Abstract
The gram-positive bacterium Staphylococcus aureus is a major pathogen responsible for a variety of diseases ranging from minor skin infections to life-threatening conditions such as sepsis. Cell wall-associated and secreted proteins (e.g., protein A, hemolysins, and phenol-soluble modulin) and cell wall components (e.g., peptidoglycan and alanylated lipoteichoic acid) have been shown to be inflammatory, and these staphylococcal components may contribute to sepsis. On the host side, many host factors have been implicated in the innate detection of staphylococcal components. One class of pattern recognition molecules, Toll-like receptor 2, has been shown to function as the transmembrane component involved in the detection of staphylococcal lipoteichoic acid and phenol-soluble modulin and is involved in the synthesis of inflammatory cytokines by monocytes/macrophages in response to these components. Nod2 (nucleotide-binding oligomerization domain 2) is the intracellular sensor for muramyl dipeptide, the minimal bioactive structure of peptidoglycan, and it may contribute to the innate immune defense against S. aureus. The staphylococcal virulence factor protein A was recently shown to interact directly with tumor necrosis factor receptor 1 in airway epithelium and to reproduce the effects of tumor necrosis factor alpha. Finally, peptidoglycan recognition protein L is an amidase that inactivates the proinflammatory activities of peptidoglycan. However, peptidoglycan recognition protein L probably plays a minor role in the innate immune response to S. aureus. Thus, several innate immunity receptors may be implicated in host defense against S. aureus.
INTRODUCTION
Staphylococcus aureus is a major pathogen in both community-acquired and nosocomial infections. S. aureus often colonizes hosts asymptomatically and lives as a commensal of the human nose. The anterior nares are the major reservoir of S. aureus: 20% of persons are persistently colonized and 60% are intermittent carriers, whereas 20% never carry S. aureus (86). The primary site of infection is often a breach in the skin that may lead to minor skin and wound infections, but S. aureus can also infect any tissue of the body, causing life-threatening diseases such as osteomyelitis, endocarditis, pneumonia, and septicemia.
The pathogenicity of S. aureus is due to the repertoire of toxins, exoenzymes, adhesins, and immune-modulating proteins that it produces. With the exception of diseases caused by specific toxins, such as enterotoxins and exfoliative or toxic shock syndrome toxins, no single virulence factor has been shown to be sufficient to provoke a staphylococcal infection. Such infection is rather promoted by the coordinated action of various virulence factors, which are cell wall associated and secreted bacterial proteins. Indeed, both localized infections, such as soft-tissue abscesses, and life-threatening systemic diseases, such as sepsis, result from the ability of this pathogen to attach to cells or tissues; escape the host immune system, i.e., factors that decrease phagocytosis and factors that interact with antistaphylococcal antibodies; and elaborate proteases, exotoxins, and enzymes, factors that specifically cause cell and tissue damage allowing dissemination of S. aureus (154).
The expression of these virulence factor genes is controlled by several regulatory systems such as Agr, SarA, and Arl (26, 42, 129, 148). The accessory gene regulator (Agr) is one of the best-characterized global regulatory systems involved in the regulation of virulence factor genes. Indeed, Agr, which is a quorum-sensing regulatory system, regulates virulence by increasing the expression of exoprotein genes and decreasing the expression of cell wall-associated protein genes (129, 148).
Staphylococcal sepsis differs from enterobacterial sepsis in that S. aureus infection often begins from infected loci at the body surface such as skin or soft tissue infections rather than enteric or genitourinary sources (159). Furthermore, staphylococcal infection induces an influx of neutrophils. Indeed, S. aureus is a pyogenic pathogen capable of tissue invasion and evasion of phagocytosis by neutrophils. Tissue invasion and killing of phagocytes are involved in the inflammatory response that leads to septic shock (178). Even though mortality rates in systemic nosocomial infections associated with S. aureus are higher than those due to enterobacteria, they are also lower than those due to aerobic gram-negative bacilli such as Pseudomonas aeruginosa (5, 151).
S. aureus, followed by Enterococcus spp. in the United States and Streptococcus pneumoniae in Europe, is the organism most frequently isolated during invasive nosocomial infections (17, 41). The increasing frequency of gram-positive sepsis is probably due to the ability of S. aureus to colonize intravascular catheters or surgically implanted materials and to the spread of antibiotic-resistant S. aureus, such as methicillin-resistant S. aureus (143). The outer cell wall of staphylococci is composed of exposed peptidoglycan, lipoteichoic acids, and a range of other toxic secreted products, which are probably implicated in staphylococcal septic shock (178). However, no symptom of shock is observed when the serum concentration of tumor necrosis factor alpha (TNF-α), one of the proinflammatory cytokines responsible for septic shock in gram-negative bacteria, is increased. This suggests that, unlike gram-negative-mediated shock, induction of TNF-α is not sufficient to cause symptoms of shock in staphylococcal infection (60). Thus, the mechanisms that lead to staphylococcal septic shock are probably multifactorial (178).
Pathogens invading the host are controlled by innate and adaptive immune responses. Recognition of microorganisms is the first step of host defense. The adaptive immune system recognizes pathogens by antigen receptors that are expressed at the surface of B and T lymphocytes. These receptors are characterized by specificity and memory. However, gene rearrangement followed by T- and B-cell activation usually takes several days to be fully active and to eradicate pathogens (183). Therefore, more rapid defense mechanisms have been developed by the host through the innate immune system. This system is capable of recognizing pathogens and provides a first line of defense to the host. Indeed, the innate immune response initiates a sequence of events that results in the production and secretion of a wide range of inflammatory cytokines and chemokines, the activation of macrophages/monocytes, and the initiation of adaptive immunity. The production of proinflammatory factors is due to several cell types, including white blood cells (neutrophils and macrophages), epithelial, and endothelial cells as well as platelets. White blood cells can phagocytose, kill, and degrade the pathogen. Compounds or molecules resulting from this degradation are then presented to T cells to activate adaptive immunity (3, 169).
The role of the innate immune system is to recognize a large number of different pathogens and to discriminate them from self and also those bacteria composing the normal flora with a limited number of receptors. Furthermore, pathogens have the ability to mutate, altering phenotypic expression of virulence determinants and recognition by host receptors. Thus, the host has developed a variety of innate immune receptors that have the ability to detect different microbial motifs that are usually conserved among species (3, 77). Interestingly, the structures recognized by the innate immune system are usually essential for the invading organisms and are not present in the host cells (113).
INFLAMMATORY RESPONSE TO S. AUREUS
The complex mechanisms of the host response upon invasion by pathogens include the production and release of proinflammatory and immunomodulating cytokines. Cytokines are inducible proteins that are mainly produced on stimulation of white blood cells and other cells by pathogens (197). Thus, synthesis of cytokines is necessary for the host defense against infections. Indeed, the absence of cytokines in deficient mice has been shown to be deleterious to the host (22). However, an inflammatory response with excessive production of proinflammatory cytokines induces side effects and can even lead to multiple organ dysfunction syndrome and death (22, 197).
Cells of the monocytic lineage are essential for innate immunity and also play a critical role in the pathophysiology of bacterial sepsis. Indeed, monocytes/macrophages are the main source of inflammatory cytokines responsible for septic shock (see Fig. 3). Among the different cytokines implicated in inflammation and septic shock, TNF-α, interleukin-1β (IL-1β), and IL-6 are proinflammatory cytokines. IL-10 is an anti-inflammatory cytokine that inhibits proinflammatory cytokines such as IL-1β, IL-6, IL-8, and TNF-α. IL-8 is a strong chemoattractant for neutrophils, while MCP-1 (macrophage/monocyte chemoattractant protein-1) and MIP-1 (macrophage-inflammatory protein-1) are chemoattractive mainly for monocytes: they elicit recruitment of phagocytes to the infectious site (109) (Table 1).
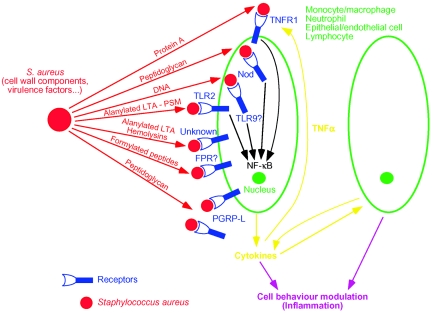
FIG. 3.
Action of staphylococcal components to promote immune responses from immune cells (data from reference 63). FPR, formylated peptide receptor.
TABLE 1.
The six cytokine familiesa
| Family | Cytokines | Biological activities |
|---|---|---|
| Interleukins | IL-1α (intracellular) and IL-1β (extracellular) | Induces growth factors and inflammatory mediators |
| Activates T cells | ||
| IL-6 | Stimulates terminal differentiation of B cells | |
| Enhances immunoglobulin secretion by B cells | ||
| Stimulates hepatocytes to produce acute-phase proteins | ||
| IL-10 | Inhibits the production of cytokines | |
| Inhibits major histocompatibility complex class II molecule expression on monocytes | ||
| Enhances B-cell proliferation and immunoglobulin synthesis | ||
| IL-12 | Stimulates cytokine synthesis (IFN-γ) | |
| Cytotoxic cytokines | TNF-α and TNF-β | Pleiotropic and multifunctional stimulators of cellular responses |
| Stimulates cytokines and cell adhesion molecules | ||
| Regulates the proliferation/differentiation of lymphocytes | ||
| Colony-stimulating factors | Granulocyte colony-stimulating factor | Stimulates proliferation and differentiation of functionally active mature neutrophils |
| Interferons | IFN-γ | Induces antiviral activity in a wide variety of cells |
| Involved in macrophage activation | ||
| Growth factors | Platelet-derived growth factor | Mitogenic |
| Stimulates chemotaxis | ||
| Chemokines | IL-8 (or CXCL8) | Chemotactic for neutrophils but not lymphocytes and monocytes |
| MIP-1α (or CCL3), MIP-1β (or CCL4), MCP-1 (or CCL2) | Chemotactic for monocytes rather than neutrophils |
Gram-positive infections such as those with S. aureus are capable of producing systemic cytokine responses. However, the peak cytokine response in gram-positive infections occurs 50 to 75 h after the challenge, whereas it occurs 1 to 5 h after in gram-negative infections (143). TNF-α, IL-1β, and IL-6 are produced from peripheral blood mononuclear cells and tissue macrophages. IL-12 is also increased in sepsis. Synthesis of the chemokine IL-8 is partly triggered by TNF-α. Gamma interferon (IFN-γ) is also produced in response to IL-12 and IL-8 (72, 106, 133, 178, 197). However, they are generally produced in lower amounts in gram-positive infections compared to gram-negative infections (43, 178). Although gram-positive and gram-negative bacteria have relatively different structural and pathogenic profiles, they induce a similar pattern of shock in the host (178).
As the systemic inflammatory response is involved in sepsis, it was suggested that treatment modulating or inhibiting inflammatory mediators would improve survival of patients with staphylococcal sepsis. In contrast to some animal models of gram-negative sepsis, pretreatment of animals with corticosteroids does not modify mortality when animals are challenged with S. aureus. Similar results were observed when patients with staphylococcal sepsis were treated with anti-inflammatory agents (143). Furthermore, it appears that anticytokine therapies have a detrimental effect in gram-positive sepsis (2, 143). Thus, these results suggest that gram-positive infections are more difficult to cure than those with gram-negative bacteria (178).
TOLL-LIKE RECEPTOR 2
The first members of the Toll family were identified in Drosophila melanogaster. Drosophila melanogaster is very resistant to microbial infections because it can synthesize antimicrobial peptides (7). Since the production of these peptides is regulated by a Toll receptor, adult flies that are mutated in Toll are susceptible to infection by fungi and bacteria (99). Thus, Toll is an essential receptor in the innate immune recognition in Drosophila melanogaster.
By a homology search of databases, 11 homologues of Toll designated Toll-like receptors (TLRs) have been found in humans (4, 114, 224). The role of TLRs in mammals is also to participate in innate immune recognition as pattern recognition receptors that detect common bacterial motifs.
Several lines of evidence support the view that TLR2 has a broad role as a pattern recognition receptor for a variety of microbes and microbial structures. These include lipoproteins from pathogens such as mycobacteria, spirochetes, and mycoplasmas, lipoarabinomannan from mycobacteria, Trypanosoma cruzi glycosylphosphatidylinositol anchor, and zymosan from fungi (4). Furthermore, TLR2 has been reported to be involved in the recognition of staphylococcal peptidoglycan and lipoteichoic acid (LTA) (see section on staphylococcal structures recognized by TLR2) (37, 61, 101, 171, 185, 194, 195, 222).
The involvement of TLR2 has been implicated in the host response to several staphylococcal infection models. For the most part, lack of TLR2 appears to increase the susceptibility of the host to staphylococcal infections. For example, TLR2-deficient mice are highly susceptible to S. aureus when inoculated intravenously (67, 184). After subcutaneous injections, staphylococci can grow intradermally in TLR2−/− mice, whereas they are cleared in wild-type mice (67). In addition, since nasal carriage is a major risk factor for staphylococcal infection, this parameter was investigated in TLR2-deficient mice (202). In a model of intranasal infection, TLR2-deficient mice showed 10-fold higher nasal carriage of S. aureus compared to that in wild-type mice (54). However, TLR2 deficiency has also been shown to be protective in certain infection models. One of the main components involved in the pathophysiology of sepsis is myocardial dysfunction, and it was found that in TLR2-deficient mice, the heart is protected against cardiac dysfunction caused by S. aureus. Furthermore, in this model, TNF-α and IL-1β production is significantly attenuated in TLR2-deficient mice (87). Thus, from these many investigations, it appears that TLR2 plays an important role as an innate immune receptor in the response to staphylococcal infections. However, depending on the infectious model, deficiency of TLR2 can be either protective or detrimental to the host organism.
TLR2 is expressed by different cells involved in the inflammatory response such as monocytes/macrophages, neutrophils, dendritic cells, astrocytes, and mast cells (38, 112, 164, 199). Interestingly, TLR2 and TLR6 are poorly expressed by human intestinal epithelial cells, which are broadly unresponsive to TLR2-dependent ligands such as staphylococcal phenol-soluble modulin or LTA (117).
Staphylococcal Structures Recognized by TLR2
Gram-positive bacterial cell walls are composed of multiple peptidoglycan layers, wall teichoic acids linked to the peptidoglycan and lipoteichoic acid (LTA) linked to the cytoplasmic membrane. In contrast, the cell envelope of a typical gram-negative bacterium is composed of a thin layer of peptidoglycan, an outer membrane, and lipopolysaccharides (LPS) and phospholipids. S. aureus does not contain lipopolysaccharide (endotoxin), which is the main cell wall component of gram-negative bacteria responsible for septic shock. However, S. aureus can cause septic shock and multiple organ failure (31). Indeed, in a canine model, infection by S. aureus provokes the same symptoms of septic shock as does Escherichia coli (134).
The pathogenicity of S. aureus is postulated to depend on the expression of a wide range of cell wall-associated and secreted bacterial proteins. We will not describe in this review the role of superantigen toxins in the inflammatory response because these superantigens are encoded by accessory genetic elements not always present in the S. aureus genome. Although cell wall-associated and secreted proteins are keys for staphylococcal virulence, their role in innate immunity remains largely unknown. On the other hand, several cell wall components such as peptidoglycan and lipoteichoic acids have been well studied.
Teichoic acids. (i) Structure.
Both wall teichoic acids and lipoteichoic acids are highly charged polymers. They concentrate cations at the cell wall surface and are associated with proteins to form complexes. They are involved in various biological activities: cation balance in the cell surface, cell division, and regulation of peptidoglycan autolysis (163).
S. aureus wall teichoic acid is a water-soluble polymer composed of 40 ribitol phosphate units that are substituted with d-alanine ester and N-acetylglucosaminyl residues (Fig. 1A). The chain is attached to peptidoglycan by a phosphodiester bond through a linkage unit that is composed of three glycerophosphate residues linked to two amino sugars (Fig. 1A).
FIG. 1.
Structures of teichoic acid (A) and lipoteichoic acid (B) of S. aureus (data from references 39 and 163). NAG, N-acetylglucosamine.
LTA is the major macroamphiphile molecule of gram-positive bacteria. The physiochemical properties of LTA are similar to those of LPS in gram-negative bacteria. Staphylococcal LTA consists of about 25 poly(1-3)-glycerol phosphate linked to a diacylglycerolipid anchor (Fig. 1B). The hydrophilic polyglycerol phosphate chain is long enough to penetrate the peptidoglycan, and the lipid moiety attaches the polymer to the surface of the cytoplasmic membrane. The glycolipid structure resembles the bacterial membrane composition and usually diverges among gram-positive bacteria in a genus-specific manner (40).
(ii) Role in the inflammatory response.
Although crucial for bacterial life, purified wall teichoic acids of S. aureus are not very inflammatory (108). However, a number of studies suggest that the bacterial LTA of S. aureus may contribute to sepsis (83). LTA from S. aureus has been shown to provoke secretion of cytokines and chemoattractants (TNF-α, IL-1β, IL-10, IL-12, IL-8, leukotriene B4, complement factor 5a, MCP-1, MIP-1α and granulocyte colony-stimulating factor) from monocytes or macrophages (14, 27, 30, 81, 179, 200). Complement factor 5a and leukotriene B4 are chemoattractants active on polymorphonuclear cells (PMNs) and monocytes. Thus, LTA induces an inflammatory response. However, very large amounts of LTA are necessary to induce responses of cells in vitro. Indeed, LTA, in the 1 to 10 μg/ml range is required to trigger cellular responses while LPS in the ng/ml range is sufficient to elicit responses (94). It must be taken into consideration that the active concentrations of LTA (1 μg or 105 to 107 CFU) as well as of LPS (20 ng or 107 CFU) are comparable when they are transposed to bacterial cell equivalents (170, 200), suggesting that LTA and LPS preparations may have similar potency.
Comparison of the activity of LPS versus LTA showed that staphylococcal LTA is able to promote the same strong induction of chemoattractants (IL-8, MIP-1α, MCP-1, complement factor 5a, and leukotriene B4), granulocyte colony-stimulating factor, and anti-inflammatory cytokines (IL-10) as LPS, whereas it is a weaker inducer of TNF-α, IL-1β, and IL-6 (200, 201). LTA also induces less IL-12 than LPS and subsequently IFN-γ (64, 91). The cytokine pattern produced by LTA is similar to that induced by the whole bacterium (200, 210). Furthermore, when LTA is inoculated intranasally in mice, a strong neutrophil and macrophage infiltration is observed in the lung, suggesting that LTA elicits granulocyte recruitment by producing chemoattractants (200). It is likely, then, that staphylococcal LTA participates in the formation of pus by recruiting neutrophils (200). Thus, staphylococcal LTA is a strong inducer of chemoattractant and granulocyte colony-stimulating factor release, suggesting that it is not just a weak LPS-like molecule but indeed displays activities distinct from LPS.
(iii) Interaction with TLR2.
LTA causes cytokine induction in mononuclear phagocytes. It was thus tempting to imagine that TLRs transduce the signal necessary for activation of immune cells by LTA. However, the role of TLRs in LTA-induced cell stimulation as well as the stimulation of cytokine production obtained in different cell systems has been controversial (14, 81, 94, 132, 171, 185). In most of these studies, commercial staphylococcal LTA preparations were used. It has been demonstrated that these preparations have a high degree of compositional heterogeneity and also contain significant amounts of endotoxin (the origin of this endotoxin is unknown) (45, 126). Furthermore, purification of LTA from S. aureus by standard methods using phenol extraction results in hydrolysis of d-alanine substituents. These substituents have been shown to be crucial for biological activity of LTA (125) (see section on alanylation of teichoic acids). Thus, taken together, the heterogeneity of the preparations, contamination by endotoxin, and inappropriate methods of purification that result in partial degradation of LTA might explain contradictory results in the literature concerning the role of TLR in the transduction of the signal and the production of cytokines after staphylococcal LTA stimulation (126).
A novel purification method using butanol extraction produces staphylococcal LTA without LPS contamination. This LTA exhibits the same efficiency, pattern of cytokine induction (TNF-α, IL-1β, IL-6, and IL-10), and recognition by TLR as a chemically synthesized LTA (45, 98, 125, 127). By using TLR2-deficient mice or monoclonal antibodies to TLR2, production of cytokines, e.g., IL-6 and TNF-α, in response to LTA stimulation requires TLR2 (38, 59, 121, 185), suggesting that TLR2 is a key receptor in response to LTA of S. aureus.
Although LTA exists as a heterogeneous family of related molecules, it appears that no relationship between LTA structure and efficiency is observed. However, several LTA compounds, such as d-Ala constituents, the glycosyl substituents, and the lipid anchor, modify LTA activity (64, 127, 128). Furthermore, LTAs from S. aureus and Bacillus subtilis exhibited similar TLR2 induction (144), whereas pneumococcal LTA is less active than staphylococcal LTA in stimulating TLR2 (59, 192). Taken together, LTA appears to constitute a broad immunostimulatory factor of gram-positive bacteria with possibly differing potencies depending on the constituents of the molecule (47, 64).
Alanylation of teichoic acids. (i) Structure.
The d-alanyl ester of teichoic acids results from a d-alanine substitution on the sugar (Fig. 1). The products of an operon of the S. aureus chromosome, dltABCD (for d-alanyl-LTA), catalyze the introduction of d-Ala into both wall teichoic acids and LTA. d-Alanylation of teichoic acids modulates the properties of the envelope. Indeed, d-alanine-esterified teichoic acids protect S. aureus against cationic antimicrobial peptides produced by host (150). Furthermore, the degree of d-alanylation is affected by growth environment (90, 135). In a culture at low salt concentrations, the degree of alanylation is 60% for wall teichoic acids and 80% for staphylococcal LTA (39).
(ii) Role in the inflammatory response.
Alanylation of teichoic acids increases the release of TNF-α and MIP-2 (93): in mice, MIP-2 and keratinocyte chemoattractant may serve as a neutrophil chemotactic factors in the recruitment of PMNs to sites of infection and inflammation because IL-8 is not present in mice (97). d-Alanine has been shown to be an important substituent for LTA activity since hydrolysis of alanine substituents of active LTA dramatically decreases cytokine induction (125). Furthermore, alanylation of teichoic acids increases the virulence of S. aureus in mice (28). The inflammatory response due to d-alanylation is correlated with the number and survival of bacteria (93). Indeed, dlt-deficient S. aureus are cleared more rapidly than the wild-type strain after administration of a similar inoculum. The lack of alanylation has three considerable consequences: increased susceptibility to defensin-like antimicrobial peptides (150), reduced adherence to host cells (1), and reduced inflammatory activity of LTA. Thus, alanylation induces multiple changes affecting the outcome of in vivo experiments.
(iii) Interaction with TLR2.
The minimum infective doses for wild-type S. aureus and dlt-deficient bacteria in TLR2−/− mice are 10-fold and 500-fold lower, respectively, than those observed in wild-type mice (93), suggesting that TLR2 is involved in murine host defense against alanylated staphylococcal teichoic acids. Since both wall teichoic acids and LTA are d-alanylated, this result confirms that TLR2 stimulates cytokine production in response to alanylated LTA (185, 201). However, the role of alanylated wall teichoic acids in TLR2 stimulation remains unknown.
Even in TLR2−/− mice, dlt-deficient bacteria have a higher minimum infective dose than the wild-type strain (93). Furthermore, release of MIP-2 and TNF-α occurs in TLR2-deficient mice. These results suggest that additional host defense mechanisms not related to TLR2 are involved. Thus, a receptor distinct from TLR2 or another mechanism also seems to be involved in the inflammatory response of mice to S. aureus alanylated teichoic acids (93).
Peptidoglycan. (i) Structure.
Peptidoglycan is a large polymer and the most conserved component of the gram-positive envelope. It provides shape-determining properties for bacteria. This polymer is constituted of glycan strands of two alternating sugar derivatives, N-acetylglucosamine and N-acetylmuramic acid, which form a dissacharide subunit. The carboxyl group of N-acetylmuramic acid is linked to a peptide subunit (or stem peptide) consisting of four or five alternating l- and d-amino acids (Fig. 2). This structure is highly cross-linked by peptide bridges (95). Whereas the structure of the glycan chains is highly conserved, the composition of the stem peptide varies among bacterial species. Indeed, most gram-negative bacteria and gram-positive bacilli possess m-diaminopimelic acid in position 3 of the stem peptide, which is usually directly cross-linked. In contrast, gram-positive cocci such as S. aureus have l-lysine in position 3 of the stem peptide that is cross-linked via an “interpeptide bridge,” the composition of which is different among bacteria (34, 167) (Fig. 2). Staphylococcal peptidoglycan belongs to this second type and has a pentaglycine interpeptide bridge (Fig. 2).
FIG. 2.
Structure of peptidoglycan of S. aureus and several other bacteria. The peptidoglycan of gram-negative bacteria and gram-positive bacilli is indicated on the right. Digestion by different enzymes (glucosaminidase, muramidase, amidase, and endopeptidase) is shown by dotted arrows. MDP structure is indicated in the dotted square. NAG, N-acetylglucosamine; NAM, N-acetylmuramic acid; m-DAP, m-diaminopimelic acid.
Peptidoglycan hydrolases are capable of hydrolyzing bonds in the cell wall peptidoglycan. They are classified into the following four classes according to their hydrolytic bond specificities: N-acetylmuramidases, N-acetylglucosaminidases, N-acetylmuramyl-l-alanine amidases, and endopeptidases (174). The first class, muramidase, catalyzes the hydrolysis of the glycosidic bond between N-acetylmuramic acid and N-acetylglucosamine (Fig. 2). The enzymes that cleave the bond between N-acetylglucosamine and the adjacent N-acetylmuramic acid in dissacharide subunits have been defined as glucosaminidases (Fig. 2). Another class of peptidoglycan hydrolases, amidases, hydrolyze the bond between the lactoyl group of N-acetylmuramic acid and l-alanine. Finally, endopeptidases cleave peptide cross-links (Fig. 2).
Peptidoglycan hydrolases are present in all bacteria, and some of them, called autolysins, digest their own protective cell wall peptidoglycan. Since peptidoglycan is essential for cellular integrity, the action of autolysins usually results in bacterial autolysis. Lysostaphin, which is an endopeptidase produced by Staphylococcus simulans biovar staphylolyticus, cleaves staphylococcal peptidoglycans in general (189) (Fig. 2). When lysing staphylococci, the enzymatic reaction of lysostaphin is the specific hydrolysis of the Gly-Gly bond in the pentaglycine bridge of the staphylococcal peptidoglycan.
Host enzymes are also implicated in peptidoglycan cleavage. Lysozyme is a muramidase present in various tissues (mucous membranes, respiratory and intestinal tracts) and fluids (serum, saliva, and tears). It is important for host defense because it can kill bacteria. Interestingly, S. aureus is resistant to lysozyme because N-acetylmuramic acid of staphylococcal peptidoglycan is O-acetylated at position C6-OH (Fig. 2) by an O-acetyltansferase that is an integral membrane protein (13). The resistance of S. aureus to lysozyme may contribute to its ability to colonize the skin and mucosal tissues such as the anterior nares. Peptidoglycan recognition protein L (PGRP-L), also known as serum amidase, displays an N-acetylmuramoyl-l-alanine amidase activity towards peptidoglycan. PGRP-L is thought to function as a peptidoglycan-scavenging molecule that likely reduces peptidoglycan-induced inflammation (209) (see section on PGRPs below).
(ii) Role in the inflammatory response.
Staphylococcal peptidoglycan has been shown to stimulate the production of proinflammatory cytokines and chemokines (TNF-α, IL-1β, IL-6, and IL-8) in monocytes and macrophages (66, 110, 190, 205). It has also been observed that primary astrocytes produce numerous cytokines such as IL-1β, TNF-α, MIP-1β, and MCP-1 in response to staphylococcal peptidoglycan (38) (Fig. 3).
Larger amounts of peptidoglycan, in the 10 to 100 μg/ml range, are necessary to stimulate cellular responses compared to LPS, which induces responses in the ng/ml range (94). Since 1 × 106 to 6 × 106 CFU correspond to 1 μg of staphylococcal peptidoglycan (192), whereas 107 CFU correspond to 20 ng of LPS, this suggests that whole peptidoglycan is about 100-fold less active than LPS. Studies of S. aureus cell walls indicate that only part of peptidoglycan is active. Indeed, insoluble and soluble peptidoglycan chains of high molecular weight are not very inflammatory. Hydrolyzing these chains to sugar and peptide monomers completely abolishes inflammation. In staphylococcal peptidoglycan, three cross-linked stem peptides appear to be the minimal structural constraint to be inflammatory (128). Furthermore, treatment of S. aureus by lysostaphin, which cleaves the pentaglycine bridge, moderately attenuates release of cytokines, whereas digestion with cellosyl, a muramidase hydrolyzing glycosidic bonds, nearly abrogates the induction of cytokine (131, 223). This suggests that the glycan strand is crucial for cytokine production, whereas stem peptide structure does not seem to be critical for the inflammatory activities of peptidoglycan.
Although peptidoglycan by itself promotes a weak induction of cytokines, it shows synergistic effects with LTA or LPS (221). Indeed, intravenous administration of staphylococcal peptidoglycan or LTA alone cannot cause shock, whereas coadministration of peptidoglycan with LTA in rats induces the production of TNF-α and IFN-γ in a synergistic way and provokes septic shock and multiple organ failure (31, 83, 188). Furthermore, although intranasally inoculated LTA does not synergize with peptidoglycan to induce the production of TNF-α and the chemoattractants MIP-2 and keratinocyte chemoattractant, these staphylococcal components act in synergy to elicit recruitment of PMNs into mouse lung (97).
Peptidoglycan of S. aureus also synergizes with low doses of LPS to cause multiple organ failure (188). Furthermore, coinjection of peptidoglycan with LPS induces synergistic production of TNF-α and IL-6 in the blood. Surprisingly, the release of IL-10 was not modified by the coadministration of peptidoglycan with LPS (188, 207). Thus, peptidoglycan seems to synergize with LPS to induce the release of proinflammatory cytokines but not the anti-inflammatory cytokines.
(iii) Interaction with TLR2.
The role of TLR2 as a peptidoglycan receptor has been investigated extensively, and until recently, it was broadly accepted that TLR2 is a receptor for staphylococcal peptidoglycan (76, 164, 171, 185). Indeed, staphylococcal peptidoglycan binds strongly to a soluble form of recombinant TLR2 composed of its putative extracellular domain, suggesting that the extracellular TLR2 domain directly interacts with peptidoglycan (76).
However, there are now contradictory results about the role of TLRs in peptidoglycan-induced cell stimulation and cytokine production. Most studies have been mainly carried out with commercial S. aureus peptidoglycan preparations, and a recent publication showed that highly purified peptidoglycan did not elicit TLR2-dependent activation and IL-6 and TNF-α production from mouse peritoneal macrophages, whereas lipoproteins and LTA did. It was hypothesized that the stimulation of TLR2 by peptidoglycan could be attributed to other inflammatory cell wall components contaminating the commercial peptidoglycan preparations (192). Indeed, treatment of peptidoglycan with hydrofluoric acid abolished TLR2 stimulation by gram-positive cell walls. As hydrofluoric acid hydrolyzes LTA, it was suggested that the peptidoglycan contaminant could be LTA (192). Thus, it seems that staphylococcal peptidoglycan is probably not recognized by TLR2 but rather by Nod2, another innate immune receptor (see section on Nod proteins).
Phenol-soluble modulin. (i) Structure.
Although infections with Staphylococcus epidermidis are less severe than those with S. aureus, S. epidermidis can cause infections ranging from localized infections to sepsis. This bacterium releases phenol-soluble modulin (PSM) in the extracellular fluid (115). PSM is composed of at least three components, PSMα, PSMβ, and PSMγ, which are very small proteins of 22 to 25 amino acids. PSMγ is identical to S. epidermidis δ-hemolysin, which is also present in S. aureus. δ-Hemolysin can be formylated (see section on formylated peptides). PSM is released into the culture medium during overnight culture and is also detected in the supernatant fluid after vigorous vortexing of washed bacteria, suggesting that PSM is also partially bound to the cell surface (85).
(ii) Role in the inflammatory response.
The δ-hemolysin of S. aureus may be involved in staphylococcal virulence. Indeed, it has been shown to lyse erythrocytes, probably by crossing the lipid membrane and inducing the release of their contents (152). It also binds to neutrophils and monocytes, inducing the production of TNF-α (168). S. epidermidis PSM also induces cytokine production (TNF-α, IL-1β, and IL-6) and activates NF-κB in macrophages. Furthermore, PSM is a chemotactic agent for both neutrophils and monocytes (102).
(iii) Interaction with TLR2.
PSM has been shown to use TLR2 to modulate the immune response (58). Indeed, human dermal endothelial cells express only very little TLR2 and do not respond to several TLR2 ligands such as PSM. On the other hand, endothelial cells that are transfected with TLR2 are capable of responding to TLR2 ligands, including PSM (19). Although further studies are required to demonstrate conclusively the role of TLR2 in PSM recognition, it appears that this staphylococcal virulence factor is involved in the inflammatory response through its activation of TLR2.
TLR2 Cooperation with Other Receptors
TLR2 has been shown to detect various specific components of pathogens. However, it is not clear how a receptor like TLR2 has the capacity to recognize such a wide spectrum of stimuli. Antibodies to CD14, TLR1, or TLR2 (but not those to TLR4) significantly reduces TNF-α production by mononuclear cells in response to staphylococcal LTA, suggesting that these receptors are involved in LTA recognition (59).
TLR1/TLR6.
Ligand recognition is more complicated with TLR2 because it has been shown to cooperate with TLR1 and/or TLR6 to increase its range of pathogen-associated molecular patterns. Ozinski et al. (145) first suggested that TLR2 mainly recognizes its ligands by forming functional heterodimers with either TLR1 or TLR6 (84, 214). TLR2 appears to require a partner to stimulate cytokine production. Indeed, dimerization of the cytoplasmic domain of TLR2 does not elicit TNF-α induction (145). TLR1 and TLR6 have been shown to mediate the discriminatory recognition of triacyl and diacyl lipopeptides by TLR2. TLR6 is necessary for TLR2 to detect MALP2 (macrophage-activating lipopeptide 2 from Mycoplasma pneumoniae), which is only diacylated (186). In contrast, TLR1 is necessary for the response to triacyl lipopeptides and mycobacterial lipoproteins (165, 187). Since staphylococci also produce a set of lipoproteins, such as the ABC transporters, it will be interesting to examine the possibility that these lipoproteins are indeed detected by TLR2 and whether their form, either diacylated or triacylated, dictates the contribution of either TLR1 or TLR6 to their recognition.
TLR6 has been shown to cooperate with TLR2 in the recognition of S. aureus (137, 145). Indeed, in the presence of a dominant negative form of TLR6, cytokine production induced by S. aureus is abolished (145). However, macrophages from TLR6-deficient mice stimulated by staphylococcal peptidoglycan still produce TNF-α, suggesting that TLR6 is not absolutely necessary for peptidoglycan recognition (4, 186). In TLR6−/− fibroblasts, the staphylococcal LTA response is significantly attenuated compared to wild-type cells, suggesting that TLR6 is required for LTA recognition by TLR2 (130). However, it has also been shown that TLR2 synergizes with TLR1 to sense LTA, consistent with the fact that this product is diacylated (192). The TLR2-mediated activation of NF-κB by PSM is increased by TLR6 but attenuated by TLR1 (58). Although definitive experiments in TLR1-deficient mice are still lacking, these results confirm a functional interaction between these receptors and interaction of staphylococcal components.
CD14.
CD14 is a member of the family of glycosylphosphatidylinositol-anchored membrane proteins lacking an intracellular signaling domain. CD14 is a key coreceptor expressed on the surface of macrophages and PMNs and is necessary for the full induction of an inflammatory response by LPS-stimulated TLR4 (217). Several studies have demonstrated that different components of S. aureus such as peptidoglycan and LTA also interact with the CD14 molecule (27, 36, 56, 59, 78, 94, 155, 170, 171, 212). Thus, it has been suggested that CD14 is a functional receptor for staphylococcal peptidoglycan and LTA. However, as it has recently been shown that LTA contamination could be involved in the inflammatory activity due to commercial preparations of peptidoglycan (192), the question is whether or not it is indeed peptidoglycan that binds to CD14.
An anti-CD14 antibody that abolishes cytokine release induced by LTA does not reduce the cytokine production caused by LPS, whereas two other antibodies have similar inhibitory effects. This suggests that the CD14 sites that recognize LTA and LPS are distinct with perhaps an overlap (56, 64). Furthermore, cytokine induction by LTA is inhibited by soluble CD14, suggesting that LTA trigger monocyte activation via CD14 (64). In addition, expression of CD14 in fibroblasts synergistically increases NF-κB activation mediated by TLR2 in response to S. aureus (222). However, the same mortality and symptoms of shock are observed in both wild-type and CD14-deficient mice challenged with various doses of S. aureus. This result suggests that CD14 does not have a significant role in septic shock caused by S. aureus (60). Thus, other CD14-independent mechanisms might be involved in TLR2 activation (60, 195).
CD36.
CD36, a single polypeptide membrane glycoprotein, is a member of a family of scavenger receptors. Recently, it has been shown that CD36-mutant mice are hypersusceptible in two models of staphylococcal infections (cutaneous and systemic) (67). Furthermore, cytokine production is abolished in CD36-deficient macrophages after stimulation by LTA and the R-enantiomer of MALP2 but is normal when stimulated by other TLR2 ligands such as S-MALP2, triacylated lipopeptide, and zymosan (67). As both LTA and MALP2 are diacylated, this suggests that CD36 is involved in the recognition of diacylglycerides. Interestingly, in TLR2-deficient cells, expression of CD36 or TLR6 alone does not induce NF-κB activation by LTA, suggesting that these receptors need a partner to transduce the LTA signal to cellular responses. Indeed, coexpression of either CD36 or TLR6 with TLR2 significantly increases the TLR2 response to LTA. When TLR2, TLR6, and CD36 are expressed together, the highest NF-κB activation is observed. Thus, it seems that CD36 may play a role analogous to that of CD14 by concentrating the diacylglyceride signal for transduction through TLR2 (67).
Asialo-GM1.
S. aureus is known to cause pulmonary infections. Once staphylococci are in the lung, they multiply and sometimes invade the epithelium of the bronchioli. The production and release of cytokines and chemokines, including IL-8, due to the presence of S. aureus elicit infiltration of PMNs and macrophages, leading to tissue damage and subsequent pneumonia (138).
In airway epithelium, asialylated glycolipids such as asialo-GM1 (asialoganglioside gangliotetraosylceramide) (153) are present on the epithelial surface and can act as receptors. Indeed, S. aureus binds to the GalNacβ1-4Gal moiety exposed at the cell surface and initiates IL-8 production through NF-κB activation (158). A recent study found small amounts of TLR2 on the apical surfaces of airway epithelial cells. After stimulation with S. aureus, TLR2 and asialo-GM1 are mobilized into lipid raft structures on the surface of the epithelial cells. The lipid raft microdomains are necessary for IL-8 production (176). Thus, asialo-GM1, similar perhaps to CD36, serves as a coreceptor that amplifies the signaling function of TLR2 (176).
Interestingly, the S. aureus strain lacking Agr, a staphylococcal virulence regulator (see the Introduction), shows attenuated IL-8 production through asialo-GM1, suggesting that the staphylococcal ligand recognized by asialo-GM1 is probably a surface molecule regulated by Agr such as these adhesins that interact with extracellular matrices (158).
Structure of TLR2
Human TLRs are type I transmembrane proteins with an extracellular domain, a transmembrane domain, and an intracellular domain (Fig. 4A).
FIG. 4.
Domain structure of TLR2 (A) and Nod2 (B). Numbers correspond to amino acid residues. A. TLR2 structure (data from references 44 and 118). ECD, extracellular domain; TMD, transmembrane domain; ICD, intracellular domain; TIR, Toll/IL-1 receptor domain. The regions homologous to LRRs are indicated by square boxes, and LRR-like motifs are indicated by boxes with an asterisk. B. Nod2 structure (data from reference 141). CARD, caspase-activating and recruitment domain; NBS, nucleotide binding site and LRR domain.
The N-terminal domain of TLR2, which is located in the extracellular compartment, is composed of leucine-rich repeat (LRR) motifs. The LRR consensus sequence consists of a motif of 24 to 29 residues with a highly conserved region (12). The N-terminal domain of TLR2 possesses 10 canonical LRR sequences and 8 to 10 LRR-like motifs that are poorly defined (Fig. 4A) (118). Proteins that contain LRRs, such as the extracellular domain of TLR2, are involved in interaction with a great variety of ligands (160). The leucine residues at positions 107, 112, and 115 in an LRR motif (44) as well as the extracellular region between Ser40 and Ile64 are crucial for the detection of peptidoglycan by TLR2 (122). In another study, Meng et al. found that the seven LRR motifs located at the N terminus (Fig. 4A) were not implicated in TLR2 activation by bacterial polypeptides, whereas the integrity of the extracellular domain was necessary to induce a full response to S. aureus peptidoglycan (118). Thus, potential binding domains of bacterial polypeptides most probably differ from those of peptidoglycan. This result suggests that TLR2 probably possesses multiple binding domains for its various ligands, explaining its promiscuous nature in terms of the molecular patterns recognized (118). Although, in light of the recent data arguing that peptidoglycan is not a TLR2 ligand (192), these findings may have to be revisited in future studies.
The cytoplasmic portion of Toll-like receptors shows a high similarity to that of the IL-1 receptor family and is now called the Toll/IL-1 receptor (TIR) domain (182). Upon extracellular stimulation, the TIR domain associates with an adaptor protein, which actuates a succession of signaling proteins, and this cascade of activation mediates signal transduction. Several polymorphisms located in the TIR domain of TLR2 have been detected. The presence of the Arg677Trp or Arg753Gln mutation in the TIR domain reduces NF-κB activation and cytokine production in response to TLR2 ligands (16, 105, 201). Furthermore, the ArG753Gln polymorphism has been shown to be present in 2 out of 91 septic patients, and these patients developed staphylococcal infections (105). However, a study of 420 patients exhibiting severe S. aureus infection showed that the Arg753Gln polymorphism in the TIR domain of the TLR2 gene is not associated with susceptibility to severe disease caused by S. aureus (124). These contradictory results might be explained by a previous observation indicating that the presence of only one wild-type allele of TLR2 is sufficient in vitro to induce normal cytokine production in response to staphylococcal LTA (124, 201).
TLR2 Signaling
TLR and Nod (see section on Nod proteins) trigger the activation of the transcription factor NF-κB (nuclear factor κB), which controls the expression of genes encoding numerous cytokines, chemokines, and costimulatory molecules necessary for the activation of the defense response. NF-κB is a transcription factor regulated by nuclear-cytoplasmic shuttling. Indeed, NF-κB dimerizes, interacts with DNA of the target genes located in the nucleus, and modifies their expression. However, NF-κB contains a nuclear localization sequence that is masked when inhibitors, the IκBs (inhibitors of κB), bind to NF-κB dimers. Thus, IκBs are responsible for NF-κB retention in the cytoplasm (80). IκB phosphorylated by IκB kinases (IKKs) is degraded by the proteasome, and NF-κB is then free to move to the nucleus, where it can directly regulate gene expression (Fig. 5) (113). Thus, stimulation of the pattern recognition receptor promotes the activation of IKKs and the release of NF-κB. The molecular cascades from the receptor to the release of NF-κB have been extensively examined to identify signaling molecules implicated in the TLR response.
FIG. 5.
Different signaling pathways of TLR2, Nod, and TNFR1 in response to ligand, resulting in activation of NF-κB, the nuclear transcriptional factor responsible for regulation of the immune response genes (data from references 3, 113, and 194). RIP1, receptor-interacting protein 1; FADD, Fas-associated death domain protein; TRAF2, TNF receptor-associated factor 2; PI3K, phosphatidylinositol 3-kinase composed of two subunits (p85 and p110).
The nature of the intracellular events following the stimulation of individual TLR is dependent on the adaptor molecules that interact with the different TLRs (Fig. 5). Although intracellular signaling induced by TLR ligands can utilize a shared group of molecules, distinct cellular responses can be generated (157). While some of the intracellular responses induced downstream of TLR2 and TLR4 are similar, for example, the activation of the NF-κB pathway, distinct differences in the cellular signaling pathways activated by these two receptors are observed (21). One of the most striking differences in cell signaling downstream of TLR2 and TLR4 is the activation of the interferon response factor pathway by TLR4 stimulation. This pathway requires the adaptors TRIF (TIR domain-containing adaptor inducing IFN-β) and TRAM (TRIF-related adaptor molecule) that are not present in TLR2 pathway (147, 181).
Indeed, TLR2 represents a more simplified signaling pathway mediated mainly by the adaptors MyD88 (myeloid differentiation protein) and TIRAP/Mal (TIR-associated proteins, also termed MAL for MyD88-associated ligand), which regulate the NF-κB pathway and an additional adaptor, Tollip (Toll-interacting protein), which appears to play a regulatory role in the pathway. From these adaptors, a cascade of events involving different mediators such as IRAK (IL-1R-associated kinase), TRAF6 (TNF receptor-associated factor 6), TAK1 (Transforming growth factor-activated kinase), and IKKs leads to the activation of NF-κB (Fig. 5) (208). Since there are many excellent recent reviews describing the activation of the NF-κB pathway, the following will focus on a description of the upstream events involving the specific adaptors of the TLR2 pathways (Fig. 5).
MyD88 also possesses a C-terminal TIR domain that interacts with the TIR domain of the TLR receptor (Fig. 5). Activation of a TLR by the ligand promotes its dimerization and the TIR domains of TLR and MyD88 bind. MyD88-deficient mice are more susceptible to systemic S. aureus infection than wild-type mice (184). Furthermore, cytokine production is attenuated in MyD88-deficient macrophages after S. aureus stimulation (183, 184). In contrast to systemic and in vitro infection, however, MyD88-deficient mice intranasally inoculated with S. aureus do not show an increase susceptibility to pulmonary infections and cytokine production as well as the neutrophil recruitment is not impaired, suggesting that lung tissues do not need MyD88 to respond to staphylococcal infections (173). Thus, the importance of MyD88 in the TLR2 signaling pathway appears to be tissue specific. This suggests that the pulmonary innate immune response to S. aureus implicates TLR2 signaling pathways distinct from MyD88 or recognition of staphylococcal ligands by a receptor independent of TLR2 (173).
TIRAP/Mal, similar to MyD88, contains a C-terminal TIR domain that directly interacts with the TIR domain of TLR2 (183). Indeed, cells from TIRAP/Mal knockout mice are unresponsive to TLR2 ligands. TIRAP/Mal, therefore, seems to be essential for signaling pathways activated by TLR2 (69, 141, 219).
An additional adaptor molecule, Tollip, has also been proposed to interact with TIR domains. Tollip was first identified as a molecule that associates with the cytoplasmic domain of IL-1R (20) and was shown to bind directly with TLR2 (225). In contrast to the other adaptor molecules, Tollip appears to suppress the TLR2 signaling pathway. Indeed, cells that overexpress Tollip cannot induce NF-κB activation in response to TLR2 ligands (20). Tollip associates and interferes with IRAK, one of the key molecules involved in the TLR2 signaling pathway (Fig. 5) (20, 225).
In addition to the MyD88-Tollip-TIRAP/Mal pathway of NF-κB activation, it has been observed that activation of Rac1, which belongs to the Rho family of GTPases, is also implicated in the signaling pathway of TLR2 (Fig. 5). Indeed, stimulation by S. aureus leads to association of Rac1 with the TLR2 cytosolic domain and the activation of the phosphatidylinositol 3-kinase pathway. This event induces the activation of protein kinases such as Akt (serine/threonine kinase) mediating NF-κB transactivation (Fig. 5) (8).
NOD PROTEINS
As stated above, TLR2 is an essential receptor for the recognition of staphylococcal components. However, it is interesting that astrocytes or macrophages from TLR2-deficient mice stimulated by S. aureus still produce proinflammatory mediators (38, 100, 184), suggesting that alternative receptors are also implicated in S. aureus recognition. The TLRs are involved in recognition of pathogens in the extracellular compartment, whereas the NBS-LRR (for nucleotide-binding site and leucine-rich repeat) family of proteins is involved in intracellular sensing of microorganisms and their products (9, 23). In this family, nucleotide-binding oligomerization domain proteins Nod1 and Nod2 play a role in the innate immune response by regulating the cytokine induction initiated by bacterial ligands through pathways that are possibly independent of TLR signaling.
The 302insC frameshift mutation in the nod2 gene is associated with the inflammatory bowel disease Crohn's disease (71, 139). This disease is associated with a severe inflammatory reaction at the level of the intestinal mucosa. Contrary to the expected gain-of-function mutation that would be consistent with the auto-inflammatory nature of this disease, the frameshift mutation in Nod2 results in a loss of bacterial sensing and decreased activation of inflammatory pathways (49, 75, 139). Much research is now focused on trying to find the mechanisms of Nod2 function that reconcile the apparently paradoxical role of this pattern recognition receptor in the pathogenesis of disease as reviewed by Kelsall (82).
Although much work is still required to fully understand Nod2 function in mediating disease, two recent papers have attempted to shed light on this issue (89, 107). One study used Nod2-deficient mice that have a “knock-in” of a mutation in Nod2 corresponding to the human Nod2 frameshift mutation associated with Crohn's disease (107). Surprisingly, these mice have high background inflammatory signaling and amplified responses to muramyl dipeptide (MDP), the specific ligand of Nod2 (see section on staphylococcal structure recognized by Nod proteins). Although these observations fit very well conceptually with the phenotype of Crohn's disease, i.e., increased levels of inflammation, they are diametrically opposed to findings in Crohn's disease patients. Cells from patients with the Nod2 frameshift mutation have normal basal levels of inflammatory signaling and are refractory to MDP stimulation (75). Thus, any firm conclusions regarding this study await confirmatory findings.
The other recent paper on mechanisms of Nod2 function demonstrated that Nod2-deficient mice have lower expression of alpha-defensins, or cryptins, at the level of the intestinal mucosa, and consequently, these mice have an increased susceptibility to oral infection with Listeria monocytogenes, a gram-positive pathogen of humans that crosses the intestinal barrier to enter the host (89). Alpha-defensins are a class of antimicrobial peptides secreted by specialized cells of the intestinal epithelium called Paneth cells. Decreased expression of alpha-defensins has also been observed in Crohn's disease patients (211). Accordingly, Nod2 mutations may render the intestinal mucosa more accessible to bacterial translocation with a resulting overamplified inflammatory response in the afflicted patient.
Staphylococcal Structure Recognized by Nod Proteins
Nod1, which is ubiquitously expressed, is involved mainly in the recognition of gram-negative bacteria. Indeed, the Nod1 ligand is a bacterial peptidoglycan fragment containing an N-acetylglucosamine-N-acetylmuramic acid tripeptide motif with diaminopimelic acid (Fig. 2) (24, 48). Nod2, which is mainly expressed in monocytes/macrophages, detects peptidoglycan from gram-negative (E. coli and Shigella flexneri) and gram-positive bacteria (B. subtilis and S. aureus) (49, 140). This protein recognizes the minimal peptidoglycan motif common to both classes of bacteria, muramyl dipeptide, corresponding to N-acetylmuramic acid-l-alanyl-d-isoglutamine (Fig. 2) (49). MDP is also the smallest unit of peptidoglycan capable of providing biological activities such as immunogenicity. MDP alone induces minimal TNF-α production, and the production of TNF-α induced by MDP is independent of CD14 or TLR2 (198, 215, 221), suggesting that another receptor is involved in the recognition of MDP. Furthermore, MDP binding sites were determined to be located within the intracellular compartment (180). Thus, it seems that Nod2 is the intracellular sensor for MDP, and it is therefore likely that Nod2 participates in the intracellular innate immune response to bacterial pathogens (50).
Since Nod1 and Nod2 are cytoplasmic proteins, the question has been raised whether or not bacteria such as S. aureus could be sensed by this class of pattern recognition receptors. Indeed, highly purified peptidoglycan does not induce cytokine production in epithelial cells expressing Nod2, suggesting that peptidoglycan must penetrate the cells to stimulate Nod proteins (57, 192). Although classically thought of as an extracellular bacterium, several observations indicate that S. aureus may also be an intracellular pathogen. S. aureus has been shown to be internalized by different mammalian cells (pulmonary epithelial cells, enterocytes, fibroblasts, endothelial cells, osteoblasts, and neutrophils) (6, 10, 11, 18, 55, 65, 70, 79, 119) in a manner dependent on the expression of the virulence regulators Agr and SarA (213). Intracellular staphylococci are sometimes present within vacuoles, but the majority appear to be free and replicating within the cytoplasm, having escaped from the endosome (70, 119). Agr is necessary for endosomal escape, probably by regulating the expression of membrane-active toxins (156, 172). Interestingly, neutrophils of patients with Crohn's disease show increased intracellular survival of S. aureus (29, 216). Thus, it is imaginable that peptidoglycan fragments from cytoplasm-dwelling bacteria could be available for recognition by Nod2 and initiate Nod-dependent cellular responses.
For the most part, however, S. aureus is classically considered an extracellular pathogen because it is found within the extracellular space during the course of a bacterial infection. Once within the host, the action of host and/or bacterial enzymes somewhat modifies the structure and composition of peptidoglycan and thereby can participate in Nod-dependent detection. On the host side, lytic enzymes such as lysozyme and amidases are known to degrade peptidoglycan polymers. The peptidoglycan of S. aureus, however, is resistant to lysozyme, and amidase digestion liberates fragments that are not detected by Nod2 (51). On the bacterial side, autolysins may contribute to staphylococcal lysis. Thus, MDP that is released when bacteria lyse can then be internalized into phagocytic cells and activate Nod2. Indeed, it has been shown that MDP is also able to be taken up by peptide transporters such as human PepT1 (196). It is also possible that phagocytic cells contain intracellular hydrolases capable of digesting bacterial peptidoglycan from phagocytosed bacteria and then releasing muropeptides active towards Nod2 into the cytosol.
Although the findings discussed here suggest that Nod2 should be capable of detecting S. aureus, it is not yet clear how this intracellular pattern recognition receptor contributes to innate immunity following infection by this organism. Studies of S. aureus infection of Nod2-deficient mice have yet to be described.
Structure of Nod2
The Nod family of cytoplasmic proteins presents a tripartite domain structure with an amino-terminal domain caspase-activating and recruitment domain (CARD), which is a protein-protein interaction domain, a central nuclear-binding site (NBS) domain, and a carboxy-terminal LRR domain (Fig. 4B). The CARD domain provides homophilic interactions with other molecules carrying these motifs, and its integrity is necessary for the activation of NF-κB (140). In contrast to Nod1, which has one CARD domain, Nod2 has two of these domains. The NBS domain, which mediates oligomerization of Nod proteins, includes consensus nucleotide-binding motifs: the P-loop, which is also found in ATP/GTPases, and the Mg2+-binding site (Fig. 4B) (23, 73). Similar to TLR, the LRR domain of Nod2 is involved in ligand sensing. Indeed, in the absence of the LRR domain, Nod2 is unresponsive to the bacterial ligand (23).
Nod Signaling
RIP2 pathway.
Expression of Nod2 protein in mammalian cells is sufficient to promote NF-κB activation (140). The LRR domain of Nod2 recognizes bacterial products. RIP2 (for receptor interacting protein 2, also known as RICK or CARDIAK), a CARD-containing protein kinase, is a common downstream signaling molecule (111). There is evidence that Nod interacts directly with RIP2 through homophilic CARD-CARD interactions (Fig. 5) (140). Furthermore, in RIP2-deficient embryonic fibroblasts, NF-κB activity is not induced by Nod ligands but is restored after addition of a RIP2 expression vector (88). A central region located between the CARD and the kinase domain of RIP2 associates with IKKγ (74). IKK is the IκB kinase complex composed of two catalytic subunits, IKKα and IKKβ, and a third regulator subunit, IKKγ (80). Interaction of RIP2 with this kinase complex appears to be sufficient for its activation, leading to the subsequent activation of NF-κB (Fig. 5) (140).
Interaction with the TLR pathway.
Another pathway involved in Nod signaling has recently been described. TAK1 is required for Nod2-induced NF-κB activation. Indeed, activation of NF-κB by Nod2 is inhibited by a dominant negative form of TAK1 (25). Furthermore, the Nod2 LRR domain appears to interact directly with TAK1 and to inhibit TAK1-induced NF-κB activation. The wild-type LRR of Nod2 is more efficient to suppress TAK1-induced NF-κB activation than the LRR with a 302insC mutation (Fig. 5) (25). For the moment, however, the role of TAK1 in Nod2 sensing of bacterial ligands has not been confirmed in in vivo studies.
Interestingly, RIP2-deficient cells show reduced cytokine production when TLR2 is stimulated by its ligand, suggesting that RIP2 is necessary for optimal signaling through TLR2 (Fig. 5) (88). Taken together, these results suggest that the Nod2 and TLR2 pathways may interact with each other and explain one aspect of Nod-TLR cross talk.
TNFR1
TNF-α receptor 1 (TNFR1) is a receptor for TNF-α that is widely expressed on the airway epithelium. An exciting recent study showed that protein A interacts directly with TNFR1. Protein A, which is a major surface protein of S. aureus strains, is covalently anchored to the peptidoglycan and belongs to the cell wall-associated virulence factors of S. aureus. Purified protein A elicits release of IL-1β, IL-4, IL-6, IL-8, and IFN-γ and weak release of TNF-α from monocytes and fibroblasts (149, 193). This virulence factor has also been shown to contribute to staphylococcal sepsis. Indeed, intravenous administration of protein A-deficient S. aureus to mice causes lower mortality than the wild-type strain (146).
It has been shown that TNFR1 recognizes S. aureus and its cell wall-associated protein A. Indeed, protein A binds to TNFR1 and reproduces the effects of TNF-α, the ligand of TNFR1. Activation of the TNFR1 pathway by protein A induces mobilization and shedding of TNFR1 into the extracellular compartment. It also induces IL-8 production and PMN recruitment through NF-κB activation (Fig. 5) (53). In the presence of a dominant-negative form of TLR2 and TLR4, protein A still induces NF-κB activation in airway epithelial cells, suggesting that TLR2 or TLR4 agonists do not contaminate protein A preparations (53). Challenge of TNFR1-deficient mice resulted in reduced pneumonia and mortality in a mouse pneumonia model of infection with wild-type S. aureus. Similarly, the absence of staphylococcal protein A also reduces pneumonia and mortality in the same model with wild-type mice (53). Moreover, protein A expression is induced in S. aureus isolated from patients with pulmonary infections (52). Thus, these results suggest that protein A may be involved in the pathophysiology of pneumonia caused by S. aureus by activating TNFR1 and inducing a strong inflammatory response characteristic of PMN infiltration that is deleterious to the host. Although molecular studies on the interaction of protein A with TNFR1 are still lacking, this study opens up the possibility of novel therapeutic strategies against staphylococcal disease.
PEPTIDOGLYCAN RECOGNITION PROTEINS
Peptidoglycan is an obvious target of the innate immune system since it is a structural cell wall molecule that is conserved among bacterial species and is not found in the host. Furthermore, it is essential for the survival of bacteria such as S. aureus (34).
Peptidoglycan recognition proteins (PGRPs) were isolated first in Drosophila melanogaster because they are able to interact with peptidoglycan with high affinity and are implicated in resistance to gram-positive bacteria (120). The family of peptidoglycan recognition proteins is conserved from insects to mammals. Peptidoglycan is detected in different compartments depending on the type of the mammalian PGRPs, which can be membrane bound, stored in vesicles, or secreted into the extracellular space. Four PGRPs are present in humans, PGRP-S, PGRP-L, PGRP-Iα, and PGRPIβ, each containing at least three conserved peptidoglycan-binding domains (34, 50, 104).
PGRP-S, the best-studied mammalian PGRP, is found in the neutrophil tertiary granules, attenuates the growth of gram-positive bacteria, and induces their intracellular killing (35, 103). PGRP-S-deficient mice that are intraperitoneally inoculated are more susceptible to infections with nonpathogen gram-positive bacteria such as B. subtilis, but they show no enhanced susceptibility to virulent gram-positive bacteria such as S. aureus (35). Thus, PGRP-S is probably another antibacterial protein present in PMNs that contributes to innate immune activity against low-virulence gram-positive bacteria. Surprisingly, however, it does not seem to act on S. aureus.
PGRP-L is the only PGRP that has the full complement of conserved amino acids necessary for enzyme activity; PGRP-L has N-acetylmuramoyl-l-alanine amidase activity (46, 209). The minimum peptidoglycan structure cleaved by PGRP-L is N-acetylmuramic acid tripeptide (209). PGRP-L resembles the serum amidase in its molecular mass and specificity of the substrate. Although PGRP-L is predicted to be a transmembrane protein, it has been found in the serum (104, 218). The serum amidase is present in the extracellular compartment but is also tissue bound. This suggests that PGRP-L is likely to be the serum/tissue amidase (34, 209).
Predigestion with lysozyme is required for mouse PGRP-L to effectively hydrolyze staphylococcal peptidoglycan (46). Amidases inactivate the proinflammatory activities of peptidoglycan by degrading its structure (68, 116). Thus, the combined action of lysozyme and PGRP-L might inactivate staphylococcal peptidoglycan and reduce peptidoglycan-induced inflammation. However, a recent study shows that no differences in mortality are observed between wild-type and PGRP-L-deficient mice challenged with S. aureus. Furthermore, S. aureus stimulation induces similar IL-6 and TNF-α production in PGRP-L-deficient and wild-type peritoneal macrophages (218). Therefore, these results suggest that PGRP-L probably plays a minor role in the innate immune response to S. aureus.
OTHER STAPHYLOCOCCAL COMPONENTS INVOLVED IN THE INFLAMMATORY RESPONSE
From the discussion presented above, it can be concluded that peptidoglycan and LTA are certainly important S. aureus components involved in the inflammatory response (Fig. 3). However, peptidoglycan from S. aureus causes cytokine production similar to that from B. subtilis and Curtobacterium flaccumfaciens, which are not pathogens. This suggests that the inflammatory property of peptidoglycan does not correlate with the pathogenicity of the bacteria (128, 131). Furthermore, several studies found a specificity between the bacterial species and the biological activity of LTA (31, 59, 83), whereas others do not observe any differences (45, 64, 144, 170). Thus, it is possible that peptidoglycan and LTA mainly act to enhance the response promoted by other staphylococcal products (131). Indeed, several other cytokine-stimulating cell components different from peptidoglycan and LTA have been described in staphylococcal species.
Hemolysins
Much less is known about the impact of virulence factors on the inflammatory response to S. aureus. We previously discussed the important role of cell wall-associated protein A in the inflammatory response. However, the overall in vivo effect of other virulence factors on the innate immune system is not clearly understood. S. aureus produces several secreted virulence factors, among which are hemolysins such as δ-hemolysin (see the section on PSM).
Alpha-toxin is another hemolysin secreted into the extracellular supernatant during the postexponential phase of growth. This toxin associates to form pores in the cell membrane, inducing lysis of several types of mammalian cells such as erythrocytes and monocytes. It is an important pathogenicity factor of S. aureus. At sublytic concentrations, S. aureus alpha-toxin can induce the production of IL-1β, IL-6, and IL-8, and a weak release of TNF-α (15, 33, 142). After intraperitoneal inoculation, alpha-toxin also elicits neutrophil recruitment into the mouse peritoneal cavity (142). Furthermore, it has been shown that IL-8 production induced by alpha-toxin is dependent on NF-κB (33).
β-Hemolysin is also secreted during the postexponential phase and is an Mg2+-dependent sphingomyelinase C. It degrades sphingomyelin present in the phospholipid layer of the cell membrane, lysing sheep erythrocytes and human monocytes, but it has no action against human granulocytes, fibroblasts, lymphocytes, or erythrocytes. In contrast to alpha-toxin, which stimulates inflammatory cytokine and chemokine production at sublytic doses by activating NF-κB, it seems that β-hemolysin provides an inflammatory response by lysing cells containing mediators. Indeed, cytolysis of monocytes induced by β-hemolysin releases IL-1β (206). To date, how these hemolysins induce an inflammatory response and which innate immune receptor they activate is not known (Fig. 3). However, as it is difficult to purify hemolysins without any contamination of inflammatory cell wall components such as lipopeptides or LTA, it is possible that these contaminants themselves rather than hemolysins cause cytokine induction (96).
Interestingly, PSM production has been shown to be upregulated by Agr (203). Agr also upregulates production of hemolysins such as alpha-toxin and β-hemolysin in S. aureus (129, 148). The culture supernatant from an agr mutant strain of S. epidermidis which does not contain either PSM or hemolysins does not induce TNF-α production, whereas the supernatant of the wild-type strain promotes significant cytokine production (203). These results suggest that the components that are present in staphylococcal culture supernatant and mediate cytokine production are regulated by Agr.
Formylated Peptides
Bacteria produce formylmethionyl peptides that are chemoattractants for neutrophils and macrophages (166). It has been shown that S. aureus releases formylated peptides into the culture supernatant (161). One of these peptides was purified and its amino acid composition was determined to be fMet-Ile-Leu-Phe. This formylated peptide can inhibit the ability of a labeled formylmethionyl chemoattractant to bind human monocytes, suggesting that this peptide may bind to the formylated peptide receptor (Fig. 3) (162). S. aureus produces δ-hemolysin in both formylated and deformylated forms. Interestingly, only the formylated form, which represents 90% of the δ-hemolysin present in the culture supernatant, is able to elicit migration of PMNs (175). Thus, formylated peptides are important inflammatory mediators, although the exact nature of their innate immune receptor is not formally established.
Staphylococcal DNA
Bacterial DNA has inflammatory properties (191). Indeed, it is able to induce the production of IL-6, IL-12, IFN-γ, and TNF-α (92, 136). Staphylococcal DNA causes arthritis (32). Furthermore, injection of staphylococcal DNA in a mouse model of cutaneous inflammation causes a strong inflammatory response (32, 123). These results suggest that staphylococcal DNA may be an important inflammatory molecule of S. aureus. Staphylococcal DNA has been shown to induce the production of TNF-α, IL-12, and IFN-γ, although its inflammatory effect is lower than that of E. coli (136, 177, 220). The inflammatory property of bacterial DNA is abolished by treatment with DNase but not RNase, confirming the deoxynucleotide nature of the stimulus. The inflammatory activity of bacterial DNA results from cytosine-phosphate-guanosine (CpG) motifs that are unmethylated at cytosine residues. In contrast, eukaryotic genomic DNA, which is not inflammatory, is mostly methylated and has less CpG than the bacterial genome (92, 220).
TLR9 is a key receptor for bacterial DNA. Indeed, it has been shown that activation of B cells in TLR9-deficient mice is abolished when stimulated by CpG (Fig. 3) (62). TLR9 requires an adaptor protein such as MyD88 and promotes mitogen-activated protein kinase and NF-κB activation. Interestingly, TLR9 is present in the intracellular endosomal compartment, whereas TLR2 is anchored to the cell surface (Fig. 3) (92, 204). Although DNA is an important inflammatory component of S. aureus, the role of TLR9 in staphylococcal infections is still not clear and awaits further study in S. aureus-challenged TLR9-deficient mice.
CONCLUDING REMARKS
It is clear that a large number of staphylococcal molecules (and probably many more than those that have been discovered so far) interact with the innate immune system to induce cytokine production and the inflammatory response, suggesting a complex interaction between Staphylococcus and eukaryotic cells. Although much work has been conducted with peptidoglycan and LTA on the level of responses to TLR2 and Nod2, much less is known about the impact of virulence factors such as hemolysins and adhesins on the inflammatory response to S. aureus. Indeed, the overall in vivo effect of these proteins on the innate immune system needs to be studied further. Furthermore, TLR2, Nod2, and TNFR1 probably have a key role in the inflammatory response to S. aureus since these receptors are necessary for full responses to S. aureus or its inflammatory components. However, other less-studied receptors such as TLR9 and formylated peptide receptor may also contribute to the activation of NF-κB and production of cytokines in response to staphylococcal infection.
Acknowledgments
We thank Ivo G. Boneca, Minou Adib-Conquy, and André Klier for critical reading of the manuscript.
REFERENCES
- 1.Abachin, E., C. Poyart, E. Pellegrini, E. Milohanic, F. Fiedler, P. Berche, and P. Trieu-Cuot. 2002. Formation of d-alanyl-lipoteichoic acid is required for adhesion and virulence of Listeria monocytogenes. Mol. Microbiol. 43:1-14. [DOI] [PubMed] [Google Scholar]
- 2.Abraham, E. 2002. Anti-cytokine therapy, p. 719-728. In J. L. Vincent, J. Carlet, and S. M. Opal (ed.), The sepsis text. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 3.Aderem, A., and R. J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406:782-787. [DOI] [PubMed] [Google Scholar]
- 4.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 5.Alberti, C., C. Brun-Buisson, S. V. Goodman, D. Guidici, J. Granton, R. Moreno, M. Smithies, O. Thomas, A. Artigas, and J. R. Le Gall. 2003. Influence of systemic inflammatory response syndrome and sepsis on outcome of critically ill infected patients. Am. J. Respir. Crit. Care Med. 168:77-84. [DOI] [PubMed] [Google Scholar]
- 6.Almeida, R. A., K. R. Matthews, E. Cifrian, A. J. Guidry, and S. P. Oliver. 1996. Staphylococcus aureus invasion of bovine mammary epithelial cells. J. Dairy Sci. 79:1021-1026. [DOI] [PubMed] [Google Scholar]
- 7.Anderson, K. V. 2000. Toll signaling pathways in the innate immune response. Curr. Opin. Immunol. 12:13-19. [DOI] [PubMed] [Google Scholar]
- 8.Arbibe, L., J. P. Mira, N. Teusch, L. Kline, M. Guha, N. Mackman, P. J. Godowski, R. J. Ulevitch, and U. G. Knaus. 2000. Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat. Immunol. 1:533-540. [DOI] [PubMed] [Google Scholar]
- 9.Athman, R., and D. Philpott. 2004. Innate immunity via Toll-like receptors and Nod proteins. Curr. Opin. Microbiol. 7:25-32. [DOI] [PubMed] [Google Scholar]
- 10.Bayles, K. W., C. A. Wesson, L. E. Liou, L. K. Fox, G. A. Bohach, and W. R. Trumble. 1998. Intracellular Staphylococcus aureus escapes the endosome and induces apoptosis in epithelial cells. Infect. Immun. 66:336-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beekhuizen, H., J. S. van de Gevel, B. Olsson, I. J. van Benten, and R. van Furth. 1997. Infection of human vascular endothelial cells with Staphylococcus aureus induces hyperadhesiveness for human monocytes and granulocytes. J. Immunol. 158:774-782. [PubMed] [Google Scholar]
- 12.Bell, J. K., G. E. Mullen, C. A. Leifer, A. Mazzoni, D. R. Davies, and D. M. Segal. 2003. Leucine-rich repeats and pathogen recognition in Toll-like receptors. Trends Immunol. 24:528-533. [DOI] [PubMed] [Google Scholar]
- 13.Bera, A., S. Herbert, A. Jakob, W. Vollmer, and F. Gotz. 2005. Why are pathogenic staphylococci so lysozyme resistant? The peptidoglycan O-acetyltransferase OatA is the major determinant for lysozyme resistance of Staphylococcus aureus. Mol. Microbiol. 55:778-787. [DOI] [PubMed] [Google Scholar]
- 14.Bhakdi, S., T. Klonisch, P. Nuber, and W. Fischer. 1991. Stimulation of monokine production by lipoteichoic acids. Infect. Immun. 59:4614-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhakdi, S., M. Muhly, S. Korom, and F. Hugo. 1989. Release of interleukin-1 beta associated with potent cytocidal action of staphylococcal alpha-toxin on human monocytes. Infect. Immun. 57:3512-3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bochud, P. Y., T. R. Hawn, and A. Aderem. 2003. Cutting edge: a Toll-like receptor 2 polymorphism that is associated with lepromatous leprosy is unable to mediate mycobacterial signaling. J. Immunol. 170:3451-3454. [DOI] [PubMed] [Google Scholar]
- 17.Bone, R. C. 1994. Gram-positive organisms and sepsis. Arch. Intern. Med. 154:26-34. [PubMed] [Google Scholar]
- 18.Buggy, B. P., D. R. Schaberg, and R. D. Swartz. 1984. Intraleukocytic sequestration as a cause of persistent Staphylococcus aureus peritonitis in continuous ambulatory peritoneal dialysis. Am. J. Med. 76:1035-1040. [DOI] [PubMed] [Google Scholar]
- 19.Bulut, Y., E. Faure, L. Thomas, O. Equils, and M. Arditi. 2001. Cooperation of Toll-like receptor 2 and 6 for cellular activation by soluble tuberculosis factor and Borrelia burgdorferi outer surface protein A lipoprotein: role of Toll-interacting protein and IL-1 receptor signaling molecules in Toll-like receptor 2 signaling. J. Immunol. 167:987-994. [DOI] [PubMed] [Google Scholar]
- 20.Burns, K., J. Clatworthy, L. Martin, F. Martinon, C. Plumpton, B. Maschera, A. Lewis, K. Ray, J. Tschopp, and F. Volpe. 2000. Tollip, a new component of the IL-1RI pathway, links IRAK to the IL-1 receptor. Nat. Cell Biol. 2:346-351. [DOI] [PubMed] [Google Scholar]
- 21.Carl, V. S., K. Brown-Steinke, M. J. Nicklin, and M. F. Smith, Jr. 2002. Toll-like receptor 2 and 4 (TLR2 and TLR4) agonists differentially regulate secretory interleukin-1 receptor antagonist gene expression in macrophages. J. Biol. Chem. 277:17448-17456. [DOI] [PubMed] [Google Scholar]
- 22.Cavaillon, J. M. 2003. Proinflammatory and anti-inflammatory cytokines as mediators of Gram-negative sepsis, p. 33-58. In M. Kotb and T. Calandra (ed.), Cytokines and chemokines in infectious diseases handbook. Humana press, Totowa, N.J.
- 23.Chamaillard, M., S. E. Girardin, J. Viala, and D. J. Philpott. 2003. Nods, Nalps and Naip: intracellular regulators of bacterial-induced inflammation. Cell Microbiol. 5:581-592. [DOI] [PubMed] [Google Scholar]
- 24.Chamaillard, M., M. Hashimoto, Y. Horie, J. Masumoto, S. Qiu, L. Saab, Y. Ogura, A. Kawasaki, K. Fukase, S. Kusumoto, M. A. Valvano, S. J. Foster, T. W. Mak, G. Nunez, and N. Inohara. 2003. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 4:702-707. [DOI] [PubMed] [Google Scholar]
- 25.Chen, C. M., Y. Gong, M. Zhang, and J. J. Chen. 2004. Reciprocal cross-talk between Nod2 and TAK1 signaling pathways. J. Biol. Chem. 279:25876-25882. [DOI] [PubMed] [Google Scholar]
- 26.Cheung, A. L., J. M. Koomey, C. A. Butler, S. J. Projan, and V. A. Fischetti. 1992. Regulation of exoprotein expression in Staphylococcus aureus by a locus (sar) distinct from agr. Proc. Natl. Acad. Sci. USA 89:6462-6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cleveland, M. G., J. D. Gorham, T. L. Murphy, E. Tuomanen, and K. M. Murphy. 1996. Lipoteichoic acid preparations of gram-positive bacteria induce interleukin-12 through a CD14-dependent pathway. Infect. Immun. 64:1906-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collins, L. V., S. A. Kristian, C. Weidenmaier, M. Faigle, K. P. Van Kessel, J. A. Van Strijp, F. Gotz, B. Neumeister, and A. Peschel. 2002. Staphylococcus aureus strains lacking d-alanine modifications of teichoic acids are highly susceptible to human neutrophil killing and are virulence attenuated in mice. J. Infect. Dis. 186:214-219. [DOI] [PubMed] [Google Scholar]
- 29.Couper, R., J. Kapelushnik, and A. M. Griffiths. 1991. Neutrophil dysfunction in glycogen storage disease Ib: association with Crohn's-like colitis. Gastroenterology 100:549-554. [DOI] [PubMed] [Google Scholar]
- 30.Danforth, J. M., R. M. Strieter, S. L. Kunkel, D. A. Arenberg, G. M. VanOtteren, and T. J. Standiford. 1995. Macrophage inflammatory protein-1 alpha expression in vivo and in vitro: the role of lipoteichoic acid. Clin. Immunol. Immunopathol. 74:77-83. [DOI] [PubMed] [Google Scholar]
- 31.De Kimpe, S. J., M. Kengatharan, C. Thiemermann, and J. R. Vane. 1995. The cell wall components peptidoglycan and lipoteichoic acid from Staphylococcus aureus act in synergy to cause shock and multiple organ failure. Proc. Natl. Acad. Sci. USA 92:10359-10363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deng, G. M., I. M. Nilsson, M. Verdrengh, L. V. Collins, and A. Tarkowski. 1999. Intra-articularly localized bacterial DNA containing CpG motifs induces arthritis. Nat. Med. 5:702-705. [DOI] [PubMed] [Google Scholar]
- 33.Dragneva, Y., C. D. Anuradha, A. Valeva, A. Hoffmann, S. Bhakdi, and M. Husmann. 2001. Subcytocidal attack by staphylococcal alpha-toxin activates NF-κB and induces interleukin-8 production. Infect. Immun. 69:2630-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dziarski, R. 2004. Peptidoglycan recognition proteins (PGRPs). Mol. Immunol. 40:877-886. [DOI] [PubMed] [Google Scholar]
- 35.Dziarski, R., K. A. Platt, E. Gelius, H. Steiner, and D. Gupta. 2003. Defect in neutrophil killing and increased susceptibility to infection with nonpathogenic gram-positive bacteria in peptidoglycan recognition protein-S (PGRP-S)-deficient mice. Blood 102:689-697. [DOI] [PubMed] [Google Scholar]
- 36.Dziarski, R., R. I. Tapping, and P. S. Tobias. 1998. Binding of bacterial peptidoglycan to CD14. J. Biol. Chem. 273:8680-8690. [DOI] [PubMed] [Google Scholar]
- 37.Dziarski, R., Q. Wang, K. Miyake, C. J. Kirschning, and D. Gupta. 2001. MD-2 enables Toll-like receptor 2 (TLR2)-mediated responses to lipopolysaccharide and enhances TLR2-mediated responses to Gram-positive and Gram-negative bacteria and their cell wall components. J. Immunol. 166:1938-1944. [DOI] [PubMed] [Google Scholar]
- 38.Esen, N., F. Y. Tanga, J. A. DeLeo, and T. Kielian. 2004. Toll-like receptor 2 (TLR2) mediates astrocyte activation in response to the Gram-positive bacterium Staphylococcus aureus. J. Neurochem. 88:746-758. [DOI] [PubMed] [Google Scholar]
- 39.Fischer, W. 1994. Lipoteichoic acid and lipids in the membrane of Staphylococcus aureus. Med. Microbiol. Immunol. (Berlin) 183:61-76. [DOI] [PubMed] [Google Scholar]
- 40.Fischer, W. 1988. Physiology of lipoteichoic acids in bacteria. Adv. Microb. Physiol. 29:233-302. [DOI] [PubMed] [Google Scholar]
- 41.Fluit, A. C., F. J. Schmitz, and J. Verhoef. 2001. Frequency of isolation of pathogens from bloodstream, nosocomial pneumonia, skin and soft tissue, and urinary tract infections occurring in European patients. Eur. J. Clin. Microbiol. Infect. Dis. 20:188-191. [DOI] [PubMed] [Google Scholar]
- 42.Fournier, B., A. Klier, and G. Rapoport. 2001. The two-component system ArlS-ArlR is a regulator of virulence gene expression in Staphylococcus aureus. Mol. Microbiol. 41:247-261. [DOI] [PubMed] [Google Scholar]
- 43.Freudenberg, M. A., and C. Galanos. 1991. Tumor necrosis factor alpha mediates lethal activity of killed gram-negative and gram-positive bacteria in d-galactosamine-treated mice. Infect. Immun. 59:2110-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujita, M., T. Into, M. Yasuda, T. Okusawa, S. Hamahira, Y. Kuroki, A. Eto, T. Nisizawa, M. Morita, and K. Shibata. 2003. Involvement of leucine residues at positions 107, 112, and 115 in a leucine-rich repeat motif of human Toll-like receptor 2 in the recognition of diacylated lipoproteins and lipopeptides and Staphylococcus aureus peptidoglycans. J. Immunol. 171:3675-3683. [DOI] [PubMed] [Google Scholar]
- 45.Gao, J. J., Q. Xue, E. G. Zuvanich, K. R. Haghi, and D. C. Morrison. 2001. Commercial preparations of lipoteichoic acid contain endotoxin that contributes to activation of mouse macrophages in vitro. Infect. Immun. 69:751-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gelius, E., C. Persson, J. Karlsson, and H. Steiner. 2003. A mammalian peptidoglycan recognition protein with N-acetylmuramoyl-l-alanine amidase activity. Biochem. Biophys. Res. Commun. 306:988-994. [DOI] [PubMed] [Google Scholar]
- 47.Ginsburg, I. 2002. Role of lipoteichoic acid in infection and inflammation. Lancet Infect. Dis. 2:171-179. [DOI] [PubMed] [Google Scholar]
- 48.Girardin, S. E., I. G. Boneca, L. A. Carneiro, A. Antignac, M. Jehanno, J. Viala, K. Tedin, M. K. Taha, A. Labigne, U. Zahringer, A. J. Coyle, P. S. DiStefano, J. Bertin, P. J. Sansonetti, and D. J. Philpott. 2003. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science 300:1584-1587. [DOI] [PubMed] [Google Scholar]
- 49.Girardin, S. E., I. G. Boneca, J. Viala, M. Chamaillard, A. Labigne, G. Thomas, D. J. Philpott, and P. J. Sansonetti. 2003. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 278:8869-8872. [DOI] [PubMed] [Google Scholar]
- 50.Girardin, S. E., and D. J. Philpott. 2004. Mini-review: the role of peptidoglycan recognition in innate immunity. Eur. J. Immunol. 34:1777-1782. [DOI] [PubMed] [Google Scholar]
- 51.Girardin, S. E., L. H. Travassos, M. Herve, D. Blanot, I. G. Boneca, D. J. Philpott, P. J. Sansonetti, and D. Mengin-Lecreulx. 2003. Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J. Biol. Chem. 278:41702-41708. [DOI] [PubMed] [Google Scholar]
- 52.Goerke, C., S. Campana, M. G. Bayer, G. Doring, K. Botzenhart, and C. Wolz. 2000. Direct quantitative transcript analysis of the agr regulon of Staphylococcus aureus during human infection in comparison to the expression profile in vitro. Infect. Immun. 68:1304-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gomez, M. I., A. Lee, B. Reddy, A. Muir, G. Soong, A. Pitt, A. Cheung, and A. Prince. 2004. Staphylococcus aureus protein A induces airway epithelial inflammatory responses by activating TNFR1. Nat. Med. 10:842-848. [DOI] [PubMed] [Google Scholar]
- 54.Gonzalez-Zorn, B., J. P. Senna, L. Fiette, S. Shorte, A. Testard, M. Chignard, P. Courvalin, and C. Grillot-Courvalin. 2005. Bacterial and host factors implicated in nasal carriage of methicillin-resistant Staphylococcus aureus in mice. Infect. Immun. 73:1847-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gresham, H. D., J. H. Lowrance, T. E. Caver, B. S. Wilson, A. L. Cheung, and F. P. Lindberg. 2000. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J. Immunol. 164:3713-3722. [DOI] [PubMed] [Google Scholar]
- 56.Gupta, D., T. N. Kirkland, S. Viriyakosol, and R. Dziarski. 1996. CD14 is a cell-activating receptor for bacterial peptidoglycan. J. Biol. Chem. 271:23310-23316. [DOI] [PubMed] [Google Scholar]
- 57.Gutierrez, O., C. Pipaon, N. Inohara, A. Fontalba, Y. Ogura, F. Prosper, G. Nunez, and J. L. Fernandez-Luna. 2002. Induction of Nod2 in myelomonocytic and intestinal epithelial cells via nuclear factor-kappa B activation. J. Biol. Chem. 277:41701-41705. [DOI] [PubMed] [Google Scholar]
- 58.Hajjar, A. M., D. S. O'Mahony, A. Ozinsky, D. M. Underhill, A. Aderem, S. J. Klebanoff, and C. B. Wilson. 2001. Cutting edge: functional interactions between toll-like receptor (TLR) 2 and TLR1 or TLR6 in response to phenol-soluble modulin. J. Immunol. 166:15-19. [DOI] [PubMed] [Google Scholar]
- 59.Han, S. H., J. H. Kim, M. Martin, S. M. Michalek, and M. H. Nahm. 2003. Pneumococcal lipoteichoic acid (LTA) is not as potent as staphylococcal LTA in stimulating Toll-like receptor 2. Infect. Immun. 71:5541-5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haziot, A., N. Hijiya, K. Schultz, F. Zhang, S. C. Gangloff, and S. M. Goyert. 1999. CD14 plays no major role in shock induced by Staphylococcus aureus but down-regulates TNF-alpha production. J. Immunol. 162:4801-4805. [PubMed] [Google Scholar]
- 61.Heine, H., C. J. Kirschning, E. Lien, B. G. Monks, M. Rothe, and D. T. Golenbock. 1999. Cutting edge: cells that carry A null allele for toll-like receptor 2 are capable of responding to endotoxin. J. Immunol. 162:6971-6975. [PubMed] [Google Scholar]
- 62.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 63.Henderson, B., S. Poole, and M. Wilson. 1996. Bacterial modulins: a novel class of virulence factors which cause host tissue pathology by inducing cytokine synthesis. Microbiol. Rev. 60:316-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hermann, C., I. Spreitzer, N. W. Schroder, S. Morath, M. D. Lehner, W. Fischer, C. Schutt, R. R. Schumann, and T. Hartung. 2002. Cytokine induction by purified lipoteichoic acids from various bacterial species-role of LBP, sCD14, CD14 and failure to induce IL-12 and subsequent IFN-gamma release. Eur. J. Immunol. 32:541-551. [DOI] [PubMed] [Google Scholar]
- 65.Hess, D. J., M. J. Henry-Stanley, E. A. Erickson, and C. L. Wells. 2003. Intracellular survival of Staphylococcus aureus within cultured enterocytes. J. Surg. Res. 114:42-49. [DOI] [PubMed] [Google Scholar]
- 66.Heumann, D., C. Barras, A. Severin, M. P. Glauser, and A. Tomasz. 1994. Gram-positive cell walls stimulate synthesis of tumor necrosis factor alpha and interleukin-6 by human monocytes. Infect. Immun. 62:2715-2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hoebe, K., P. Georgel, S. Rutschmann, X. Du, S. Mudd, K. Crozat, S. Sovath, L. Shamel, T. Hartung, U. Zahringer, and B. Beutler. 2005. CD36 is a sensor of diacylglycerides. Nature 433:523-527. [DOI] [PubMed] [Google Scholar]
- 68.Hoijer, M. A., M. J. Melief, R. Debets, and M. P. Hazenberg. 1997. Inflammatory properties of peptidoglycan are decreased after degradation by human N-acetylmuramyl-l-alanine amidase. Eur. Cytokine Netw. 8:375-381. [PubMed] [Google Scholar]
- 69.Horng, T., G. M. Barton, R. A. Flavell, and R. Medzhitov. 2002. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature 420:329-333. [DOI] [PubMed] [Google Scholar]
- 70.Hudson, M. C., W. K. Ramp, N. C. Nicholson, A. S. Williams, and M. T. Nousiainen. 1995. Internalization of Staphylococcus aureus by cultured osteoblasts. Microb. Pathog. 19:409-419. [DOI] [PubMed] [Google Scholar]
- 71.Hugot, J. P., M. Chamaillard, H. Zouali, S. Lesage, J. P. Cezard, J. Belaiche, S. Almer, C. Tysk, C. A. O'Morain, M. Gassull, V. Binder, Y. Finkel, A. Cortot, R. Modigliani, P. Laurent-Puig, C. Gower-Rousseau, J. Macry, J. F. Colombel, M. Sahbatou, and G. Thomas. 2001. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 411:599-603. [DOI] [PubMed] [Google Scholar]
- 72.Hultgren, O. H., M. Stenson, and A. Tarkowski. 2001. Role of IL-12 in Staphylococcus aureus-triggered arthritis and sepsis. Arthritis Res. 3:41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Inohara, N., T. Koseki, L. del Peso, Y. Hu, C. Yee, S. Chen, R. Carrio, J. Merino, D. Liu, J. Ni, and G. Nunez. 1999. Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-κB. J. Biol. Chem. 274:14560-14567. [DOI] [PubMed] [Google Scholar]
- 74.Inohara, N., T. Koseki, J. Lin, L. del Peso, P. C. Lucas, F. F. Chen, Y. Ogura, and G. Nunez. 2000. An induced proximity model for NF-kappa B activation in the Nod1/RICK and RIP signaling pathways. J. Biol. Chem. 275:27823-27831. [DOI] [PubMed] [Google Scholar]
- 75.Inohara, N., Y. Ogura, A. Fontalba, O. Gutierrez, F. Pons, J. Crespo, K. Fukase, S. Inamura, S. Kusumoto, M. Hashimoto, S. J. Foster, A. P. Moran, J. L. Fernandez-Luna, and G. Nunez. 2003. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J. Biol. Chem. 278:5509-5512. [DOI] [PubMed] [Google Scholar]
- 76.Iwaki, D., H. Mitsuzawa, S. Murakami, H. Sano, M. Konishi, T. Akino, and Y. Kuroki. 2002. The extracellular toll-like receptor 2 domain directly binds peptidoglycan derived from Staphylococcus aureus. J. Biol. Chem. 277:24315-24320. [DOI] [PubMed] [Google Scholar]
- 77.Janeway, C. A., Jr. 1992. The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol. Today 13:11-16. [DOI] [PubMed] [Google Scholar]
- 78.Jorgensen, P. F., J. E. Wang, M. Almlof, C. Thiemermann, S. J. Foster, R. Solberg, and A. O. Aasen. 2001. Peptidoglycan and lipoteichoic acid modify monocyte phenotype in human whole blood. Clin. Diagn. Lab. Immunol. 8:515-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kahl, B. C., M. Goulian, W. van Wamel, M. Herrmann, S. M. Simon, G. Kaplan, G. Peters, and A. L. Cheung. 2000. Staphylococcus aureus RN6390 replicates and induces apoptosis in a pulmonary epithelial cell line. Infect. Immun. 68:5385-5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karin, M. 1999. The beginning of the end: IkappaB kinase (IKK) and NF-κB activation. J. Biol. Chem. 274:27339-27342. [DOI] [PubMed] [Google Scholar]
- 81.Keller, R., W. Fischer, R. Keist, and S. Bassetti. 1992. Macrophage response to bacteria: induction of marked secretory and cellular activities by lipoteichoic acids. Infect. Immun. 60:3664-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kelsall, B. 2005. Getting to the guts of NOD2. Nat. Med. 11:383-384. [DOI] [PubMed] [Google Scholar]
- 83.Kengatharan, K. M., S. De Kimpe, C. Robson, S. J. Foster, and C. Thiemermann. 1998. Mechanism of gram-positive shock: identification of peptidoglycan and lipoteichoic acid moieties essential in the induction of nitric oxide synthase, shock, and multiple organ failure. J. Exp. Med. 188:305-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kirschning, C. J., and R. R. Schumann. 2002. TLR2: cellular sensor for microbial and endogenous molecular patterns. Curr. Top. Microbiol. Immunol. 270:121-144. [DOI] [PubMed] [Google Scholar]
- 85.Klebanoff, S. J., F. Kazazi, W. C. Van Voorhis, and K. G. Schlechte. 1994. Activation of the human immunodeficiency virus long terminal repeat in THP-1 cells by a staphylococcal extracellular product. Proc. Natl. Acad. Sci. USA 91:10615-10619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kluytmans, J., A. van Belkum, and H. Verbrugh. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 10:505-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Knuefermann, P., Y. Sakata, J. S. Baker, C. H. Huang, K. Sekiguchi, H. S. Hardarson, O. Takeuchi, S. Akira, and J. G. Vallejo. 2004. Toll-like receptor 2 mediates Staphylococcus aureus-induced myocardial dysfunction and cytokine production in the heart. Circulation 110:3693-3698. [DOI] [PubMed] [Google Scholar]
- 88.Kobayashi, K., N. Inohara, L. D. Hernandez, J. E. Galan, G. Nunez, C. A. Janeway, R. Medzhitov, and R. A. Flavell. 2002. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature 416:194-199. [DOI] [PubMed] [Google Scholar]
- 89.Kobayashi, K. S., M. Chamaillard, Y. Ogura, O. Henegariu, N. Inohara, G. Nunez, and R. A. Flavell. 2005. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307:731-734. [DOI] [PubMed] [Google Scholar]
- 90.Koch, H. U., R. Doker, and W. Fischer. 1985. Maintenance of d-alanine ester substitution of lipoteichoic acid by reesterification in Staphylococcus aureus. J. Bacteriol. 164:1211-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kolb-Maurer, A., U. Kammerer, M. Maurer, I. Gentschev, E. B. Brocker, P. Rieckmann, and E. Kampgen. 2003. Production of IL-12 and IL-18 in human dendritic cells upon infection by Listeria monocytogenes. FEMS Immunol. Med. Microbiol. 35:255-262. [DOI] [PubMed] [Google Scholar]
- 92.Krieg, A. M. 2002. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 20:709-760. [DOI] [PubMed] [Google Scholar]
- 93.Kristian, S. A., X. Lauth, V. Nizet, F. Goetz, B. Neumeister, A. Peschel, and R. Landmann. 2003. Alanylation of teichoic acids protects Staphylococcus aureus against Toll-like receptor 2-dependent host defense in a mouse tissue cage infection model. J. Infect. Dis. 188:414-423. [DOI] [PubMed] [Google Scholar]
- 94.Kusunoki, T., E. Hailman, T. S. Juan, H. S. Lichenstein, and S. D. Wright. 1995. Molecules from Staphylococcus aureus that bind CD14 and stimulate innate immune responses. J. Exp. Med. 182:1673-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Labischinski, H., and H. Maidhof. 1994. Bacterial peptidoglycan: overview and evolving concepts, p. 23-38. In J.-M. Ghuysen and R. Hakenbeck (ed.), Bacterial cell wall. Elsevier, Amsterdam, The Netherlands.
- 96.Lee, H. K., J. Lee, and P. S. Tobias. 2002. Two lipoproteins extracted from Escherichia coli K-12 LCD25 lipopolysaccharide are the major components responsible for Toll-like receptor 2-mediated signaling. J. Immunol. 168:4012-4017. [DOI] [PubMed] [Google Scholar]
- 97.Leemans, J. C., M. Heikens, K. P. van Kessel, S. Florquin, and T. van der Poll. 2003. Lipoteichoic acid and peptidoglycan from Staphylococcus aureus synergistically induce neutrophil influx into the lungs of mice. Clin. Diagn. Lab. Immunol. 10:950-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lehner, M. D., S. Morath, K. S. Michelsen, R. R. Schumann, and T. Hartung. 2001. Induction of cross-tolerance by lipopolysaccharide and highly purified lipoteichoic acid via different Toll-like receptors independent of paracrine mediators. J. Immunol. 166:5161-5167. [DOI] [PubMed] [Google Scholar]
- 99.Lemaitre, B., E. Nicolas, L. Michaut, J. M. Reichhart, and J. A. Hoffmann. 1996. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86:973-983. [DOI] [PubMed] [Google Scholar]
- 100.Lembo, A., C. Kalis, C. J. Kirschning, V. Mitolo, E. Jirillo, H. Wagner, C. Galanos, and M. A. Freudenberg. 2003. Differential contribution of Toll-like receptors 4 and 2 to the cytokine response to Salmonella enterica serovar Typhimurium and Staphylococcus aureus in mice. Infect. Immun. 71:6058-6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lien, E., T. J. Sellati, A. Yoshimura, T. H. Flo, G. Rawadi, R. W. Finberg, J. D. Carroll, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419-33425. [DOI] [PubMed] [Google Scholar]
- 102.Liles, W. C., A. R. Thomsen, D. S. O'Mahony, and S. J. Klebanoff. 2001. Stimulation of human neutrophils and monocytes by staphylococcal phenol-soluble modulin. J. Leukoc. Biol. 70:96-102. [PubMed] [Google Scholar]
- 103.Liu, C., E. Gelius, G. Liu, H. Steiner, and R. Dziarski. 2000. Mammalian peptidoglycan recognition protein binds peptidoglycan with high affinity, is expressed in neutrophils, and inhibits bacterial growth. J. Biol. Chem. 275:24490-24499. [DOI] [PubMed] [Google Scholar]
- 104.Liu, C., Z. Xu, D. Gupta, and R. Dziarski. 2001. Peptidoglycan recognition proteins: a novel family of four human innate immunity pattern recognition molecules. J. Biol. Chem. 276:34686-34694. [DOI] [PubMed] [Google Scholar]
- 105.Lorenz, E., J. P. Mira, K. L. Cornish, N. C. Arbour, and D. A. Schwartz. 2000. A novel polymorphism in the toll-like receptor 2 gene and its potential association with staphylococcal infection. Infect. Immun. 68:6398-6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 107.Maeda, S., L. C. Hsu, H. Liu, L. A. Bankston, M. Iimura, M. F. Kagnoff, L. Eckmann, and M. Karin. 2005. Nod2 mutation in Crohn's disease potentiates NF-κB activity and IL-1beta processing. Science 307:734-738. [DOI] [PubMed] [Google Scholar]
- 108.Majcherczyk, P. A., E. Rubli, D. Heumann, M. P. Glauser, and P. Moreillon. 2003. Teichoic acids are not required for Streptococcus pneumoniae and Staphylococcus aureus cell walls to trigger the release of tumor necrosis factor by peripheral blood monocytes. Infect. Immun. 71:3707-3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mantovani, A., A. Sica, S. Sozzani, P. Allavena, A. Vecchi, and M. Locati. 2004. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25:677-686. [DOI] [PubMed] [Google Scholar]
- 110.Mattsson, E., L. Verhage, J. Rollof, A. Fleer, J. Verhoef, and H. van Dijk. 1993. Peptidoglycan and teichoic acid from Staphylococcus epidermidis stimulate human monocytes to release tumour necrosis factor-alpha, interleukin-1 beta and interleukin-6. FEMS Immunol. Med. Microbiol. 7:281-287. [DOI] [PubMed] [Google Scholar]
- 111.McCarthy, J. V., J. Ni, and V. M. Dixit. 1998. RIP2 is a novel NF-κB-activating and cell death-inducing kinase. J. Biol. Chem. 273:16968-16975. [DOI] [PubMed] [Google Scholar]
- 112.McCurdy, J. D., T. J. Olynych, L. H. Maher, and J. S. Marshall. 2003. Cutting edge: distinct Toll-like receptor 2 activators selectively induce different classes of mediator production from human mast cells. J. Immunol. 170:1625-1629. [DOI] [PubMed] [Google Scholar]
- 113.Medzhitov, R., and C. Janeway, Jr. 2000. The Toll receptor family and microbial recognition. Trends Microbiol. 8:452-456. [DOI] [PubMed] [Google Scholar]
- 114.Medzhitov, R., P. Preston-Hurlburt, and C. A. Janeway, Jr. 1997. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388:394-397. [DOI] [PubMed] [Google Scholar]
- 115.Mehlin, C., C. M. Headley, and S. J. Klebanoff. 1999. An inflammatory polypeptide complex from Staphylococcus epidermidis: isolation and characterization. J. Exp. Med. 189:907-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mellroth, P., J. Karlsson, and H. Steiner. 2003. A scavenger function for a Drosophila peptidoglycan recognition protein. J. Biol. Chem. 278:7059-7064. [DOI] [PubMed] [Google Scholar]
- 117.Melmed, G., L. S. Thomas, N. Lee, S. Y. Tesfay, K. Lukasek, K. S. Michelsen, Y. Zhou, B. Hu, M. Arditi, and M. T. Abreu. 2003. Hum. intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: implications for host-microbial interactions in the gut. J. Immunol. 170:1406-1415. [DOI] [PubMed] [Google Scholar]
- 118.Meng, G., A. Grabiec, M. Vallon, B. Ebe, S. Hampel, W. Bessler, H. Wagner, and C. J. Kirschning. 2003. Cellular recognition of tri-/di-palmitoylated peptides is independent from a domain encompassing the N-terminal seven leucine-rich repeat (LRR)/LRR-like motifs of TLR2. J. Biol. Chem. 278:39822-39829. [DOI] [PubMed] [Google Scholar]
- 119.Menzies, B. E., and I. Kourteva. 1998. Internalization of Staphylococcus aureus by endothelial cells induces apoptosis. Infect. Immun. 66:5994-5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Michel, T., J. M. Reichhart, J. A. Hoffmann, and J. Royet. 2001. Drosophila Toll is activated by Gram-positive bacteria through a circulating peptidoglycan recognition protein. Nature 414:756-759. [DOI] [PubMed] [Google Scholar]
- 121.Michelsen, K. S., A. Aicher, M. Mohaupt, T. Hartung, S. Dimmeler, C. J. Kirschning, and R. R. Schumann. 2001. The role of toll-like receptors (TLRs) in bacteria-induced maturation of murine dendritic cells (DCS). Peptidoglycan and lipoteichoic acid are inducers of DC maturation and require TLR2. J. Biol. Chem. 276:25680-25686. [DOI] [PubMed] [Google Scholar]
- 122.Mitsuzawa, H., I. Wada, H. Sano, D. Iwaki, S. Murakami, T. Himi, N. Matsushima, and Y. Kuroki. 2001. Extracellular Toll-like receptor 2 region containing Ser40-Ile64 but not Cys30-Ser39 is critical for the recognition of Staphylococcus aureus peptidoglycan. J. Biol. Chem. 276:41350-41356. [DOI] [PubMed] [Google Scholar]
- 123.Molne, L., L. V. Collins, and A. Tarkowski. 2003. Inflammatogenic properties of bacterial DNA following cutaneous exposure. J. Investig. Dermatol. 121:294-299. [DOI] [PubMed] [Google Scholar]
- 124.Moore, C. E., S. Segal, A. R. Berendt, A. V. Hill, and N. P. Day. 2004. Lack of association between Toll-like receptor 2 polymorphisms and susceptibility to severe disease caused by Staphylococcus aureus. Clin. Diagn. Lab. Immunol. 11:1194-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Morath, S., A. Geyer, and T. Hartung. 2001. Structure-function relationship of cytokine induction by lipoteichoic acid from Staphylococcus aureus. J. Exp. Med. 193:393-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Morath, S., A. Geyer, I. Spreitzer, C. Hermann, and T. Hartung. 2002. Structural decomposition and heterogeneity of commercial lipoteichoic acid preparations. Infect. Immun. 70:938-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Morath, S., A. Stadelmaier, A. Geyer, R. R. Schmidt, and T. Hartung. 2002. Synthetic lipoteichoic acid from Staphylococcus aureus is a potent stimulus of cytokine release. J. Exp. Med. 195:1635-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Moreillon, P., and P. A. Majcherczyk. 2003. Proinflammatory activity of cell-wall constituents from gram-positive bacteria. Scand. J. Infect. Dis. 35:632-641. [DOI] [PubMed] [Google Scholar]
- 129.Morfeldt, E., L. Janzon, S. Arvidson, and S. Lofdahl. 1988. Cloning of a chromosomal locus (exp) which regulates the expression of several exoprotein genes in Staphylococcus aureus. Mol. Gen. Genet. 211:435-440. [DOI] [PubMed] [Google Scholar]
- 130.Morr, M., O. Takeuchi, S. Akira, M. M. Simon, and P. F. Muhlradt. 2002. Differential recognition of structural details of bacterial lipopeptides by toll-like receptors. Eur. J. Immunol. 32:3337-3347. [DOI] [PubMed] [Google Scholar]
- 131.Myhre, A. E., J. F. Stuestol, M. K. Dahle, G. Overland, C. Thiemermann, S. J. Foster, P. Lilleaasen, A. O. Aasen, and J. E. Wang. 2004. Organ injury and cytokine release caused by peptidoglycan are dependent on the structural integrity of the glycan chain. Infect. Immun. 72:1311-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nagai, Y., S. Akashi, M. Nagafuku, M. Ogata, Y. Iwakura, S. Akira, T. Kitamura, A. Kosugi, M. Kimoto, and K. Miyake. 2002. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat. Immunol. 3:667-672. [DOI] [PubMed] [Google Scholar]
- 133.Nakane, A., M. Okamoto, M. Asano, M. Kohanawa, and T. Minagawa. 1995. Endogenous gamma interferon, tumor necrosis factor, and interleukin-6 in Staphylococcus aureus infection in mice. Infect. Immun. 63:1165-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Natanson, C., R. L. Danner, R. J. Elin, J. M. Hosseini, K. W. Peart, S. M. Banks, T. J. MacVittie, R. I. Walker, and J. E. Parrillo. 1989. Role of endotoxemia in cardiovascular dysfunction and mortality. Escherichia coli and Staphylococcus aureus challenges in a canine model of human septic shock. J. Clin. Investig. 83:243-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Neuhaus, F. C., and J. Baddiley. 2003. A continuum of anionic charge: structures and functions of d-alanyl-teichoic acids in gram-positive bacteria. Microbiol. Mol. Biol. Rev. 67:686-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Neujahr, D. C., C. F. Reich, and D. S. Pisetsky. 1999. Immunostimulatory properties of genomic DNA from different bacterial species. Immunobiology 200:106-119. [DOI] [PubMed] [Google Scholar]
- 137.Nishiya, T., and A. L. DeFranco. 2004. Ligand-regulated chimeric receptor approach reveals distinctive subcellular localization and signaling properties of the Toll-like receptors. J. Biol. Chem. 279:19008-19017. [DOI] [PubMed] [Google Scholar]
- 138.Normark, B. H., S. Normark, and A. Norrby-Teglund. 2004. Staphylococcal protein A inflames the lungs. Nat. Med. 10:780-781. [DOI] [PubMed] [Google Scholar]
- 139.Ogura, Y., D. K. Bonen, N. Inohara, D. L. Nicolae, F. F. Chen, R. Ramos, H. Britton, T. Moran, R. Karaliuskas, R. H. Duerr, J. P. Achkar, S. R. Brant, T. M. Bayless, B. S. Kirschner, S. B. Hanauer, G. Nunez, and J. H. Cho. 2001. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 411:603-606. [DOI] [PubMed] [Google Scholar]
- 140.Ogura, Y., N. Inohara, A. Benito, F. F. Chen, S. Yamaoka, and G. Nunez. 2001. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-κB. J. Biol. Chem. 276:4812-4818. [DOI] [PubMed] [Google Scholar]
- 141.O'Neill, L. A. 2002. Wanted: a molecular basis for specificity in toll-like receptor signal transduction. Mol. Cell 10:969-971. [DOI] [PubMed] [Google Scholar]
- 142.Onogawa, T. 2002. Staphylococcal alpha-toxin synergistically enhances inflammation caused by bacterial components. FEMS Immunol. Med. Microbiol. 33:15-21. [DOI] [PubMed] [Google Scholar]
- 143.Opal, S. M., and J. Cohen. 1999. Clinical gram-positive sepsis: does it fundamentally differ from gram-negative bacterial sepsis? Crit. Care Med. 27:1608-1616. [DOI] [PubMed] [Google Scholar]
- 144.Opitz, B., N. W. Schroder, I. Spreitzer, K. S. Michelsen, C. J. Kirschning, W. Hallatschek, U. Zahringer, T. Hartung, U. B. Gobel, and R. R. Schumann. 2001. Toll-like receptor-2 mediates Treponema glycolipid and lipoteichoic acid-induced NF-κB translocation. J. Biol. Chem. 276:22041-22047. [DOI] [PubMed] [Google Scholar]
- 145.Ozinsky, A., D. M. Underhill, J. D. Fontenot, A. M. Hajjar, K. D. Smith, C. B. Wilson, L. Schroeder, and A. Aderem. 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc. Natl. Acad. Sci. USA 97:13766-13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Palmqvist, N., T. Foster, A. Tarkowski, and E. Josefsson. 2002. Protein A is a virulence factor in Staphylococcus aureus arthritis and septic death. Microb. Pathog. 33:239-249. [DOI] [PubMed] [Google Scholar]
- 147.Palsson-McDermott, E. M., and L. A. O'Neill. 2004. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology 113:153-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Peng, H. L., R. P. Novick, B. Kreiswirth, J. Kornblum, and P. Schlievert. 1988. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J. Bacteriol. 170:4365-4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Perfetto, B., G. Donnarumma, D. Criscuolo, I. Paoletti, E. Grimaldi, M. A. Tufano, and A. Baroni. 2003. Bacterial components induce cytokine and intercellular adhesion molecules-1 and activate transcription factors in dermal fibroblasts. Res. Microbiol. 154:337-344. [DOI] [PubMed] [Google Scholar]
- 150.Peschel, A., M. Otto, R. W. Jack, H. Kalbacher, G. Jung, and F. Gotz. 1999. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J. Biol. Chem. 274:8405-8410. [DOI] [PubMed] [Google Scholar]
- 151.Pittet, D., N. Li, R. F. Woolson, and R. P. Wenzel. 1997. Microbiological factors influencing the outcome of nosocomial bloodstream infections: a 6-year validated, population-based model. Clin. Infect. Dis. 24:1068-1078. [DOI] [PubMed] [Google Scholar]
- 152.Pokorny, A., T. H. Birkbeck, and P. F. Almeida. 2002. Mechanism and kinetics of delta-lysin interaction with phospholipid vesicles. Biochemistry 41:11044-11056. [DOI] [PubMed] [Google Scholar]
- 153.Prince, A. 1992. Adhesins and receptors of Pseudomonas aeruginosa associated with infection of the respiratory tract. Microb. Pathog. 13:251-260. [DOI] [PubMed] [Google Scholar]
- 154.Projan, S. J., and R. P. Novick. 1997. The molecular basis of pathogenicity, p. 55-81. In K. B. Crossley and G. L. Archer (ed.), The staphylococci in human disease. Churchill Livingstone, New-York, N.Y.
- 155.Pugin, J., I. D. Heumann, A. Tomasz, V. V. Kravchenko, Y. Akamatsu, M. Nishijima, M. P. Glauser, P. S. Tobias, and R. J. Ulevitch. 1994. CD14 is a pattern recognition receptor. Immunity 1:509-516. [DOI] [PubMed] [Google Scholar]
- 156.Qazi, S. N., E. Counil, J. Morrissey, C. E. Rees, A. Cockayne, K. Winzer, W. C. Chan, P. Williams, and P. J. Hill. 2001. agr expression precedes escape of internalized Staphylococcus aureus from the host endosome. Infect. Immun. 69:7074-7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rabehi, L., T. Irinopoulou, B. Cholley, N. Haeffner-Cavaillon, and M. P. Carreno. 2001. Gram-positive and gram-negative bacteria do not trigger monocytic cytokine production through similar intracellular pathways. Infect. Immun. 69:4590-4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ratner, A. J., R. Bryan, A. Weber, S. Nguyen, D. Barnes, A. Pitt, S. Gelber, A. Cheung, and A. Prince. 2001. Cystic fibrosis pathogens activate Ca2+-dependent mitogen-activated protein kinase signaling pathways in airway epithelial cells. J. Biol. Chem. 276:19267-19275. [DOI] [PubMed] [Google Scholar]
- 159.Reuben, A. G., D. M. Musher, R. J. Hamill, and I. Broucke. 1989. Polymicrobial bacteremia: clinical and microbiologic patterns. Rev. Infect. Dis. 11:161-183. [DOI] [PubMed] [Google Scholar]
- 160.Rock, F. L., G. Hardiman, J. C. Timans, R. A. Kastelein, and J. F. Bazan. 1998. A family of human receptors structurally related to Drosophila Toll. Proc. Natl. Acad. Sci. USA 95:588-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rot, A., L. E. Henderson, and E. J. Leonard. 1986. Staphylococcus aureus-derived chemoattractant activity for human monocytes. J. Leukoc. Biol. 40:43-53. [DOI] [PubMed] [Google Scholar]
- 162.Rot, A., L. E. Henderson, R. Sowder, and E. J. Leonard. 1989. Staphylococcus aureus tetrapeptide with high chemotactic potency and efficacy for human leukocytes. J. Leukoc. Biol. 45:114-120. [DOI] [PubMed] [Google Scholar]
- 163.Ruhland, G. J., and F. Fiedler. 1990. Occurrence and structure of lipoteichoic acids in the genus Staphylococcus. Arch. Microbiol. 154:375-379. [DOI] [PubMed] [Google Scholar]
- 164.Sabroe, I., L. R. Prince, E. C. Jones, M. J. Horsburgh, S. J. Foster, S. N. Vogel, S. K. Dower, and M. K. Whyte. 2003. Selective roles for Toll-like receptor (TLR)2 and TLR4 in the regulation of neutrophil activation and life span. J. Immunol. 170:5268-5275. [DOI] [PubMed] [Google Scholar]
- 165.Sandor, F., E. Latz, F. Re, L. Mandell, G. Repik, D. T. Golenbock, T. Espevik, E. A. Kurt-Jones, and R. W. Finberg. 2003. Importance of extra- and intracellular domains of TLR1 and TLR2 in NFkappa B signaling. J. Cell Biol. 162:1099-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Schiffmann, E., B. A. Corcoran, and S. M. Wahl. 1975. N-Formylmethionyl peptides as chemoattractants for leucocytes. Proc. Natl. Acad. Sci. USA 72:1059-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Schleifer, K. H., and O. Kandler. 1972. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol. Rev. 36:407-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Schmitz, F. J., K. E. Veldkamp, K. P. Van Kessel, J. Verhoef, and J. A. Van Strijp. 1997. Delta-toxin from Staphylococcus aureus as a costimulator of human neutrophil oxidative burst. J. Infect. Dis. 176:1531-1537. [DOI] [PubMed] [Google Scholar]
- 169.Schnare, M., G. M. Barton, A. C. Holt, K. Takeda, S. Akira, and R. Medzhitov. 2001. Toll-like receptors control activation of adaptive immune responses. Nat. Immunol. 2:947-950. [DOI] [PubMed] [Google Scholar]
- 170.Schroder, N. W., S. Morath, C. Alexander, L. Hamann, T. Hartung, U. Zahringer, U. B. Gobel, J. R. Weber, and R. R. Schumann. 2003. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J. Biol. Chem. 278:15587-15594. [DOI] [PubMed] [Google Scholar]
- 171.Schwandner, R., R. Dziarski, H. Wesche, M. Rothe, and C. J. Kirschning. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J. Biol. Chem. 274:17406-17409. [DOI] [PubMed] [Google Scholar]
- 172.Shompole, S., K. T. Henon, L. E. Liou, K. Dziewanowska, G. A. Bohach, and K. W. Bayles. 2003. Biphasic intracellular expression of Staphylococcus aureus virulence factors and evidence for Agr-mediated diffusion sensing. Mol. Microbiol. 49:919-927. [DOI] [PubMed] [Google Scholar]
- 173.Skerrett, S. J., H. D. Liggitt, A. M. Hajjar, and C. B. Wilson. 2004. Cutting edge: myeloid differentiation factor 88 is essential for pulmonary host defense against Pseudomonas aeruginosa but not Staphylococcus aureus. J. Immunol. 172:3377-3381. [DOI] [PubMed] [Google Scholar]
- 174.Smith, T. J., S. A. Blackman, and S. J. Foster. 2000. Autolysins of Bacillus subtilis: multiple enzymes with multiple functions. Microbiology 146:249-262. [DOI] [PubMed] [Google Scholar]
- 175.Somerville, G. A., A. Cockayne, M. Durr, A. Peschel, M. Otto, and J. M. Musser. 2003. Synthesis and deformylation of Staphylococcus aureus delta-toxin are linked to tricarboxylic acid cycle activity. J. Bacteriol. 185:6686-6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Soong, G., B. Reddy, S. Sokol, R. Adamo, and A. Prince. 2004. TLR2 is mobilized into an apical lipid raft receptor complex to signal infection in airway epithelial cells. J. Clin. Investig. 113:1482-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sparwasser, T., T. Miethke, G. Lipford, K. Borschert, H. Hacker, K. Heeg, and H. Wagner. 1997. Bacterial DNA causes septic shock. Nature 386:336-337. [DOI] [PubMed] [Google Scholar]
- 178.Sriskandan, S., and J. Cohen. 1999. Gram-positive sepsis. Mechanisms and differences from gram-negative sepsis. Infect. Dis. Clin. N. Am. 13:397-412. [DOI] [PubMed] [Google Scholar]
- 179.Standiford, T. J., D. A. Arenberg, J. M. Danforth, S. L. Kunkel, G. M. VanOtteren, and R. M. Strieter. 1994. Lipoteichoic acid induces secretion of interleukin-8 from human blood monocytes: a cellular and molecular analysis. Infect. Immun. 62:119-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sumaroka, M. V., I. S. Litvinov, S. V. Khaidukov, T. N. Golovina, M. V. Kamraz, R. L. Komal'eva, T. M. Andronova, E. A. Makarov, V. A. Nesmeyanov, and V. T. Ivanov. 1991. Muramyl peptide-binding sites are located inside target cells. FEBS Lett. 295:48-50. [DOI] [PubMed] [Google Scholar]
- 181.Takeda, K. 2005. Evolution and integration of innate immune recognition systems: the Toll-like receptors. J. Endotoxin Res. 11:51-55. [DOI] [PubMed] [Google Scholar]
- 182.Takeda, K., and S. Akira. 2001. Roles of Toll-like receptors in innate immune responses. Genes Cells 6:733-742. [DOI] [PubMed] [Google Scholar]
- 183.Takeda, K., and S. Akira. 2003. Toll receptors and pathogen resistance. Cell Microbiol. 5:143-153. [DOI] [PubMed] [Google Scholar]
- 184.Takeuchi, O., K. Hoshino, and S. Akira. 2000. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 165:5392-5396. [DOI] [PubMed] [Google Scholar]
- 185.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 186.Takeuchi, O., T. Kawai, P. F. Muhlradt, M. Morr, J. D. Radolf, A. Zychlinsky, K. Takeda, and S. Akira. 2001. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 13:933-940. [DOI] [PubMed] [Google Scholar]
- 187.Takeuchi, O., S. Sato, T. Horiuchi, K. Hoshino, K. Takeda, Z. Dong, R. L. Modlin, and S. Akira. 2002. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J. Immunol. 169:10-14. [DOI] [PubMed] [Google Scholar]
- 188.Thiemermann, C. 2002. Interactions between lipoteichoic acid and peptidoglycan from Staphylococcus aureus: a structural and functional analysis. Microbes Infect. 4:927-935. [DOI] [PubMed] [Google Scholar]
- 189.Thumm, G., and F. Gotz. 1997. Studies on prolysostaphin processing and characterization of the lysostaphin immunity factor (Lif) of Staphylococcus simulans biovar staphylolyticus. Mol. Microbiol. 23:1251-1265. [DOI] [PubMed] [Google Scholar]
- 190.Timmerman, C. P., E. Mattsson, L. Martinez-Martinez, L. De Graaf, J. A. Van Strijp, H. A. Verbrugh, J. Verhoef, and A. Fleer. 1993. Induction of release of tumor necrosis factor from human monocytes by staphylococci and staphylococcal peptidoglycans. Infect. Immun. 61:4167-4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tokunaga, T., H. Yamamoto, S. Shimada, H. Abe, T. Fukuda, Y. Fujisawa, Y. Furutani, O. Yano, T. Kataoka, T. Sudo, et al. 1984. Antitumor activity of deoxyribonucleic acid fraction from Mycobacterium bovis BCG. I. Isolation, physicochemical characterization, and antitumor activity. J. Natl. Cancer Inst. 72:955-962. [PubMed] [Google Scholar]
- 192.Travassos, L. H., S. E. Girardin, D. J. Philpott, D. Blanot, M. A. Nahori, C. Werts, and I. G. Boneca. 2004. Toll-like receptor 2-dependent bacterial sensing does not occur via peptidoglycan recognition. EMBO Rep. 10:1000-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tufano, M. A., G. Cipollaro de l'Ero, R. Ianniello, M. Galdiero, and F. Galdiero. 1991. Protein A and other surface components of Staphylococcus aureus stimulate production of IL-1 alpha, IL-4, IL-6, TNF and IFN-gamma. Eur. Cytokine Netw. 2:361-366. [PubMed] [Google Scholar]
- 194.Underhill, D. M., and A. Ozinsky. 2002. Toll-like receptors: key mediators of microbe detection. Curr. Opin. Immunol. 14:103-110. [DOI] [PubMed] [Google Scholar]
- 195.Underhill, D. M., A. Ozinsky, A. M. Hajjar, A. Stevens, C. B. Wilson, M. Bassetti, and A. Aderem. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401:811-815. [DOI] [PubMed] [Google Scholar]
- 196.Vavricka, S. R., M. W. Musch, J. E. Chang, Y. Nakagawa, K. Phanvijhitsiri, T. S. Waypa, D. Merlin, O. Schneewind, and E. B. Chang. 2004. hPepT1 transports muramyl dipeptide, activating NF-κB and stimulating IL-8 secretion in human colonic Caco2/bbe cells. Gastroenterology 127:1401-1409. [DOI] [PubMed] [Google Scholar]
- 197.Verhoef, J., and E. Mattsson. 1995. The role of cytokines in gram-positive bacterial shock. Trends Microbiol. 3:136-140. [DOI] [PubMed] [Google Scholar]
- 198.Vidal, V. F., N. Casteran, C. J. Riendeau, H. Kornfeld, E. C. Darcissac, A. Capron, and G. M. Bahr. 2001. Macrophage stimulation with Murabutide, an HIV-suppressive muramyl peptide derivative, selectively activates extracellular signal-regulated kinases 1 and 2, C/EBPbeta and STAT1: role of CD14 and Toll-like receptors 2 and 4. Eur. J. Immunol. 31:1962-1971. [DOI] [PubMed] [Google Scholar]
- 199.Visintin, A., A. Mazzoni, J. H. Spitzer, D. H. Wyllie, S. K. Dower, and D. M. Segal. 2001. Regulation of Toll-like receptors in human monocytes and dendritic cells. J. Immunol. 166:249-255. [DOI] [PubMed] [Google Scholar]
- 200.von Aulock, S., S. Morath, L. Hareng, S. Knapp, K. P. van Kessel, J. A. van Strijp, and T. Hartung. 2003. Lipoteichoic acid from Staphylococcus aureus is a potent stimulus for neutrophil recruitment. Immunobiology 208:413-422. [DOI] [PubMed] [Google Scholar]
- 201.von Aulock, S., N. W. Schroder, S. Traub, K. Gueinzius, E. Lorenz, T. Hartung, R. R. Schumann, and C. Hermann. 2004. Heterozygous Toll-like receptor 2 polymorphism does not affect lipoteichoic acid-induced chemokine and inflammatory responses. Infect. Immun. 72:1828-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.von Eiff, C., K. Becker, K. Machka, H. Stammer, and G. Peters. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N. Engl. J. Med. 344:11-16. [DOI] [PubMed] [Google Scholar]
- 203.Vuong, C., M. Durr, A. B. Carmody, A. Peschel, S. J. Klebanoff, and M. Otto. 2004. Regulated expression of pathogen-associated molecular pattern molecules in Staphylococcus epidermidis: quorum-sensing determines pro-inflammatory capacity and production of phenol-soluble modulins. Cell Microbiol. 6:753-759. [DOI] [PubMed] [Google Scholar]
- 204.Wagner, H. 2004. The immunobiology of the TLR9 subfamily. Trends Immunol. 25:381-386. [DOI] [PubMed] [Google Scholar]
- 205.Wakabayashi, G., J. A. Gelfand, W. K. Jung, R. J. Connolly, J. F. Burke, and C. A. Dinarello. 1991. Staphylococcus epidermidis induces complement activation, tumor necrosis factor and interleukin-1, a shock-like state and tissue injury in rabbits without endotoxemia. Comparison to Escherichia coli. J. Clin. Investig. 87:1925-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Walev, I., U. Weller, S. Strauch, T. Foster, and S. Bhakdi. 1996. Selective killing of human monocytes and cytokine release provoked by sphingomyelinase (beta-toxin) of Staphylococcus aureus. Infect. Immun. 64:2974-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wang, J. E., P. F. Jorgensen, E. A. Ellingsen, M. Almiof, C. Thiemermann, S. J. Foster, A. O. Aasen, and R. Solberg. 2001. Peptidoglycan primes for LPS-induced release of proinflammatory cytokines in whole human blood. Shock 16:178-182. [DOI] [PubMed] [Google Scholar]
- 208.Wang, Q., R. Dziarski, C. J. Kirschning, M. Muzio, and D. Gupta. 2001. Micrococci and peptidoglycan activate TLR2->MyD88->IRAK->TRAF->NIK->IKK->NF-κB signal transduction pathway that induces transcription of interleukin-8. Infect. Immun. 69:2270-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wang, Z. M., X. Li, R. R. Cocklin, M. Wang, K. Fukase, S. Inamura, S. Kusumoto, D. Gupta, and R. Dziarski. 2003. Hum. peptidoglycan recognition protein-L is an N-acetylmuramoyl-l-alanine amidase. J. Biol. Chem. 278:49044-49052. [DOI] [PubMed] [Google Scholar]
- 210.Wang, Z. M., C. Liu, and R. Dziarski. 2000. Chemokines are the main proinflammatory mediators in human monocytes activated by Staphylococcus aureus, peptidoglycan, and endotoxin. J. Biol. Chem. 275:20260-20267. [DOI] [PubMed] [Google Scholar]
- 211.Wehkamp, J., J. Harder, M. Weichenthal, M. Schwab, E. Schaffeler, M. Schlee, K. R. Herrlinger, A. Stallmach, F. Noack, P. Fritz, J. M. Schroder, C. L. Bevins, K. Fellermann, and E. F. Stange. 2004. NOD2 (CARD15) mutations in Crohn's disease are associated with diminished mucosal alpha-defensin expression. Gut. 53:1658-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Weidemann, B., H. Brade, E. T. Rietschel, R. Dziarski, V. Bazil, S. Kusumoto, H. D. Flad, and A. J. Ulmer. 1994. Soluble peptidoglycan-induced monokine production can be blocked by anti-CD14 monoclonal antibodies and by lipid A partial structures. Infect. Immun. 62:4709-4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wesson, C. A., L. E. Liou, K. M. Todd, G. A. Bohach, W. R. Trumble, and K. W. Bayles. 1998. Staphylococcus aureus Agr and Sar global regulators influence internalization and induction of apoptosis. Infect. Immun. 66:5238-5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wetzler, L. M. 2003. The role of Toll-like receptor 2 in microbial disease and immunity. Vaccine 21:S55-60. [DOI] [PubMed] [Google Scholar]
- 215.Wolfert, M. A., T. F. Murray, G. J. Boons, and J. N. Moore. 2002. The origin of the synergistic effect of muramyl dipeptide with endotoxin and peptidoglycan. J. Biol. Chem. 277:39179-39186. [DOI] [PubMed] [Google Scholar]
- 216.Worsaae, N., K. Staehr Johansen, and K. C. Christensen. 1982. Impaired in vitro function of neutrophils in Crohn's disease. Scand. J. Gastroenterol. 17:91-96. [DOI] [PubMed] [Google Scholar]
- 217.Wright, S. D., R. A. Ramos, P. S. Tobias, R. J. Ulevitch, and J. C. Mathison. 1990. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249:1431-1433. [DOI] [PubMed] [Google Scholar]
- 218.Xu, M., Z. Wang, and R. M. Locksley. 2004. Innate immune responses in peptidoglycan recognition protein L-deficient mice. Mol. Cell. Biol. 24:7949-7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yamamoto, M., S. Sato, H. Hemmi, H. Sanjo, S. Uematsu, T. Kaisho, K. Hoshino, O. Takeuchi, M. Kobayashi, T. Fujita, K. Takeda, and S. Akira. 2002. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature 420:324-329. [DOI] [PubMed] [Google Scholar]
- 220.Yamamoto, S., T. Yamamoto, S. Shimada, E. Kuramoto, O. Yano, T. Kataoka, and T. Tokunaga. 1992. DNA from bacteria, but not from vertebrates, induces interferons, activates natural killer cells and inhibits tumor growth. Microbiol. Immunol. 36:983-997. [DOI] [PubMed] [Google Scholar]
- 221.Yang, S., R. Tamai, S. Akashi, O. Takeuchi, S. Akira, S. Sugawara, and H. Takada. 2001. Synergistic effect of muramyldipeptide with lipopolysaccharide or lipoteichoic acid to induce inflammatory cytokines in human monocytic cells in culture. Infect. Immun. 69:2045-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yoshimura, A., E. Lien, R. R. Ingalls, E. Tuomanen, R. Dziarski, and D. Golenbock. 1999. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J. Immunol. 163:1-5. [PubMed] [Google Scholar]
- 223.Yoshimura, A., H. Takada, T. Kaneko, I. Kato, D. Golenbock, and Y. Hara. 2000. Structural requirements of muramylpeptides for induction of Toll-like receptor 2-mediated NF-κB activation in CHO cells. J. Endotoxin Res. 6:407-410. [PubMed] [Google Scholar]
- 224.Zhang, D., G. Zhang, M. S. Hayden, M. B. Greenblatt, C. Bussey, R. A. Flavell, and S. Ghosh. 2004. A toll-like receptor that prevents infection by uropathogenic bacteria. Science 303:1522-1526. [DOI] [PubMed] [Google Scholar]
- 225.Zhang, G., and S. Ghosh. 2002. Negative regulation of toll-like receptor-mediated signaling by Tollip. J. Biol. Chem. 277:7059-7065. [DOI] [PubMed] [Google Scholar]