | 2 |
| G-421 | Donaldson | 9,840 | 9 | 12 | 105.4 | 7 |
| G-371 | Donaldson | 10,080 | 11 | 17 | 106.0 | 12 |
| G-462 | Donaldson | 10,350 | 9 | 15 | 104.6 | 2 |
| G-296 | Donaldson | 10,620 | 12 | 13 | 104.4 | 2 |
| G-469 | Donaldson | 10,980 | 13 | 12 | 106.0 | 4 |
| S-1106 | Donaldson | 11,780 | 14 | 7 | 105.8 | 1 |
| G-458 | Donaldson | 12,120 | 10 | 2 | 104.0 | 1 |
| G-436 | Donaldson | 12,600 | 18 | 10 | 104.6 | 3 |
| G-341 | Donaldson | 12,600 | 12 | 8 | 106.6 | 6 |
| G-390 | Donaldson | 12,960 | 17 | 9 | 105.6 | 3 |
| G-482 | Donaldson | 13,080 | 11 | 6 | 105.0 | 2 |
| G-328 | Donaldson | 13,320 | 20 | 3 | 103.8 | 0 |
| G-413 | Donaldson | 14,400 | 9 | NAa | NA | NA |
| G-395 | Donaldson | 14,688 | 4 | 6 | 106.0 | 4 |
| G-471 | Liberian | 15,120 | 14 | 10 | 107.8 | 6 |
| G-435 | Donaldson | 15,120 | 12 | 12 | 106.0 | 5 |
| S-1305 | Donaldson | 15,153 | 21 | 15 | 105.2 | 2 |
| G-321 | Donaldson | 15,408 | 17 | NA | NA | NA |
| G-475 | Liberian | 18,000 | 13 | 18 | 105.6 | 7 |
| G-298 | Donaldson | 18,180 | 12 | 13 | 106.0 | 4 |
| G-473 | Donaldson | 18,576 | 15 | 9 | 104.6 | 2 |
| G-463 | Donaldson | 18,900 | 10 | 17 | 105.2 | 6 |
| S-1083 | Donaldson | 19,100 | 12 | 12 | 106.8 | 9 |
| G-320 | Donaldson | 19,440 | 23 | 9 | 105.0 | 3 |
| G-456 | Donaldson | 24,480 | 18 | 13 | 105.0 | 6 |
| G-451 | Donaldson | 24,960 | 19 | 15 | 105.2 | 6 |
| G-429 | Donaldson | 25,200 | 21 | 11 | 105.2 | 7 |
| G-336 | Donaldson | 25,440 | 18 | 3 | 103.8 | 0 |
NA, no fever chart available.
Parasitemia.
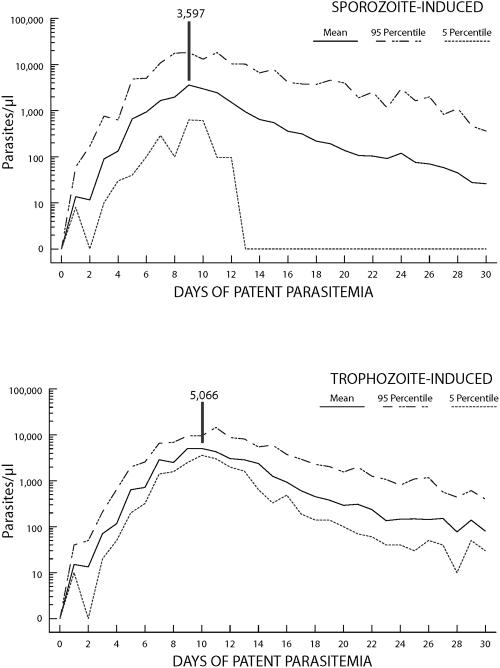
Maximum parasite counts are usually low compared to those of patients infected with P. falciparum or P. vivax (19), no doubt reflecting the restriction of P. ovale to development in younger erythrocytes. An examination of records from 90 patients (Tables 1 and 2) indicated maximum parasite levels ranging from 380 to 27,600/μl. The geometric mean maximum parasite level was 6,944/μl for sporozoite-induced infections and 7,310/μl for trophozoite-induced infections; median maximum parasite levels were 7,312 and 9,532/μl, respectively. Higher density parasitemia (≥1,000/μl) averaged 10.2 days and 12.4 days, respectively. The mean parasitemia curves for 30 sporozoite-induced and 60 trophozoite-induced infections (Fig. 1) indicated maximum parasite levels of 3,597/μl on day 9 for sporozoite-induced and 5,066/μl on day 10 for trophozoite-induced infections.
FIG. 1.
Mean, 5th-percentile, and 95th-percentile parasitemia curves for 30 sporozoite-induced and 60 trophozoite-induced infections with Plasmodium ovale.
Previous infection with P. ovale did not prevent reinfection but resulted in reduced levels of parasitemia and fever. Previous infection with P. vivax (Table 3), P. falciparum, and P. malariae (Table 4) did not prevent infection; there was some reduction in the frequency and intensity of fever and parasite counts. Glynn and Bradley (42) reviewed archival records on 80 induced infections with P. ovale in nonimmune patients as regards inoculum size and severity of the resulting malaria. Patients with shorter prepatent periods had higher and more peaks of fever and longer-lasting infections.
TABLE 3.
Route of inoculation, prepatent period, maximum parasite count, days of parasitemia of ≥1,000/μl, days of fever of ≥101 and ≥104°F, and maximum fever for 26 patients with Plasmodium ovale following infection with P. vivax
| Patient | Strain | Route | Prepatent period (days) | Parasites/μl
|
Fever
|
|||
|---|---|---|---|---|---|---|---|---|
| Maximum no. | Days ≥1,000 | Days ≥101°F | Maximum fever (°F) | Days ≥104°F | ||||
| G-332 | Donaldson | Sporo | 16 | 1,684 | 2 | 9 | 105.0 | 2 |
| S-1017 | Donaldson | Sporo | 18 | 2,704 | 4 | 6 | 106.0 | 1 |
| G-373 | Donaldson | Sporo | 16 | 4,320 | 3 | 5 | 106.4 | 4 |
| G-190 | Donaldson | Sporo | 15 | 5,376 | 7 | 7 | 106.0 | 2 |
| G-450 | Donaldson | Sporo | 14 | 7,272 | 9 | 10 | 105.2 | 4 |
| G-267 | Donaldson | Sporo | 16 | 7,992 | 19 | 7 | 104.4 | 1 |
| G-91 | Donaldson | Sporo | 16 | 12,000 | 14 | 8 | 105.0 | 7 |
| G-223 | Donaldson | Sporo | 16 | 21,780 | 9 | 6 | 102.6 | 0 |
| G-459 | Donaldson | Sporo | 14 | 23,040 | 4 | 4 | 104.6 | 1 |
| S-1146 | Donaldson | Blood | 464 | 0 | 8 | 104.2 | 1 | |
| S-629 | Donaldson | Blood | 760 | 0 | 3 | 106.0 | 1 | |
| S-1100 | Donaldson | Blood | 1,080 | 1 | 10 | 106.0 | 4 | |
| S-533 | Donaldson | Blood | 1,520 | 2 | 12 | 106.6 | 10 | |
| S-1095 | Donaldson | Blood | 1,880 | 3 | 9 | 106.0 | 8 | |
| S-1134 | Donaldson | Blood | 2,100 | 3 | 4 | 106.0 | 2 | |
| S-1148 | Donaldson | Blood | 2,512 | 4 | 0 | 0 | ||
| S-768 | Donaldson | Blood | 2,856 | 5 | 11 | 106.2 | 5 | |
| S-1131 | Donaldson | Blood | 3,648 | 3 | 0 | 0 | ||
| S-1060 | Donaldson | Blood | 3,744 | 6 | 4 | 105.4 | 2 | |
| G-432 | Donaldson | Blood | 4,280 | 7 | 4 | 106.2 | 2 | |
| S-670 | Donaldson | Blood | 4,420 | 6 | 5 | 105.8 | 3 | |
| S-1057 | Donaldson | Blood | 5,024 | 4 | 7 | 107.0 | 4 | |
| G-171 | Donaldson | Blood | 5,712 | 7 | 6 | 104.4 | 2 | |
| G-454 | Liberian | Blood | 6,180 | 4 | 4 | 104.6 | 1 | |
| G-348 | Donaldson | Blood | 19,440 | 6 | 6 | 105.4 | 3 | |
| G-325 | Donaldson | Blood | 35,520 | 11 | 10 | 105.0 | 3 | |
TABLE 4.
Route of inoculation, prepatent period, maximum parasite count, days of parasitemia of ≥1,000/μl, days of fever of ≥101 and ≥104°F, and maximum fever for 26 patients infected with Plasmodium ovale following infection with P. falciparum (17 subjects) or P. malariae (9 subjects)
| Patient | Strain | Previous malaria | Route | Prepatent period (days) | Parasites/μl
|
Fever
|
|||
|---|---|---|---|---|---|---|---|---|---|
| Maximum no. | Days >1,000 | Days >101°F | Maximum fever (°F) | Days ≥104°F | |||||
| S-1129 | Donaldson | Falciparum | Sporo | 17 | 9,400 | 25 | 5 | 104.0 | 1 |
| S-1323 | Donaldson | Falciparum | Blood | 930 | 0 | 4 | 104.4 | 2 | |
| G-289 | Donaldson | Falciparum | Blood | 1,590 | 5 | 2 | 105.0 | 2 | |
| S-1114 | Donaldson | Falciparum | Blood | 1,600 | 2 | 8 | 105.0 | 8 | |
| G-308 | Donaldson | Falciparum | Blood | 1,854 | 3 | 5 | 105.0 | 3 | |
| S-1144 | Donaldson | Falciparum | Blood | 1,900 | 5 | 6 | 106.0 | 4 | |
| S-830 | Donaldson | Falciparum | Blood | 1,970 | 4 | 6 | 106.0 | 2 | |
| S-1249 | Donaldson | Falciparum | Blood | 2,100 | 3 | 1 | 103.6 | 0 | |
| S-1274 | Donaldson | Falciparum | Blood | 2,596 | 10 | 16 | 106.0 | 7 | |
| S-1299 | Donaldson | Falciparum | Blood | 3,131 | 7 | 12 | 106.0 | 5 | |
| S-1320 | Donaldson | Falciparum | Blood | 3,725 | 11 | 10 | 105.0 | 4 | |
| G-268 | Donaldson | Falciparum | Blood | 4,320 | 4 | 2 | 105.6 | 1 | |
| S-1297 | Donaldson | Falciparum | Blood | 5,040 | 9 | 7 | 106.0 | 5 | |
| S-1161 | Donaldson | Falciparum | Blood | 7,480 | 11 | 8 | 105.8 | 4 | |
| S-1124 | Donaldson | Falciparum | Blood | 7,700 | 16 | 7 | 105.6 | 3 | |
| S-1326 | Donaldson | Falciparum | Blood | 12,811 | 7 | 6 | 105.4 | 3 | |
| S-1316 | Donaldson | Falciparum | Blood | 14,653 | 9 | NAa | NA | NA | |
| S-1276 | Donaldson | Malariae | Blood | 820 | 0 | 0 | |||
| S-1172 | Donaldson | Malariae | Blood | 850 | 0 | NA | NA | NA | |
| S-1259 | Donaldson | Malariae | Blood | 2,315 | 5 | 4 | 102.4 | 0 | |
| S-1277 | Donaldson | Malariae | Blood | 3,895 | 6 | 7 | 105.0 | 3 | |
| S-1112 | Donaldson | Malariae | Blood | 4,000 | 11 | NA | NA | NA | |
| S-1052 | Donaldson | Malariae | Blood | 4,466 | 8 | 8 | 105.0 | 3 | |
| S-1119 | Donaldson | Malariae | Blood | 6,150 | 5 | NA | NA | NA | |
| S-1290 | Donaldson | Malariae | Blood | 7,216 | 16 | 12 | 106.0 | 6 | |
| S-1008 | Donaldson | Malariae | Blood | 7,920 | 13 | 2 | 105.0 | 2 | |
NA, no fever chart available.
The Duffy blood group does not appear to be a controlling factor for infections with P. ovale as it does with P. vivax. There appears to be no difference in susceptibility to infection between Caucasians and African-Americans (58, 59).
Relapse.
Plasmodium ovale is a relapsing infection in that secondary infections can be generated from latent parasites in the liver. These are often asymptomatic infections that are detected only by the continued examination of peripheral blood films. Relapses occurred as early as 17 days after treatment of the primary attack to as late as 255 days (16). Delayed primary attacks occur when the primary attack has been eliminated, usually with antimalarial drugs. Such infections have been reported after 4 years (94) and 1.3 years (17). A relapse of P. ovale after 45 months of incubation has been reported (65). However, Shute and Maryon (87) reported that of 200 cases of P. ovale experimentally induced by mosquito bite, only one patient had a detectable relapse of the infection.
Exoerythrocytic stages.
The only demonstration of exoerythrocytic stages of P. ovale in the liver of a human was that of Garnham et al. (37, 38). A volunteer was fed upon by Anopheles maculipennis atroparvus mosquitoes infected with a Liberian strain of P. ovale. Infected mosquitoes were allowed to feed on the patient on three different days, 5, 6, and 9 days before a liver biopsy was performed. Exoerythrocytic bodies at different stages of development were demonstrated in liver tissue; parasites were observed in the blood of the volunteer 10 days after initial feeding. Only 17 schizonts were observed in the examination of over 4,000 serial sections. The size of the schizont was taken to indicate the age of the developing parasite. Eight schizonts, presumed to be 5-day forms, ranged in length from 28 μm to 60 μm. Nuclei were large, approximately 2 μm in diameter. Nine-day tissue stages measured from 70 to 80 μm by 50 μm. The nuclei of the exoerythrocytic stages had an uneven margin and the cytoplasm was granular. The cytoplasm was sometimes clumped around each nucleus so that it appeared to contain clefts. The merozoite of the schizont was large, spherical, and consisted of two portions, a larger portion of cytoplasm and a smaller portion being the nucleus.
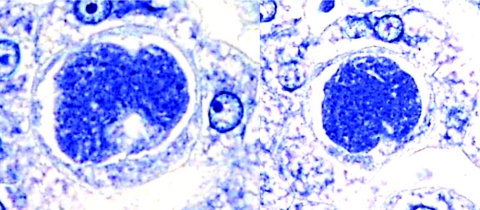
Subsequently, exoerythrocytic stages were demonstrated in the liver tissue of chimpanzees following inoculation of sporozoites from Anopheles gambiae mosquitoes (11, 12). Seven-day exoerythrocytic stages in the liver measured an average of 36.6 by 30.3 μm. Three characteristics that have not been shown in the tissue stages of P. vivax or P. falciparum were a definite limiting membrane or periplast; peripheral nuclear bars tangential rather than radial; and a minor but distinct hypertrophy of the host cell nucleus. In a subsequent study, biopsy on the 19th day revealed bursting and mature schizonts suggesting the existence of a delayed generation (11). Exoerythrocytic bodies were also demonstrated in hepatic tissue of Saimiri monkeys (Fig. 2), 7 days following injection of sporozoites dissected from Anopheles dirus mosquitoes (75).
FIG. 2.
Exoerythrocytic stages of Plasmodium ovale in sections of liver from Saimiri boliviensis monkeys taken 7 days after injection of sporozoites.
Sporozoites of P. ovale from Anopheles stephensi, Anopheles gambiae, and Anopheles dirus were introduced into primary cultures of human hepatocytes, rat hepatocytes, and cultures of a human hepatoma clone, Hep 5 A-1 (69). Maturation only occurred in primary cultured human hepatocytes. Parasites developed to 60 μm in length by day 8. The exoerythocytic stages of P. ovale were subsequently grown in primary cultures of hepatocytes from Saimiri sciureus boliviensis monkeys following introduction of sporozoites dissected from A. dirus mosquitoes (75). The morphology and size of the liver stages were similar to those previously described from humans and chimpanzees. By day 7, parasites contained over 100 nuclei; by day 9, parasites had a mean diameter of 68 μm and contained mature merozoites.
Mosquito Host
Anopheles gambiae and A. funestus are the likely natural vectors, based on enzyme-linked immunosorbent assay-based detection of infected mosquitoes (9); Bray demonstrated their infection while working with chimpanzees in the Gambia (11, 12). Experimentally, A. atroparvus was shown to be an effective mosquito host and capable of transmitting the infection to humans (38, 48, 49, 86, 88). Other proven experimental hosts are A. albimanus (53, 58, 59), A. quadrimaculatus (53, 59), A. freeborni (18), A. maculatus (18), and A. subpictus (36); A. stephensi and A. balabacensis balabacensis (= A. dirus) have also been shown to be experimentally infected (20). In studies with the Donaldson strain of P. ovale, A. quadrimaculatus was the most susceptible to infection, followed by A. albimanus from the Florida Keys and A. albimanus from Panama (53). In comparative studies with the West African strain, A. stephensi was the most susceptible, followed by A. freeborni, A. dirus, A. quadrimaculatus, A. maculatus, and A. albimanus (20). Anopheles farauti has also been experimentally infected with P. ovale (29).
The comparative rate of oocyst development of P. ovale in five species of anopheline mosquitoes (Anopheles balabacensis [= A. dirus], A. maculatus, A. freeborni, A. quadrimaculatus and A. stephensi) was determined (24). When held at 25°C, sporozoites were present in the salivary glands after 13 to 14 days. The mean diameter measurements of oocysts indicated that P. ovale was smaller than P. vivax and P. schwetzi (a parasite of chimpanzees and gorillas). A line of A. gambiae refractory for infection with P. cynomolgi was fed through a membrane on heparinized blood from a chimpanzee infected with P. ovale (21). There was 66% encapsulation of oocysts in the refractory line versus none in the susceptible line.
The development of monoclonal antibodies to detect mosquitoes infected with P. ovale has allowed a number of longitudinal entomological studies to determine the presence and biology of vectors of this parasite. Konate et al. (61) conducted a longitudinal survey in Senegal of Anopheles gambiae sensu lato and A. funestus in an area of Sudan-type savanna. Sporozoite typing indicated that 8.2% of the infected salivary glands were infected with P. ovale. This was calculated to represent eight infective bites per human the first year of observation and 25 infective bites the second year. In another report on the same study (95) it was estimated that the inoculation rate for P. ovale was 0.04 infective bites per person per night.
DISTRIBUTION
Many reports have been made on the presence of P. ovale throughout the world. However, a critical analysis of these reports by Lysenko and Bejaev (63) indicated that the natural distribution is in sub-Saharan Africa and the islands of the western Pacific. The parasite has been reported in New Guinea (5, 46, 68, 70) and the Philippines (3); it is apparently rare in the Philippines and only found on the island of Palawan (14). According to McMillan and Kelley (71), Heydon recorded P. ovale from the Duke of York Islands in 1923. Jackson (46) described two cases in Australian servicemen who had acquired their infections in New Guinea. It was also reported in Timor, Indonesia, for the first time in 1975 (43). The parasite was reported from Irian Jaya, two sites in West Flores and East Timor, Indonesia, but not present in Sumatra, Kalimantan, Java, and Sulawesi (7). Plasmodium ovale was reported in Moscow from a patient who had been infected in Melanesia (78). Reports from Southeast Asia suggest that P. ovale has been introduced to areas such as Vietnam (41), Thailand (60), and India (15). Whether or not it will be established on the mainland of Southeast Asia remains to be seen.
There are many reports of its distribution in sub-Saharan Africa. Lacan and Peel (62) in 1958 reported the presence of P. ovale in 25 children in French Equatorial Africa. In the neighborhood of Brazzaville, Republic of Congo, in 1978 to 1979, surveys among schoolchildren revealed a 24.5% infection rate with Plasmodium (1.9% of which was P. ovale) (74). In Gabon, in children 5 to 10 years of age, P. ovale was found in 2.4% of cases found infected with Plasmodium, while overall, the prevalence of infections with Plasmodium was 30% (84). Among 500 febrile children examined in the Pediatric Department of the General Hospital in Libreville, 29.2% had malaria, but P. ovale was “sparsely present” (85). In the Manyemen forest region of Cameroon, the prevalence of P. ovale was 10.5% (31). The parasite has been repeatedly reported from Nigeria (100). Fairley (34) reported P. ovale from a patient who returned to England after traveling to Nigeria, Gold Coast, Gambia, and Sierra Leone. In Sierra Leone, malaria infections have been reported to be due to P. ovale in from 0.5 to 1.0% of infected individuals (31, 100).
Because of the resistance of individuals with negative Duffy blood group to infection with P. vivax and the high prevalence of negativity in populations of West Africa, surveys reporting P. vivax may actually represent infections with P. ovale. Young and Johnson (101) found 2% of cases in Liberia to be P. vivax. It is probable that these were actually cases of P. ovale. Bjorkman et al. (10) conducted studies in an area of Liberia and found a prevalence rate in children for P. ovale of 9%. James et al. (50) reported that they had worked with strains of P. ovale from Nigeria and Belgian Congo. Afari (1) reported 2.7% of malarial infections due to P. ovale during a survey in a rural community in the central region of Ghana. Chin and Contacos (17) established a strain of P. ovale from a patient over a year after returning from service in Ghana.
Plasmodium ovale was reported to be extremely rare in southern Sudan and was absent in the north (80). Onori (81) carried out a survey in Uganda where, among 251 infections with P. ovale, the parasite was more often found in infants and adolescents. Infections with P. ovale have been reported in Zimbabwe (44, 93), Ethiopia (6), Zambia (99), Tanzania (66), and Natal (45). A relapse in an American after his return to the United States from Kenya has also been reported (82).
LABORATORY DIAGNOSIS
Diagnosis of P. ovale is usually made by the examination of peripheral blood films stained with Giemsa stain. Differentiation from the human malaria parasite, P. vivax, is most difficult. A detailed comparison was made by Wilcox et al. (97) of two strains of P. vivax (Chesson and St. Elizabeth) and the Donaldson strain of P. ovale. Cellular enlargement is a characteristic of both species. Erythrocytes containing ring stages of Chesson and St. Elizabeth showed enlargements of 10.3 and 11.5%, respectively, whereas in the Donaldson strain parasitized cells were the same as uninfected cells. With the binucleate schizont, the Chesson increased in size by 52.9%, the St. Elizabeth by 44.0%, and the Donaldson by 26.8%. Erythrocytes containing mature schizonts increased in size for Chesson 55.8%, for St. Elizabeth 50.7%, and for Donaldson 27.4%. The average number of merozoites for Chesson was 17.3, for St. Elizabeth 14.1, and for Donaldson 7.8. Thus, in comparison with P. vivax, P. ovale does not enlarge the infected erythrocyte as much and produces much fewer merozoites.
About 20% of erythrocytes infected with P. vivax were elliptical, with 2% definitely elongated. In contrast, 35% of P. ovale-infected erythrocytes were elliptical and 16% had a definitely long, narrow, oval or otherwise elongated form. When ring-infected erythrocytes were examined for the presence of Schüffner's stippling, it was much more numerous in P. ovale than in either strain of P. vivax.
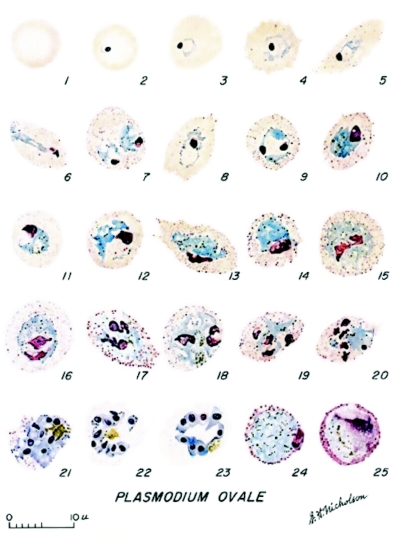
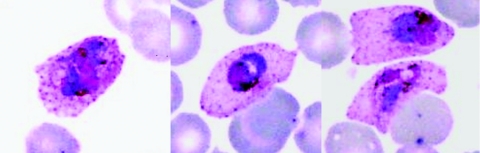
As described by Coatney et al. (20) (Fig. 3), the young ring forms of P. ovale have a prominent circular nucleus with a wisp of cytoplasm. As the parasite grows, the erythrocyte becomes enlarged; older trophozoites occupy about half the erythrocyte. The host cell may appear oval with fimbriated edges. This is especially marked in erythrocytes of splenectomized chimpanzees infected with P. ovale (Fig. 4). The pigment is initially in the form of dust-like grains that later come together to form greenish-brown beads; eventually they mass together in yellowish-brown patches. The most distinctive characteristic is the stippling. This appears early and becomes intense as the parasite develops. The stippling is more intense than that of P. vivax.
FIG. 3.
Development of the erythrocytic stages of Plasmodium ovale. Sexual forms: macrogametocyte (panel 24) and microgametocyte (panel 25). Reproduced from Coatney et al. (20).
FIG. 4.
Erythrocytes from chimpanzees infected with Plasmodium ovale, showing marked distortion due to infection.
Gametocytes grow to fill the enlarged host cell. The macrogametocyte stains blue with Giemsa. The pigment is in granules arranged like a string of beads. Stippling is prominent and is arranged in a ring around the parasite. The microgametocyte takes a lighter stain and the nucleus occupies half the parasite. The color with Giemsa appears light pink toward the edge. The parasite is completely enclosed in a prominent circle of eosinophilic stippling.
Molecular techniques for the differentiation of P. ovale from other species of human malaria parasites have been developed using PCR. Snounou et al. (89) were the first to apply the two-step nested PCR technique to the separation of all four human infecting species using the P. ovale primers rOVA 1 (ATC TCT TTT GCT ATC TTT TTT TAG TAT TGG AGA) and rOVA 2 (GGA AAA GGA CAC ATT AAT TGT ATC CTA GTG). In the first step (PCR1), extracted DNA is amplified using genus-specific primers; in the second step (PCR2), the PCR1 amplification product is further amplified using species-specific primers. Then, each PRC2-amplified DNA product is separated by 2% agarose gel electrophoresis, stained with ethidium bromide, and visualized by UV illumination. The migration position on the gel identifies the species of Plasmodium present. The P. ovale primers described on the Centers for Disease Control and Prevention DPDx Website are those presented by Snounou et al.
Oliveira et al. (79) reported a procedure where the target region of the 18S rRNA gene is amplified by PCR using an 18S rRNA, genus-specific, biotinylated (5′) and an unlabeled primer (3′) pair. The detection probes were digoxigenin-labeled DNA oligonucleotides derived from species-specific rRNA sequences. The amplified fragment complex is allowed to hybridize with the species-specific, digoxigenin-labeled oligonucleotide probes. The oligo/DNA complex is allowed to bind onto streptavidin-peroxidase substrate. The two different pairs of primers were used to detect P. ovale were DIG 11 (5′ AAT AAG AAC ACA TTT TGC A) and DIG 12 (3′ CAG ATA CGT TGT ATT GTC) and DIG 13 (5′ AAT AGC AAA AGA GAT TTT) and DIG 14 (3′ CAT CTT ATA GCA AAA GTA).
Preservation
The preservation of viable malaria parasites was a major breakthrough in the study of these organisms. In 1955, Jeffery and Rendtorff (56) reported the frozen preservation of both blood and sporozoite stages of P. ovale. Blood stages were stored for up to 234 days at a temperature of 70°C. Sporozoites were also readily preserved following dissection into human plasma and subsequent storage at −70°C. Suspensions of sporozoites were quickly thawed and then injected intravenously into recipient volunteers. Prepatent periods were similar to those of patients receiving infection via mosquito bite. In a subsequent report, (54) frozen preservation of the Donaldson strain of P. ovale was reported for periods of 399 and 997 days. Most infections with P. ovale in chimpanzees have been induced by the injection of infected erythrocytes that has been stored frozen over liquid nitrogen, often for many years (25, 76). Parasites are usually stored in Glycerolyte and are expected to be viable for decades when held at extremely low temperatures. Thick and thin blood films for immunofluorescence studies and teaching can be stored unfixed and frozen for extended periods. Frozen blood is unsuitable for preparation of blood films.
SEROLOGIC STUDIES
Serologic tests with malaria parasites are basically epidemiologic tools and not specific enough to be used for diagnostic purposes. The fluorescent antibody technique has been used to measure the presence of antibodies to P. ovale. However, extensive studies have been limited due to limited availability of the antigen. The pattern of fluorescence for P. ovale was similar to that of P. malariae (26). In a study with patients that had induced infections with P. falciparum, P. malariae, and P. ovale, antibodies to P. ovale persisted for a period of 6 years after treatment (28). Meuwissen found a high degree of cross-reactivity of sera from patients with P. ovale infections and the monkey malaria parasite P. fieldi (72). In a later study, it was shown that such antisera also cross-reacted to P. cynomolgi bastianellii, but at a lower level than the homologous antigen or P. fieldi (73).
In an initial field study with 498 sera collected from Nigerians, 22.3% had positive responses to P. ovale (27). A serologic survey was conducted in Ethiopia using a strain of P. ovale from Ghana as the antigen (30). Maximum responses were highest to P. falciparum (45%), followed by P. ovale (41%), P. malariae (36%), and then, P. vivax (9%); this included individuals in whom maximum responses were equal for some species of Plasmodium. An indirect fluorescent antibody study was subsequently conducted to evaluate patterns of antibody response in remote populations of the New Hebrides, Solomon, and Western Caroline islands and New Guinea (13). Maximum titers to P. ovale occurred most frequently in the eastern and southern Solomon Islands, although P. ovale had never been reported in either the New Hebrides or Solomon islands. In West New Guinea (Irian Jaya) and Papua New Guinea, serologic responses were highest to P. falciparum, followed by P. ovale, P. malariae, and P. vivax, a pattern similar to that observed in the survey of samples from Ethiopia.
A serologic survey of urban and rural populations of Ghana indicated the proportion of positive titers against P. falciparum rose rapidly with age, with more than 50% of children 1 to 2 years old being positive (35). In comparison, titers against P. ovale rose more slowly, reaching 50% in the 7- to 8-year-old group. A survey was also made of a remote population living in the Star Mountains in the Western Province of Papua New Guinea (22). Highest responses were to P. falciparum, followed by P. malariae, P. vivax, and P. ovale; here, only 5 of 614 samples examined had the highest titers to P. ovale.
MOLECULAR STUDIES
Erythrocytes infected with the Nigerian strain of P. ovale were concentrated from chimpanzee blood using Percoll gradients (4). The greatest concentration and separation from white blood cells was obtained when the buffy coat was removed before centrifugation of the Percoll gradients. Band 1 of the gradient contained 99% infected erythrocytes with less than 1% white blood cells. Monoclonal antibodies were subsequently produced against the asexual stages of P. ovale. Four distinct patterns were observed using the indirect fluorescent antibody assay, a spotted fluorescence pattern within the infected erythrocyte, fluorescence of the parasite itself, a diffuse pattern of fluorescence over the entire infected erythrocyte, and a diffuse pattern over the entire cell plus the parasite itself. Three monoclonal antibodies produced against P. ovale reacted only with P. ovale, whereas others reacted either with all four human malaria parasites or with P. falciparum, P. vivax, and P. ovale.
Antisporozoite monoclonal antibody 110-54.3 was used to characterize the circumsporozoite protein of P. ovale (83). In Western blot analysis with P. ovale sporozoites, three distinct species-specific polypeptides were recognized. A single-antibody, two-site enzyme-linked immunosorbent assay demonstrated the presence of a repeating epitope. However, the sequence of the repeating epitope has yet to be determined. This antibody was used to demonstrate the circumsporozoite protein in midgut oocysts by immunoelectron microscopy (77). The monoclonal antibody bound primarily to the plasma membrane of sporoblasts that contained budding sporozoites. Gold particles were not found in immature, nonvacuolated oocysts. An enzyme-linked immunosorbent assay has been developed for the identification of mosquitoes infected with P. ovale. This test has been used successfully to identify mosquitoes infected with P. ovale in Kenyan field studies (9).
Analyses have indicated that there are two types of P. ovale based on nucleotide deletions and substitutions in the 18S rRNA gene, and these parasites have been found to coexist in Vietnam, Thailand, and Myanmar (60, 102, 98). Two types of P. ovale were shown to have distinct sequences for ookinete surface proteins that suggested that there may be two subspecies of the parasites (94).
INFECTIONS IN CHIMPANZEES AND MONKEYS
The first demonstration of infection of chimpanzees with P. ovale indicated that splenectomy was necessary for the animals to support significant parasitemia (11, 12).
Infections with the Nigerian I/CDC strain of P. ovale have been induced in splenectomized chimpanzees (25, 76). Animals previously infected with P. vivax or P. malariae were readily susceptible to infection via intravenous inoculation of infected erythrocytes that had been stored frozen. Maximum parasite counts ranged from 1,240 to 127,224/μl. Anopheles stephensi, A. gambiae, A. freeborni, and A. dirus mosquitoes were infected by feeding through parafilm membranes on heparinized blood from chimpanzees. Mean oocyst counts ranged from 1 to 85.1 per mosquito midgut. One infection was induced in a chimpanzee via the bites of infected A. gambiae; the prepatent period was 16 days.
Attempts to infect intact rhesus monkeys (Macaca mulatta) have been unsuccessful (19, 55). Subsequent attempts to infect splenectomized rhesus monkeys were also unsuccessful. Sporozoites from the salivary glands of infected A. maculipennis mosquitoes were injected into an intact mona monkey (Cercopithicus mona), but no parasitemia developed (37). Attempts to infect splenectomized New World Aotus trivirgatus griseimembra monkeys were also unsuccessful (20). Although Saimiri sciureus boliviensis monkeys were shown to support the development of exoerythrocytic stages, parasitemia was not demonstrated (75). Five splenectomized S. s. boliviensis monkeys were injected with from 77,000 to 500,000 sporozoites dissected from A. dirus mosquitoes; none developed detectable parasitemia during 3 months of observation.
ULTRASTRUCTURE
A limited number of studies have been conducted on the changes that occur when erythrocytes are infected with P. ovale (2, 67, 68). These changes appear to be similar to those seen with the other species of Plasmodium, particularly P. vivax. Asexual parasites possess acristate mitochondria surrounded by a single-membrane pellicle in addition to a parasitophorous vacuole membrane. The gametocytes possess cristate mitochondria surrounded by a three-membrane pellicle in addition to a parasitophorous vacuole membrane (67). Caveola-vesicle complexes are formed along the host cell plasmalemma, probably corresponding to Schüffner's dots. Nodules were observed on the erythrocytes infected with asexual parasites of P. ovale. These nodules had not been described on any other species of malaria parasites (68).
RELATIONSHIPS TO OTHER SPECIES
Malaria parasites of primates are clustered together based on certain biologic and morphological characteristics that assist in their identification and their selection as models for research. Plasmodium ovale is a relapsing malaria parasite with a latent liver stage that often persists for many months; all stages of the asexual cycle of the parasite are present in the peripheral circulation; the asexual parasite count rarely reaches high density, indicating restriction to specific population of erythrocytes; the course of parasitemia is short compared to other human-infecting malaria parasites; unlike P. vivax, host susceptibility is not controlled by the absence of the Duffy gene blood group; geographically restricted to sub-Saharan Africa and islands of the western Pacific; infectious to anopheline mosquitoes outside its geographic distribution, and thus the reasons for geographic isolation are not due to vector incompetence; and it is the only human-infecting malaria parasite that has not infected (experimentally) New World monkeys.
Biologically, P. ovale has latent liver stages and is thus classified as one of the relapsing malaria parasites. These include the primate-infecting malaria species P. vivax, P. cynomolgi, P. fieldi, and P. simiovale. Infected erythrocytes of these species all exhibit Schüffner's dots. However, other primate-infecting species such as P. simium and P. gonderi, which also exhibit Schüffner's dots, have not been shown to have latent liver forms. Of the Old World monkey malaria parasites, the ones that appear to be most similar biologically and morphologically to P. ovale are P. fieldi from Malaysia and P. simiovale from Sri Lanka. There is also morphological similarity in the blood stages between the chimpanzee parasite P. schwetzi and P. ovale. However, the sporogonic stages of these two species are markedly different in size and rate of development (24).
A phylogenetic analysis of all the primate malaria parasites tested, based on the gene encoding the cytochrome b protein from the mitochondrial genome, indicated that they formed a monophyletic group with the exception of P. falciparum and P. reichenowi. There is no molecular evidence suggesting that it is closely related to any other of the primate malaria parasites that have been examined so far. Plasmodium ovale appears to represent an independent colonization of humans by malaria parasites (33).
REFERENCES
- 1.Afari, E. A., T. Nakano, F. Binka, S. Owusu-Agyei, and J. Asigbee. 1993. Seasonal characteristics of malaria infection in under-five children in a rural community in southern Ghana. W. Afr. J. Med. 12:39-42. [PubMed] [Google Scholar]
- 2.Aikawa, M., C. L. Hsieh, and L. H. Miller. 1977. Ultrastructural changes of the erythrocytic membrane in ovale-type malarial parasites. J. Parasitol. 63:152-154. [PubMed] [Google Scholar]
- 3.Alves. W., L. A. Schinazi, and F. Aniceto. 1968. Plasmodium ovale infections in the Philippines. Bull. W. H. O. 39:494-495. [PMC free article] [PubMed] [Google Scholar]
- 4.Andrysiak, P. M., W. E. Collins, and G. H. Campbell. 1986. Stage-specific and species-specific antigens of Plasmodium vivax and P. ovale defined by monoclonal antibodies. Infect. Immun. 54:609-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anthony, R. L., M. J. Bangs, N. Hamzah, H. Basri, Purnomo, and B. Subianto. 1992. Heightened transmission of stable malaria in an isolated population in the highlands of Irian Jaya, Indonesia. Am. J. Trop. Med. Hyg. 47:346-356. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong, J. C. 1969. Plasmodium ovale endemic in Ethiopia. Trans. R. Soc. Trop. Med. Hyg. 63:287-288. [DOI] [PubMed] [Google Scholar]
- 7.Baird, J. K., Purnomo, and S. Masbar. 1990. Plasmodium ovale in Indonesia. Southeast Asian J. Trop. Med. Public Health 21:541-544. [PubMed] [Google Scholar]
- 8.Barnish, G., G. H. Maude, M. J. Bockarie, O. A. Erunkulu, M. S. Dumbuya, and B. M. Greenwood. 1993. Malaria in a rural area of Sierra Leone. II. Parasitological and related results from pre- and post-rains clinical surveys. Ann. Trop. Med. Parasitol. 87:137-148. [DOI] [PubMed] [Google Scholar]
- 9.Beier, M. S., I. K. Schwartz, J. C. Beier, P. V. Perkins, F. Onyango, J. K. Koros, G. H. Campbell, P. M. Andrysiak, and A. D. Brandling-Bennett. 1988. Identification of malaria species by ELISA in sporozoite and oocyst infected Anopheles from western Kenya. Am. J. Trop. Med. Hyg. 39:323-327. [DOI] [PubMed] [Google Scholar]
- 10.Bjorkman, A., P. Hedman, J. Brohult, M. Willcox, I. Diamant, P. O. Pehrsson, L. Rombo, and E. Bengtsson. 1985. Different malaria control activities in an area of Liberia-effects on malariometric parameters. Ann. Trop. Med. Parasitol. 79:239-246. [DOI] [PubMed] [Google Scholar]
- 11.Bray, R. S. 1957. Studies on malaria in chimpanzees. IV. Plasmodium ovale. Am. J. Trop. Med. Hyg. 6:638-645. [DOI] [PubMed] [Google Scholar]
- 12.Bray, R. S., R. W. Burgess, and J. R. Baker. 1963. Studies on malaria in chimpanzees. X. The presumed second generation of the tissue phase of Plasmodium ovale. Am. J. Trop. Med. Hyg. 12:1-12. [PubMed] [Google Scholar]
- 13.Brown, P., W. E. Collins, D. C. Gajdusek, and L. H. Miller. 1976. An evaluation of malaria fluorescent antibody patterns in several remote island populations of the New Hebrides, Solomons, Western Carolines, amd New Guinea. Am. J. Trop. Med. Hyg. 25:775-783. [DOI] [PubMed] [Google Scholar]
- 14.Cabrera, B. D., and P. V. Arumbulo III. 1977. Malaria of the Philippines, a review. Acta Trop. 34:265-279. [PubMed] [Google Scholar]
- 15.Cadigan, F. C., and R. S. Desowitz. 1969. Two cases of Plasmodium ovale from central Thailand. Trans. R. Soc. Trop. Med. Hyg. 63:681-682. [DOI] [PubMed] [Google Scholar]
- 16.Chin, W., and G. R. Coatney. 1971. Relapse activity of mosquito-induced infections with a West African strain of Plasmodium ovale. Am. J. Trop. Med. Hyg. 20:825-827. [DOI] [PubMed] [Google Scholar]
- 17.Chin, W., and P. G. Contacos. 1966. A recently isolated West African strain of Plasmodium ovale. Am. J. Trop. Med. Hyg. 15:1-2. [DOI] [PubMed] [Google Scholar]
- 18.Chin, W., P. G. Contacos, and J. N. Buxbaum. 1966. The transmission of a West African strain of Plasmodium ovale by Anopheles freeborni and Anopheles maculatus. Am. J. Trop. Med. Hyg. 15:690-693. [DOI] [PubMed] [Google Scholar]
- 19.Christophers, R. 1934. Malaria from a zoological point of view. Proc. R. Soc. Med. 27:991-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coatney, G. R., W. E. Collins, M. Warren, and P. G. Contacos. 1971. The primate malarias. U. S. Government Printing Office, Washington, D.C.
- 21.Collins, F. H., R. K. Sakai, K. D. Vernick, S. Paskewitz, D. C. Seeley, L. H. Miller, W. E. Collins, C. C. Campbell, and R. W. Gwadz. 1986. Genetic selection of a Plasmodium-refractory strain of the malaria vector Anopheles gambiae. Science 234:607-610. [DOI] [PubMed] [Google Scholar]
- 22.Collins, W. E., J. Cattani, J. A. Lourie, T. Taufa, W. Anderson, J. C. Skinner, P. S. Stanfill, and A. Y. Huong. 1988. Antibody responses to malarial antigens in the Wopkaimin population of the Star Mountains, Papua New Guinea. Am. J. Trop. Med. Hyg. 39:241-245. [DOI] [PubMed] [Google Scholar]
- 23.Collins, W. E., and G. M. Jeffery. 2002. A retrospective examination of sporozoite- and trophozoite-induced infections with Plasmodium ovale: development of parasitologic and clinical immunity during primary infection. Am. J. Trop. Med. Hyg. 66:492-502. [DOI] [PubMed] [Google Scholar]
- 24.Collins, W. E., T. C. Orihel, P. G. Contacos, M. H. Jeter, and L. S. Gell. 1969. Some observations of the sporogonic cycle of Plasmodium schwetzi, P. vivax, and P. ovale in five species of Anopheles. J. Protozool. 16:589-596. [Google Scholar]
- 25.Collins, W. E., M. Pappaioanou, H. M. McClure, R. B. Swenson, E. Strobert, J. C. Skinner, V. Filipski, P. S. Stanfill, F. H. Collins, and C. C. Campbell. 1987. Infection of chimpanzees with Nigerian I/CDC strain of Plasmodium ovale. Am. J. Trop. Med. Hyg. 37:455-459. [DOI] [PubMed] [Google Scholar]
- 26.Collins, W. E., and J. C. Skinner. 1972. The indirect fluorescent antibody test for malaria. Am. J. Trop. Med. Hyg. 21:690-695. [DOI] [PubMed] [Google Scholar]
- 27.Collins, W. E., J. C. Skinner, and R. F. Coifma. 1967. Fluorescent antibody studies in human malaria V. Response of sera from Nigerians to five Plasmodium antigens. Am. J. Trop. Med. Hyg. 16:568-571. [DOI] [PubMed] [Google Scholar]
- 28.Collins, W. E., J. C. Skinner, and G. M. Jeffery. 1968. Studies on the persistence of malarial antibody response. Am. J. Epidemiol. 87:592-598. [DOI] [PubMed] [Google Scholar]
- 29.Collins, W. E., J. S. Sullivan, D. Nace, T. Williams, J. Kendall, J. J. Sullivan, G. G. Galland, K. K. Grady, and A. Bounngaseng. 2002. Experimental infection of Anopheles farauti with different species of Plasmodium. J. Parasitol. 88:295-298. [DOI] [PubMed] [Google Scholar]
- 30.Collins, W. E., M. Warren, and J. C. Skinner. 1971. Serological malaria survey in the Ethiopian highlands. Am. J. Trop. Med. Hyg. 20:199-205. [DOI] [PubMed] [Google Scholar]
- 31.Cornu, M., A. Combe, B. Couprie, R. Moyou-Somo, B. Carteron, W. H. Van Harten, J. Tribouley, and C. Ripert. 1986. Epidemiological aspects of malaria in 2 villages in the Manyemen forest region (Cameroon, southwest province). Med. Trop. 46:131-140. [PubMed] [Google Scholar]
- 32.Craig, C. F. 1900. Report bacteriological lab. U.S. Army General Hospital, Presidio of San Francisco, California for 1899-1900. Surgeon-General's Report, U. S. Army.
- 33.Escalante, A. A., D. F. Freeland, W. E. Collins, and A. A. Lal. 1988. The evolution of primate malaria parasites based on the gene encoding cytochrome b from the linear mitochrondrial genome. Proc. Natl. Acad. Sci. USA 95:8124-8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fairley, N. M. 1933. A case of malaria due to Plasmodium ovale Stephens 1922. Br. Med. J. July 15:1-4. [DOI] [PMC free article] [PubMed]
- 35.Gardiner, C., R. J. Bigger, W. E. Collins, and F. K. Nkrumah. 1984. Malaria in urban and rural areas of southern Ghana: a survey of parasitemia, antibodies, and antimalarial practices. Bull. W. H. O. 62:607-613. [PMC free article] [PubMed] [Google Scholar]
- 36.Garnham, P. C. C. 1966. Malaria parasites and other haemosporidia. Blackwell Scientific Publications, Oxford, England.
- 37.Garnham, P. C. C., R. S. Bray, W. Cooper, R. Lainson, F. I. Awad, and J. Williamson. 1955. Pre-erythrocytic stages of human malaria: Plasmodium ovale. Br. Med. J. i:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garnham, P. C. C., R. S. Bray, W. Cooper, R. Lainson, F. I. Awad, and J. Williamson. 1954. Pre-erythrocytic stages of human malaria: Plasmodium ovale. Trans. R. Soc. Trop. Med. Hyg. 49:158-167. [DOI] [PubMed] [Google Scholar]
- 39.Gbakima, A. A. 1994. Inland valley swamp rice development: malaria, schistosomiasis, onchocerciasis in south central Sierra Leone. Public Health 108:149-157. [DOI] [PubMed] [Google Scholar]
- 40.Giovannola, A. 1935. Plasmodium ovale considered as a modification of Plasmodium vivax after long residence in the human host. Am. J. Trop. Med. 15:175-186 [Google Scholar]
- 41.Gleason, N. N., G. U. Fisher, R. Blumhardt, A. E. Roth, and G. W. Gaffney. 1970. Plasmodium ovale malaria acquired in Viet-Nam. Bull. W. H. O. 39:947-948. [PMC free article] [PubMed] [Google Scholar]
- 42.Glynn, J. R., and D. J. Bradley. 1995. Inoculum size and severity of malaria induced with Plasmodium ovale. Acta Trop. 59:65-70.20. [DOI] [PubMed] [Google Scholar]
- 43.Gundelfinger, B. F. 1975. Observations on malaria in Indonesian Timor. Am. J. Trop. Med. Hyg. 24:393-396. [DOI] [PubMed] [Google Scholar]
- 44.Harwin, R. M. 1971. Malaria due to Plasmodium ovale in Rhodesia. Centr. Afr. J. Med. 17:146-148. [PubMed] [Google Scholar]
- 45.Herbst, J. M., L. A. Taylor, and S. M. Joubert. 1987. Plasmodium ovale infections in the lower Rufiji basin, Tanzania. Trop. Geog. Med. 37:102-107. [Google Scholar]
- 46.Jackson, A. V. 1944. Plasmodium ovale malaria: a report of two cases contracted in New Guinea. Med. J. Aust. 2:278-279. [Google Scholar]
- 47.Jambulingam, P., S. S. Mohapatra, L. K. Das, P. K. Das, and P. K. Rajagopalan. 1989. Detection of Plasmodium ovale in Koraput district, Orissa state. Indian J. Med. Res. 89:115-116. [PubMed] [Google Scholar]
- 48.James, S. P., W. D. Nicol, and P. G. Shute. 1932. P. ovale passage through mosquitoes and successful transmission by their bites. Ann. Trop. Med. Parasitol. 26:139-145. [Google Scholar]
- 49.James, S. P., W. D. Nicol, and P. G. Shute. 1933. Plasmodium ovale Stephens 1922. Parasitology 25:87-95. [Google Scholar]
- 50.James, S. P., W. D. Nicol, and P. G. Shute. 1935. The specific status of Plasmodium ovale Stephens. Am. J. Trop. Med. 15:187-188. [Google Scholar]
- 51.James, S. P., W. D. Nicol, and P. G. Shute. 1949. Ovale malaria, in Boyd's Malariology, Vol. II, Chapter 44. W. B. Sanders Company, Philadelphia, Pennsylvania.
- 52.James, S. P., and W. Yorke. 1932. Cycle of Plasmodium ovale Stephens, in man and Anopheles maculipennis. Trans. R. Soc. Trop Med. Hyg. 26:3-4.
- 53.Jeffery, G. M. 1954. The Donaldson strain of malaria 3. The infection in the mosquito. Am. J. Trop. Med. Hyg. 3:651-659. [DOI] [PubMed] [Google Scholar]
- 54.Jeffery, G. M. 1957. Extended low-temperature preservation of human malaria parasites. J. Parasitol. 43:488. [PubMed] [Google Scholar]
- 55.Jeffery, G. M. 1961. Inoculation of human malaria into a simian host. J. Parasitol. 47:90. [Google Scholar]
- 56.Jeffery, G. M., and R. C. Rendtorff. 1955. Preservation of viable human malaria sporozoites by low-temperature freezing. Exp. Parasitol. 4:445-454. [DOI] [PubMed] [Google Scholar]
- 57.Jeffery, G. M., and M. D. Young. 1954. The Donaldson strain of malaria 4. An evaluation and status. Am. J. Trop. Med. Hyg. 3:660-664. [DOI] [PubMed] [Google Scholar]
- 58.Jeffery, G. M., A. Wilcox, and M. D. Young. 1955. A comparison of West African and West Pacific strains of Plasmodium ovale. Trans. R. Soc. Trop. Med. Hyg. 49:168-175. [DOI] [PubMed] [Google Scholar]
- 59.Jeffery, G. M., M. D. Young, and A. Wilcox. 1954. The Donaldson strain of malaria 1. History and characterization of the infection in man. Am. J. Trop. Med. Hyg. 3:628-637. [DOI] [PubMed] [Google Scholar]
- 60.Kawamoto, F., H. Miyake, O. Kaneko, M. Kimura, N. T. Dung, N. T. Dung, O. Liu, M. Zhou, L. D. Dao, S. Kawai, S. Isomura, and Y. Wataya. 1996. Sequence variation in the 18S rRNA gene, a target for PCR-based malaria diagnosis, in Plasmodium ovale from southern Vietnam. Microbiology 34:2287-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Konate, L. N. Diagne, K. Brahimi, O. Faye, F. Legros, C. Rogier, V. Petrarca, and J. F. Trape. 1994. Biology of the vectors and transmission of Plasmodium falciparum, P. malariae and P. ovale in a village in the savanna of west Africa (Dielma, Senegal). Parasite 1:325-333. [DOI] [PubMed] [Google Scholar]
- 62.Lacan, A., and E. Peel. 1958. Plasmodium ovale Stephens 1922 en Afrique Equatoriale Française. Bull. Soc. Pathol. Exot. 51:167-169. [PubMed] [Google Scholar]
- 63.Lysenko, A. J. A., and A. E. Beljaev. 1969. An analysis of the geographical distribution of Plasmodium ovale. Bull. W. H. O. 40:383-394. [PMC free article] [PubMed] [Google Scholar]
- 64.Macfie, J. W. S., and A. Ingram. 1917. Observations on malaria in the Gold Coast colony, West Africa. Ann. Trop. Med. Parasitol. 11:1-23. [Google Scholar]
- 65.Marty, P., B. Chapdelaine, Y. Le Fichoux, and J. M. Chabert. 1987. Paludisme anémiant à Plasmodium ovale après 45 mois d'incubation. Presse Med. 16:357. [PubMed] [Google Scholar]
- 66.Matola, Y. G. 1985. Prospects of human malaria and Bancroftian filariasis infections in the lower Rufiji basin, Tanzania. I. Malaria. Trop. Geogr. Med. 37:102-107. [PubMed] [Google Scholar]
- 67.Matsumoto, Y., S. Matsuda, and Y. Yoshida. 1986. Ultrastructure of erythrocytic stages of Plasmodium ovale in humans. Am. J. Trop. Med. Hyg. 35:689-696. [DOI] [PubMed] [Google Scholar]
- 68.Matsumoto, Y., S. Matsuda, and Y. Yoshida. 1986. Ultrastructure of human erythrocytes infected with Plasmodium ovale. Am. J. Trop. Med. Hyg. 35:697-703. [DOI] [PubMed] [Google Scholar]
- 69.Mazier, D., W. E. Collins, S. Mellock, P. M. Andrysiak, N. Berbiguier, G. H. Campbell, F. Miltgen, R. Bertolotti, P. Langois, and M. Gentilini. 1987. Plasmodium ovale: in vitro development of hepatic stages. Exp. Parasitol. 54:393-400 [DOI] [PubMed] [Google Scholar]
- 70.McMillan, B. 1968. Further observations on ovale malaria in New Guinea. Trop. Geogr. Med. 20:172-176. [PubMed] [Google Scholar]
- 71.McMillan, B., and A. Kelly. 1967. Ovale malaria in eastern New Guinea. Trop. Geogr. Med. 19:172-176. [PubMed] [Google Scholar]
- 72.Meuwissen, J. H. E. T. 1966. Fluorescent antibodies in human malaria, especially in Plasmodium ovale. Trop. Geogr. Med. 18:250-259. [PubMed] [Google Scholar]
- 73.Meuwissen, J. H. E. T. 1968. Antibody responses of patients with natural malaria to human and simian Plasmodium antigens measured by the fluorescent antibody test. Trop. Geogr. Med. 20:137-140. [PubMed] [Google Scholar]
- 74.Michel, R., P. Carnevale, M. F. Bosseno, J. F. Molez, O. Brandicourt, A. Zoulani, and Y. Michel. 1981. Plasmodium falciparum and drepanocytic gene in Popular Republic of Congo. I. Prevalence of malaria and drepanocytic trait among school children in Brazzaville area. Med. Trop. 41:403-412. [PubMed] [Google Scholar]
- 75.Millet, P., C. Nelson, G. G. Galland, J. S. Sullivan, C. L. Morris, B. B. Richardson, and W. E. Collins. 1994. Plasmodium ovale: observations on the parasite development in Saimiri monkey hepatocytes in vivo and in vitro, in contrast with its ability to induce parasitemia. Exp. Parasitol. 78:394-399. [DOI] [PubMed] [Google Scholar]
- 76.Morris, C. L., J. S. Sullivan, H. M. McClure, E. Strobert, B. B. Rchardson, G. G. Galland, I. F. Goldman, and W. E. Collins. 1996. The Nigerian I/CDC strain of Plasmodium ovale in chimpanzees. J. Parasitol. 82:444-449. [PubMed] [Google Scholar]
- 77.Nagasawa, H., P. M. Procell, C. T. Atkinson, G. H. Campbell, W. E. Collins, and M. Aikawa. 1987. Localization of circumsporozoite protein of Plasmodium ovale in midgut oocysts. Infect. Immun. 65:2928-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nemirovskaia, A. I., E. A. Pavlova, and A. E. Beliaev. 1973. Detection of Plasmodium ovale in a patient infected in Melanesia. Med. Parazitol. (Moskow) 42:182-185. [In Russian.] [PubMed] [Google Scholar]
- 79.Oliveira, D. A., B. P. Holloway, E. L. Durigon, W. E. Collins, and A. A. Lal. 1995. Polymerase chain reaction and a liquid-phase nonisotopic hybridization for species-specific and sensitive detection of malaria infection. Am. J. Trop. Med. Hyg. 52:139-144. [DOI] [PubMed] [Google Scholar]
- 80.Omer, A. H. 1978. Species prevalence of malaria in northern and southern Sudan and control by mass chemoprophylaxis. Am. J. Trop. Med. Hyg. 27:858-863. [DOI] [PubMed] [Google Scholar]
- 81.Onori, E. 1967. Distribution of Plasmodium ovale in the eastern, western, and northern regions of Uganda. Bull. W. H. O. 37:665-668. [PMC free article] [PubMed] [Google Scholar]
- 82.Patterson, J. E., F. J. Bia, J. Miller, and P. McPhedra. 1987. Relapsing malaria infection acquired in Kenya. Yale J. Biol. Med. 60:245-253. [PMC free article] [PubMed] [Google Scholar]
- 83.Procell, P. M., W. E. Collins, and G. H. Campbell. 1988. Circumsporozoite protein of the human malaria parasite Plasmodium ovale identified with monoclonal antibodies. Infect. Immun. 56:376-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Richard-lenoble, D., M. Kombila, J. Chamdenier, E. Engohan, M. Gannier, and C. Dubourg. 1986. Malaria in Gabon. I. Study of 500 children with fever in Libreville. Bull. Soc. Pathol. Exot. 79:284-287. [PubMed] [Google Scholar]
- 85.Richard-Lenoble, D., M. Kombila, J. Chandenier, F. Gy, X. Billiault, C. Nguiri, M. Martz, F. Boyer, and M. Bauzou. 1987. Malaria in Gabon. II. Evaluation of the quantitative and qualitative prevalence of parasites in the total school and preschool population of the country. Bull. Soc. Path. Exotic. 80:532-542. [PubMed] [Google Scholar]
- 86.Shute, P. G., and M. Maryon. 1952. A study of human malaria oocysts as an aid to species diagnosis. Trans. R. Soc. Trop. Med. Hyg. 46:275-292. [DOI] [PubMed] [Google Scholar]
- 87.Shute, P. G., and M. Maryon. 1969. Imported malaria in the United Kingdom. Br. Med. J. 2:781-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sinton, J. A., E. L. Hutton, and P. G. Shute. 1939. Studies of infections with Plasmodium ovale. I. Natural resistance to ovale infections. Trans. R. Soc. Trop. Med. Hyg. 32:751-762. [Google Scholar]
- 89.Snounou, G., S. Viriyakosol, W. Jarra, S. Thaithong, and K. N. Brown. 1993. Identification of the four human malaria parasites species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol. Biochem. Parasitol. 58:283-292. [DOI] [PubMed] [Google Scholar]
- 90.Stephens, J. W. W. 1922. A new malaria parasite of man. Ann. Trop. Med. Parasitol. 16:383-388. [Google Scholar]
- 91.Stephens, J. W. W. and D. U. Owen. 1927. Plasmodium ovale. Ann. Trop. Med. Parasitol. 21:293-302. [Google Scholar]
- 92.Tachibana, M., T. Takafumi, O. Kaneko, B. Khuntirat, and M. Torii. 2002. Two types of Plasmodium ovale defined by SSU rRNA have distinct sequences for ookinete surface proteins. Mol. Biochem. Parasitol. 122:223-226. [DOI] [PubMed] [Google Scholar]
- 93.Taylor, P., and S. L. Mutambu. 1986. A review of the malaria situation in Zimbabwe in the lower Rufiji Basin, Tanzania. I. Malaria. Trop. Geog. med. 37:102-107. [Google Scholar]
- 94.Trager, W., and H. Most. 1963. A long-delayed primary attack of ovale malaria. Am. J. Trop. Med. Hyg. 12:837-839. [DOI] [PubMed] [Google Scholar]
- 95.Trape, J. F., C. Rogier, L. Konate, N. Fiagne, H. Bougananali, B. Canque, F. Legros, A. Badji, G. Ndiaye, P. Ndiaye, H. Bouganali, B. Canque, F. Legros, A. Badji, G. Ndiaye, P. Ndiaye, K. Brahimi, O. Faya, P. Druilhe, and L. Pereira da Silva. 1994. The Dielmo project: a longitudinal study of natural malaria infection and the mechanisms of protective immunity in a community living in a holoendemic area of Senegal. Am. J. Trop. Med. Hyg. 51:123-137. [DOI] [PubMed] [Google Scholar]
- 96.Weijer, C. 1999. Another Tuskegee? Am. J. Trop. Med. Hyg. 61:103. [DOI] [PubMed] [Google Scholar]
- 97.Wilcox, A., G. M. Jeffery, and M. D. Young. 1954. The Donaldson strain of malaria. 2. Morphology of the erythrocytic parasites. Am. J. Trop. Med. Hyg. 3:638-649. [DOI] [PubMed] [Google Scholar]
- 98.Win, T. T., K. Lin, S. Mizuno, M. Zhou, Q. Liu, M. U. Ferreira, I. S. Tantular, S. Kojima, A. Ishii, and F. Kawamoto. 2002. Wide distribution of Plasmodium ovale in Myanmar. Trop. Med. Int. Health 7:231-239. [DOI] [PubMed]
- 99.Wolfe, H. L. 1968. Plasmodium ovale in Zambia. Bull. W. H. O. 39:947-948. [PMC free article] [PubMed] [Google Scholar]
- 100.Yorke, W., and D. U. Owen. 1930. Plasmodium ovale. Ann. Trop. Med. Parasitol. 24:593-599. [Google Scholar]
- 101.Young, M. D., and T. H. Johnson, Jr. 1949. A malaria survey of Liberia. J. Natl. Malaria Soc. 8:247-266. [PubMed] [Google Scholar]
- 102.Zhou, M. Q. Liu, C. Wongsrichanalai, W. Suwonkerd, K. Panart, S. Prajakwong, A. Pensiri, M. Kimura, H. Matsuoka, M. U. Ferreira, S. Isomura, and F. Kawamoto. 1998. High prevalence of Plasmodium malariae and Plasmodium ovale in malaria patients along the Thai-Myanmar border, as revealed by acridine staining and PCR-based diagnoses. Top. Med. Int. Health 3:304-312. [DOI] [PubMed] [Google Scholar]