Abstract
We have identified a Helicobacter pylori d-glycero-d-manno-heptosyltransferase gene, HP0479, which is involved in the biosynthesis of the outer core region of H. pylori lipopolysaccharide (LPS). Insertional inactivation of HP0479 resulted in formation of a truncated LPS molecule lacking an α-1,6-glucan-, dd-heptose-containing outer core region and O-chain polysaccharide. Detailed structural analysis of purified LPS from HP0479 mutants of strains SS1, 26695, O:3, and PJ1 by a combination of chemical and mass spectrometric methods showed that HP0479 likely encodes α-1,2-d-glycero-d-manno-heptosyltransferase, which adds a d-glycero-d-manno-heptose residue (DDHepII) to a distal dd-heptose of the core oligosaccharide backbone of H. pylori LPS. When the wild-type HP0479 gene was reintegrated into the chromosome of strain 26695 by using an “antibiotic cassette swapping” method, the complete LPS structure was restored. Introduction of the HP0479 mutation into the H. pylori mouse-colonizing Sydney (SS1) strain and the clinical isolate PJ1, which expresses dd-heptoglycan, resulted in the loss of colonization in a mouse model. This indicates that H. pylori expressing a deeply truncated LPS is unable to successfully colonize the murine stomach and provides evidence for a critical role of the outer core region of H. pylori LPS in colonization.
Lipopolysaccharide (LPS) is a major surface antigen of gram-negative bacteria and is essential for the physical integrity and function of the bacterial outer membrane. The basic structure of Helicobacter pylori LPS is similar to those of other bacterial species and consists of three distinct regions: an O-chain polysaccharide composed of repeating units, a core oligosaccharide, and a hydrophobic lipid A moiety (40). H. pylori LPS may play several roles in pathogenesis and has been implicated in causing abnormal acid secretion and in inducing apoptosis of epithelial cells and gastritis in mice (22, 36-39, 42). More recently, the focus has been on the ability of many strains of H. pylori to express O-polysaccharide chains that mimic Lewis blood group antigens (33). These antigens are naturally expressed on the surface of epithelial cells of several human tissues, including the gastric mucosa (30).
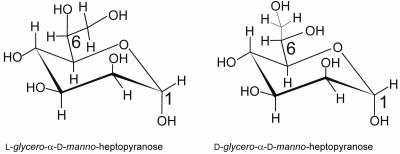
A majority of the core regions of LPSs from different gram-negative bacteria contain l-glycero-d-manno-heptopyranose (ld-heptose) (Fig. 1). One of the unique features of H. pylori LPS is the abundance of d-glycero-d-manno-heptose (dd-heptose) (Fig. 1) residues in its core region (33). The occurrence of dd-heptose in bacterial LPS is relatively uncommon. A limited number of bacterial species are known to contain dd-heptose residues in their core region, including Proteus mirabilis R110/1959, Yersinia enterocolitica, several strains of Yersinia pestis, Aeromonas hydrophila, and Rhodocyclus spp. (18). In Klebsiella pneumoniae strain 01/R20, the outer core region of LPS contains an α-1,2-linked dd-heptoglycan (48). Other bacteria, such as Haemophilus ducreyi (18), Vibrio parahaemolyticus (18), Vibrio salmonicida (18), Coxiella burnetii (18), Proteus penneri (54), Actinobacillus pleuropneumoniae (45), and Mannheimia hemolytica (4), have one or two internal dd-heptose residues in their core regions. Additionally, the glycan chain of the surface layer glycoprotein of Aneurinibacillus thermoaerophilus DSM 10155 was reported to contain dd-heptose residues (57). To date, only the dd-heptosyltransferase gene of H. ducreyi has been cloned and characterized (12, 15, 53).
FIG. 1.
Chemical structures of l-glycero-d-manno-heptopyranose and d-glycero-d-manno-heptopyranose.
In H. pylori LPS, dd-heptose residues form an integral component of the core oligosaccharide as well as the linking region that connects the outer core oligosaccharide to the O chain. In some H. pylori isolates the linking region is composed of a long dd-heptoglycan polymer, while in other strains a single dd-heptose residue links the O chain to the core (33). Recently, nontypeable strains of H. pylori expressing dd-heptoglycan but lacking traditional Lewis antigens have been identified (2, 20, 44). The presence of the outer core region consisting of multiple dd-heptose residues and the dd-heptoglycan domain along with the Lewis blood group antigen-containing O-chain polysaccharide distinguishes H. pylori LPS from those of other gram-negative bacteria (33).
To continue our work directed towards elucidating the genetic basis of LPS biosynthesis in H. pylori, we have cloned and characterized the key outer core biosynthetic enzyme encoded by the HP0479 gene through insertional mutagenesis studies and structural analysis of the resulting LPS.
MATERIALS AND METHODS
Bacterial strains and media.
H. pylori strain 26695 (51) was obtained from R. A. Alm. H. pylori strain SS1 was obtained from A. Lee. H. pylori strains ATCC 43504 (type strain) and serotype O:3 were obtained from J. Penner. H. pylori strain PJ1 was a clinical isolate of unknown clinical history. H. pylori strain O:1 was an isolate from Health Canada that was originally typed as the type strain but had undergone phenotypic changes after several passages. H. pylori cultures were grown either on solid Columbia blood agar (Difco) supplemented with horse blood (5%), vancomycin (10 mg/liter), nalidixic acid (1.1 mg/liter), Bacitracin (20 mg/liter), polymyxin B (0.33 mg/liter), and amphotericin A (5 mg/liter) or in brucella broth supplemented with fetal bovine calf serum (5 to 10%), vancomycin (10 mg/liter), nalidixic acid (1.1 mg/liter), Bacitracin (20 mg/liter), polymyxin B (0.33 mg/liter), and amphotericin A (5 mg/liter). All H. pylori cultures were incubated at 37°C in a Tri-Gas incubator (Nuaire) with a gas mixture of 85% N2, 10% CO2, and 5% O2 until the desired amount of growth was obtained (normally 2 to 3 days). Small-scale liquid medium cultures were shaken at 100 rpm in the Tri-Gas incubator at 37°C. For propagation and maintenance of plasmids, Escherichia coli DH5α was used. E. coli cultures were grown in Luria broth supplemented, when appropriate, with ampicillin (100 mg/liter; Sigma), X-Gal (Gibco/BRL), IPTG (isopropyl-β-d-thiogalactopyranoside) (100 mM; Gibco/BRL), or kanamycin (20 mg/liter; Sigma).
Large-scale growth of H. pylori for LPS purification.
For 1-liter flask growth, brucella broth cultures were gassed with a gas mixture as described above and inoculated with two 100-ml volumes of bacterial stocks at an optical density (OD) of 0.5 to 0.6 to reach the starting OD at 600 nm (OD600) of 0.1. Cultures were gassed again for 15 min, sealed, and incubated for 48 h in a shaker. Flasks were flushed repeatedly with the gas mixture every 2 to 3 h.
Three to four liters of bacterial stock was used to inoculate each fermentor run, giving an OD600 of 0.1 to 0.15. The fermentor, a New Brunswick Scientific MF-128S Microferm, was modified to take the gas mixture through the headspace. The medium used was 2.8% brucella broth containing 7.5% fetal bovine serum. Dissolved oxygen was maintained at around 2%, the pH was adjusted to 7.2, the agitation rate was set at 210 rpm, and the temperature was a nominal 37°C. OD600 was measured hourly. The fermentor run lasted 48 h. The contents were killed by the addition of 2% (wt/vol) phenol, and the cells were harvested by centrifugation at 5,000 × g.
Identification of putative H. pylori dd-heptosyltransferases.
Computer database searches of the genome of H. pylori 26695 were conducted using the BLAST search engine (3, 35) for genes that showed structural homology to heptosyltransferases. Several different heptosyltransferases were used as the query sequences for these searches, including the WaaC genes from E. coli, Salmonella enterica serovar Typhimurium, Campylobacter coli, Campylobacter jejuni, Campylobacter hyoilei, and H. pylori 26695 (HP0279). Reciprocal BLAST searches were conducted using the HP0479 sequence as the query sequence.
Cloning of the HP0479 gene.
The PCR-amplified HP0479 gene from H. pylori 26695, as well as the HP0479 homologs from the genomes of the type strain (ATCC 43504) and the O:3, PJ1, and SS1 strains were cloned by blunt-end ligation into pUC19 cut with SmaI restriction enzyme. The primers used for amplification of the HP0479 gene were HP0479-F1 (5′-GCCTTTATCAAGCTAGAG-3′) and HP0479-R1 (5′-CATAAATGTCCTAACAAGC-3′). Standard DNA manipulation methodologies were used to isolate the plasmids (43).
Mutagenesis of the cloned HP0479 gene.
Mutagenesis of the cloned HP0479 gene of pHP0479G.1 was carried out by the insertion of a kanamycin resistance marker from C. coli (24) as previously described (29), generating plasmid pHP0479G.1kan.
Transformation of H. pylori.
Natural transformation of H. pylori was carried out according to the protocol of Haas and coworkers (16), using pHP0479G.1kan. Chromosomal DNA was isolated from kanamycin-resistant transformants, and PCR using Taq DNA polymerase (Roche Molecular Biochemicals) was used to ascertain if the C. coli kanamycin marker had been inserted into the HP0479 gene. For this purpose, the primers HP0479-GF1 (5′-ATGCATGTTGCTTGTCTTTTGG-3′) and HP0479-GR1 (5′-TTATAATAGCCCCAAATGGC-3′) were used.
Mutagenesis of HP0480.
Mutation of the HP0480 gene immediately downstream of HP0479 was also investigated. This gene was amplified from the H. pylori 26695 genome by using the primers HP0480-F1 (5′-GATAACCTCATCACGCTTAG-3′) and HP0480-R1 (5′-TTCAATCCATTCTAACGC-3′) with Pwo-. The PCR amplified HP0480 gene was cloned by blunt-end ligation into pUC19 cut with SmaI to make pHP0480G.1. The HP0480 gene of pHP0480G.1 was mutated by insertion of the C. coli chloramphenicol resistance cassette from the plasmid pRY109 (58) into a unique SmaI restriction site in the HP0480 gene. The construct pHP0480GmC.1 carrying the mutated HP0480 gene was transformed into 26695 and SS1 as described above. The LPSs from whole cells were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Reintegration of wild-type HP0479 gene into HP0479 mutant strains.
A wild-type copy of the HP0479 gene was put back into the chromosome by “antibiotic cassette swapping.” The H. pylori 26695::HP0479kan mutant strain was transformed with the plasmid p479480GmC.1, which contained a wild-type copy of the HP0479 gene and the HP0480 gene into which a chloramphenicol resistance cassette had been inserted. Transformants were selected initially on chloramphenicol, followed by replica plating onto kanamycin. Chloramphenicol-resistant and kanamycin-sensitive colonies indicated a double recombination event, where the insertionally inactivated copy of HP0479 was replaced with the plasmid-encoded wild-type copy and the chromosomally encoded HP0480 wild-type gene was replaced with the insertionally inactivated copy from the plasmid.
SDS-PAGE and Western blotting.
The LPS profiles of the parental and mutant H. pylori strains were analyzed by SDS-PAGE of whole-cell LPS preparations, using methods modified from those of Hitchcock and Brown (17). Samples of proteinase K-digested whole-cell lysates or purified LPS were electrophoresed on 12% polyacrylamide gels in the presence of SDS according to the method of Laemmli (25), using a mini-slab gel apparatus (Bio-Rad). LPS was visualized using the silver staining technique described by Tsai and Frasch (52) or transferred to nitrocellulose for immunological detection as previously described (28). The antibodies (Signet Laboratories) were used at a 1:500 dilution for anti-LeY monoclonal antibody (MAb) and a 1:250 dilution for anti-LeX MAb.
Membrane fraction analysis.
Membrane fractions were prepared from overnight (18-h) liquid cultures of HP0479 mutant strains and parental strains by using the protocols described by Logan and coworkers (29). The fractions were analyzed by one-dimensional SDS-PAGE and stained using Coomassie blue.
Isolation and purification of LPS.
The wet cell mass obtained by centrifugation of the bacterial growth was washed successively once with ethanol, twice with acetone, and twice with light petroleum ether and air dried. LPS was extracted from the air-dried cellular material by the hot-phenol-water extraction procedure of Westphal and Jann (56). LPS was obtained from the aqueous phase after extensive dialysis and lyophilization. H. pylori LPS from parental strains was further purified by ultracentrifugation and the pellet suspended in distilled water and lyophilized.
Preparation of core oligosaccharides.
LPS (25 to 30 mg) was hydrolyzed in sodium acetate buffer, pH 4.2, for 2 h at 100°C; the solution was cooled and the precipitated lipid A was removed by low-speed centrifugation. The supernatant solution was lyophilized, and the water-soluble components were fractionated by gel filtration chromatography on a Bio-Gel P-2 column (1.6 cm by 95 cm) equilibrated with pyridinium acetate (0.05 M, pH 4.5). Elution was performed with pyridinium acetate (0.05 M, pH 4.5). The fractions (1 ml) were monitored for neutral glycoses (9), and those a giving positive reaction were combined and lyophilized.
Analytical methods.
Glycoses were determined by gas-liquid chromatography (GLC) as their alditol acetate derivatives. Samples (0.2 to 0.5 mg) were hydrolyzed with 2 M trifluoroacetic acid for 16 h at 100°C and evaporated to dryness under a stream of nitrogen. The liberated glycoses were reduced with sodium borohydride and acetylated with acetic anhydride-pyridine as previously described (59). The configuration of peracetylated heptitol derivatives was determined to be l-glycero-d-manno or d-glycero-d-manno by comparison of their GLC retention times with those of authentic standards. Hexoses were determined to have the d configuration by GLC analysis of their acetylated (R)-2-butyl glycoside derivatives (14).
Methylation analysis was performed on lipopolysaccharide samples (1 to 3 mg) or dried bacterial cells with iodomethane in dimethyl sulfoxide containing an excess of sodium hydroxide (6), and permethylated alditol acetates were characterized by GLC-mass spectrometry (MS) in the electron impact mode as previously described (2). Fast atom bombardment (FAB)-MS analysis in a positive mode and electrospray mass spectrometry were performed as previously described (2, 27).
Mouse colonization.
Mouse colonization was performed as described by Logan and coworkers (29). For each inoculum, two sets of 10 mice were inoculated by gavage twice 5 days apart with bacterial suspensions of approximately 109 organisms of H. pylori SS1, H. pylori SS1::HP0479kan, H. pylori PJ1, or H. pylori PJ1::HP0479kan. Five mice from each group were sacrificed at 2 weeks and 4 weeks. The stomachs were excised and homogenized. Dilutions of the homogenate were plated to obtain bacterial counts.
RESULTS
Identification of putative heptosyltransferase genes of H. pylori.
HP0479 was identified through BLASTP analysis as a putative heptosyltransferase and a member of carbohydrate active enzyme family 9 (CAZY9) (5, 8) by using the amino acid sequences of several heptosyltransferase genes from other organisms as query sequences (13). Most notably, HP0479 displayed 29% identity to Hp0279 (waaC, ld-heptosyltransferase), which is the protein responsible for addition of ld-Hep to 2-keto-3-deoxyoctulosonic acid (KDO) in the inner cores of a number of gram-negative bacterial species. HP0479 also showed 22% identity with a functionally characterized dd-heptosyltransferase of H. ducreyi. In addition, it was noted that the HP0479 sequence contained a conserved domain (K203 to Y478) commonly found in many WaaC and WaaF heptosyltransferase protein sequences.
Cloning and mutagenesis of HP0479 genes from H. pylori strains.
The HP0479 gene of H. pylori 26695 and the HP0479 homologs from H. pylori strains Sydney (SS1), O:3, ATCC 43504 (type strain), and PJ1 were amplified by PCR and cloned. The nucleotide sequences of the HP0479 genes from PJ1, SS1, and O:3 showed between 91.5% and 94.2% identity with the HP0479 nucleotide sequence. The deduced amino acid sequences of the HP0479 homologs had 88.3% to 93.6% identity with HP0479 (data not shown; GenBank accession numbers AY885679, AY885680, and AY885681).
The HP0479 gene carried on plasmid pHP0479G.1 (from genome strain 26695) was mutated by insertion of a kanamycin resistance cassette as described in Materials and Methods. The cassette was in the nonpolar orientation as determined by restriction enzyme digestion (data not shown). The mutant construct pHP0479GmK.1 was used to generate HP0479 mutant strains of 26695, SS1, O:3, ATCC 43504 (type strain), and PJ1 (16). In all strains, the incorporation of the mutant HP0479 allele by double recombination was confirmed by PCR (data not shown). Membrane preparations of the mutants and the parental strain showed that the mutation of the HP0479 gene did not alter the membrane protein profile as seen by one-dimensional SDS-PAGE and Coomassie blue staining (data not shown). The mutant H. pylori strains were designated H. pylori 26695::HP0479kan, H. pylori SS1::HP0479kan, H. pylori O:3::HP0479kan, H. pylori ATCC 43504::HP0479kan, and H. pylori PJ1::HP0479kan.
Analysis of the LPSs of H. pylori strains by SDS-PAGE.
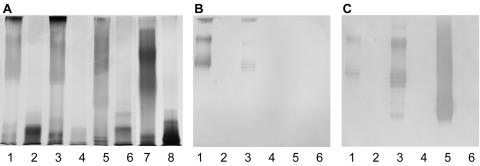
The LPS profiles of the parental and HP0479 mutant strains were analyzed by SDS-PAGE of whole-cell lysates and purified LPS preparations from 26695, O:3, SS1, and PJ1. In all cases, the mutation of the HP0479 gene resulted in truncation of the LPS. LPS from parental strains 26695, O:3, and SS1 exhibited a typical high-molecular-weight ladder associated with S-type LPS, while their corresponding HP0479 mutants no longer produced O chain (Fig. 2A, lanes 1 to 6). In comparison, LPS from PJ1, although producing a unique pattern of migration by SDS-PAGE (lane 7), was Le negative (data not shown). However, as was observed with the other HP0479 mutants, the corresponding HP0479 mutant of this strain also failed to produce any high-molecular-weight material (lane 8). Immunoblotting with anti-LeX and anti-LeY MAbs confirmed that LPSs from 26695::HP0479kan, O:3::HP0479kan, and SS1::HP0479kan strains no longer displayed Le antigens, although the respective parent strains were LeX/Y positive (26695 and O:3) and LeY positive (SS1) (Fig. 2B and C).
FIG. 2.
SDS-PAGE and immunoblot analysis of LPS from H. pylori HP0479 isogenic mutants. (A) Lane 1, H. pylori 26695; lane 2, H. pylori 26695::HP479kan; lane 3, H. pylori O:3; lane 4, H. pylori O:3::HP0479kan; lane 5, H. pylori strain SS1; lane 6, H. pylori SS1::HP0479kan; lane 7, H. pylori strain PJ1; lane 8, H. pylori PJ1::HP0479kan. (B) Immunoblot using anti-LeX monoclonal antibody at a 1:250 dilution for lanes 1 to 6 from panel A. (C) Immunoblot using anti-LeY monoclonal antibody at a 1:500 dilution for lanes 1 to 6 from panel A.
Mutagenesis of HP0480.
The HP0480 gene has been annotated as encoding a putative yihK GTP-binding protein (51) and was not expected to be involved in LPS biosynthesis. Similarly, HP0481 and HP0482 were not expected to contribute to LPS biosynthesis, although they may be linked to HP0479 as part of a transcriptional unit (51). Mutation of the HP0480 gene did not affect LPS expression as evidenced by SDS-PAGE (data not shown), and no change in growth rate was observed over 48 h in broth culture.
Reintegration of wild-type HP0479 in the genome by “antibiotic swapping.”
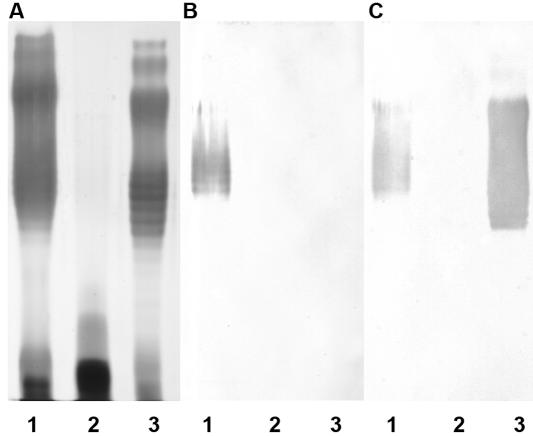
A wild-type copy of the HP0479 gene was reintegrated into the chromosomes of H. pylori strains 26695::HP0479kan and O:3::HP0479kan by using an “antibiotic swapping” technique. When the wild-type copy of the HP0479 gene was restored in the 26695::HP0479kan (Fig. 3) and O:3::HP0479kan (data not shown) strains, an extended LPS structure with O chain was observed; however, it was noted that the major LPS species in this strain appeared to migrate further into the gel, indicating a reduction in length of the O chain. It is well known that cultures grown on solid media will produce LPS of lower molecular mass (34). In addition, while reactivity with anti-LeY was restored in the complemented strain (Fig. 3C), we were unable to detect reactivity with anti-LeX antibodies. These antibodies detect only terminal LeX structures (Fig. 3B). To determine if the parental structure had been restored, we completed extensive structural analysis of LPS from this strain. Structural analysis (see below) indicated that the complemented cells produced smooth-type LPS carrying both LeX and LeY structures in quantities comparable to those for the 26695 parent, although most chains now appeared to be capped by terminal LeY.
FIG. 3.
SDS-PAGE and immunoblot analysis of LPS from complemented H. pylori HP0479 mutant. (A) Lane 1, H. pylori 26695; lane 2, H. pylori 26695::HP479kan; lane 3, H. pylori 26695::HP0479-HP0480::cam. (B) Corresponding immunoblot using anti-LeX monoclonal antibody at a 1:250 dilution. (C) Corresponding immunoblot using anti-LeY monoclonal antibody at a 1:500 dilution.
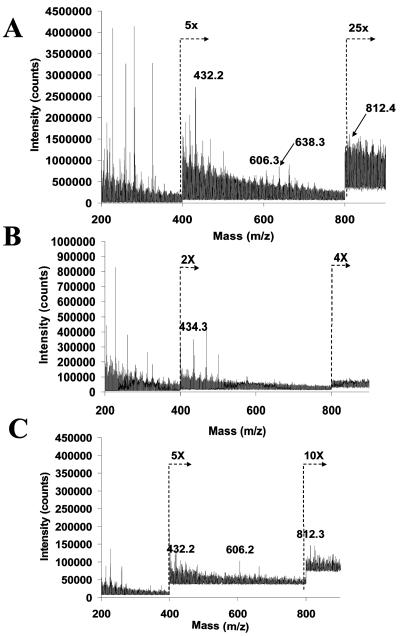
Structural characterization of H. pylori HP0479 LPS mutants of strains 26695, O:3, SS1, and PJ1.
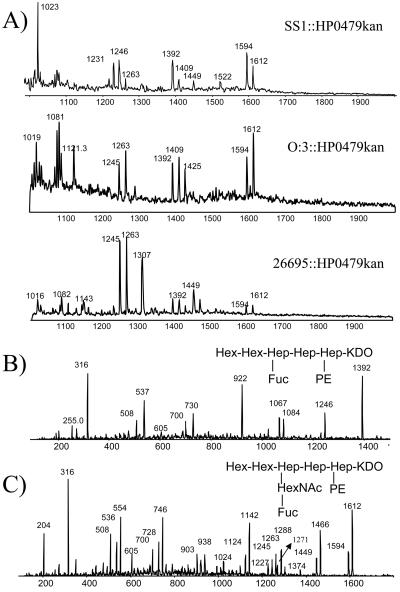
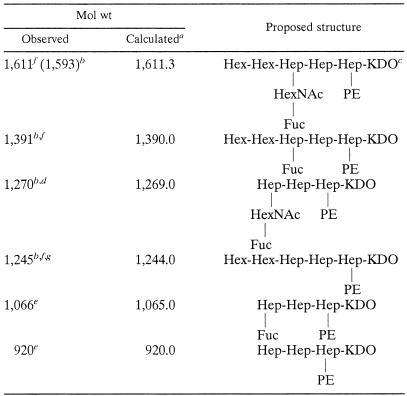
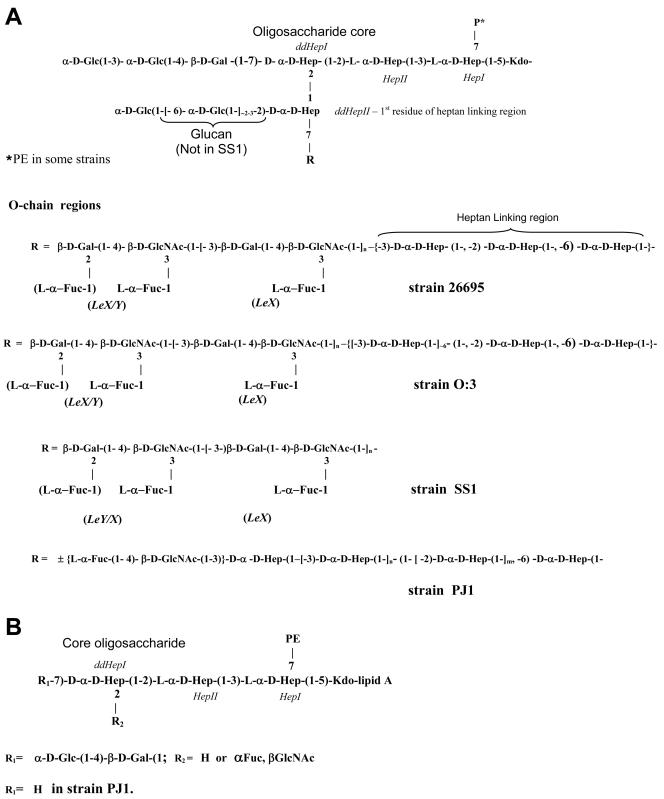
Sugar analysis of the HP0479 LPS mutants indicated reductions in the amounts of O-chain components, namely, l-Fuc, d-Gal, d-GlcNAc, and dd-Hep (Table 1), compared with parent LPS. Methylation analysis of the intact mutant LPS from each strain indicated an absence of 3,4-linked d-GlcNAc; a significant reduction in terminal l-Fuc; an absence of 3-substituted and 6-substituted d-Glc, 3-substituted dd-Hep (for H. pylori O:3::HP0479kan), and 6-substituted dd-Hep (for H. pylori O:3::HP0479kan and H. pylori 26695::HP0479kan); and a significant reduction in 2-substituted dd-Hep compared to the intact LPSs from corresponding parental strains, suggesting deficiencies in the core and O-chain biosyntheses. In addition, FAB-MS analysis in the positive mode of the permethylated LPS from each HP0479 mutant indicated a loss of the major glycosyl oxonium ions at m/z 432, m/z 606, m/z 638, and m/z 812, which were present in all parent strains. Primary glycosyl oxonium ions at m/z 260 [GlcNAc]+ and m/z 434 [Fuc, GlcNAc]+ and secondary glycosyl oxonium ions at m/z 228 (m/z 260 − 32) [GlcNAc]+ and m/z 402 (m/z 434 − 32) [Fuc, GlcNAc]+ were present in each mutant LPS. The data in Fig. 4 represent FAB-MS spectra of the permethylated LPS from 26695 (Fig. 4A) and 26695::HP0479kan (Fig. 4B). This evidence together with the absence of the primary glycosyl oxonium ion at m/z 682 [Fuc, GlcNAc, Hep]+ suggested that the mutant LPS structure was lacking the dd-Hep residue which bridges the O chain and the core oligosaccharide in the respective parent LPS (29, 33). LPSs from H. pylori SS1::HP0479kan, 26695::HP0479kan, and O:3::HP0479kan were delipidated and desalted following gel filtration chromatography on a Bio-Gel P-2 column. Fractions containing core oligosaccharide components were subjected to mass spectrometric analysis using on-line capillary electrophoresis-mass spectrometry (CE-MS) (Fig. 5A), followed by MS/MS analysis of the most abundant oligosaccharide fragments (Fig. 5B and C). The product ion spectra of delipidated LPSs from SS1, 26695, and O:3 mutants (Fig. 5C) showed singly charged fragment ions at m/z 1612 and m/z 1594, containing an anhydro-KDO. These fragment ions could be assigned to the glycoform HexHexHep(HexNAcFuc)HepHep(PE)KDO, based on the linkage and FAB-MS analyses data and recent structural studies (33). Further structural evidence was obtained in a MS/MS experiment where the singly charged ion at m/z 1392 was selected as a precursor (Fig. 5B). Observation of the diagnostic ion at m/z 1246, arising from the loss of Fuc, indicated the structure of the precursor ion m/z 1392 to be HexHexHep(Fuc)HepHep(PE)KDO, while fragment ions m/z 1271 (Fig. 5C, arrow) and m/z 1288 (NH4 adduct) indicated the existence of the fragment Hep(HexNAcFuc)HepHep(PE)KDO (data not shown). A pseudo-MS/MS/MS strategy was used to confirm sequence information for the second-generation fragment ion at m/z 1271 generated through in-source collision-induced dissociation (IS-CID), i.e., increasing the orifice voltage from 30 V to 200 V (50). A similar CE-MS/MS strategy was applied for the characterization of fragment ions at m/z 1270, m/z 1246, m/z 1066, and m/z 920 (Table 2). A summary of the glycoforms present in HP0479 mutants of H. pylori strains 26695, SS1, O:3, and PJ1 is presented in Table 2. These structural assignments were consistent with the methylation data, which showed the presence of 2,7-substituted dd-Hep, 7-substituted dd-Hep, and 2-substituted dd-Hep in the methylation analysis of H. pylori LPS mutants 26695::HP0479kan, SS1::HP0479kan, and O:3::HP0479kan. Sugar analysis of the PJ1::HP0479kan mutant revealed a significant reduction in the amounts of dd-Hep and Glc, compared with the parent PJ1 strain (Table 1). Similarly, methylation analysis of the PJ1::HP0479kan mutant performed on purified intact LPS confirmed that this mutant LPS was devoid of 6-linked Glc and 3-linked dd-Hep and produced a significantly reduced amount of 2-linked dd-Hep, which were reported to be main components of the dd-heptoglycan-containing O chain of strain PJ1 (32). Interestingly, methylation analysis of PJ1::HP0479kan mutant LPS showed only a negligible amount of 4-linked Gal and the absence of 2,7-linked dd-Hep. A reduced amount of 4-linked Gal was also observed in the composition analysis of LPS from the parent strain PJ1, suggesting structural variability in the core oligosaccharide backbone. CE-MS analysis of the delipidated PJ1::HP0479kan LPS indicated the presence of predominant singly charged species at m/z 1269 (data not shown). Subsequent MS/MS analysis of this fragment was consistent with the glycoform Hep(HexNAcFuc)HepHep(PE)KDO (Table 2). A comparison of the LPS structures from mutant and parent H. pylori 26695, SS1, O:3, and PJ1 strains is presented in Fig. 6.
TABLE 1.
Chemical analysis of intact LPSs of isogenic mutants of H. pylori HP0479 strains 26695, SS1, O:3, and PJ1 and complemented strain 26695::HP0479-HP0480::cam
| Strain | Approx molar ratio of alditol acetate derivativea
|
|||||
|---|---|---|---|---|---|---|
| l-Fuc | d-Glc | d-Gal | d-GlcNAc | dd-Hep | ld-Hepb | |
| 26695::HP0479kanc | 0.9 (4) | 3.0 (9) | 1.4 (9) | 4.2 (15) | 0.9 (5.5) | 1.0 (1) |
| SS1::HP0479kand | 1.0 (3.5) | 1.1 (4) | 1.1 (12) | 4.0 (9) | 0.9 (2.5) | 1.0 (1) |
| O:3::HP0479kanc | 0.5 (8) | 1.2 (7) | 1.0 (12) | 4.8 (13) | 1.1 (6) | 1.0 (1) |
| PJ1::HP0479kanc | 0.3 (0.6) | 1.1 (2.8) | 1.0 (0.1) | 6.4 (2.7) | 1.1 (7.8) | 1.0 (1) |
| 26695::HP0479-HP0480::camc | 3.8 (4) | 5.3 (9) | 6.9 (9) | 9.5 (15) | 4.5 (5.5) | 1.0 (1) |
Numbers in parentheses indicate ratios obtained for respective parent strains; analyses were performed on broth-grown cells.
Ratios were normalized against ld-Hep. Inner ld-Hep (Hepl) is not released due to phosphorylation.
Broth-grown cells.
Fermentor-grown cells.
FIG. 4.
FAB-MS spectra of permethylated H. pylori LPS in the positive mode, indicating characteristic primary and secondary fragments. (A) strain 26695; (B) 26695::HP0479kan mutant strain; (C) complemented 26695::HP0479-HP480::cam strain.
FIG. 5.
CE-MS and CE-MS/MS (positive-ion mode) analysis of delipidated LPSs from H. pylori SS1::HP0479kan, 26695::HP0479kan, and O:3::HP0479kan mutants. Separation conditions: 10 mM ammonium acetate containing 5% methanol, pH 9.0, 25 kV. (A) Extracted mass spectra at 14.6 min for strains SS1::HP0479kan, 26695::HP0479kan, and O:3::HP0479kan. (B) Tandem mass spectrum of precursor ions at m/z 1392. (C) Tandem mass spectrum of precursor ions at m/z 1612. Separation conditions were as for panel A except nitrogen collision gas, Elab: 60 eV (laboratory frame of reference).
TABLE 2.
Positive-ion CE-MS of LPSs from H. pylori SS1, 26695, O:3, and PJ1 HP0479 mutants
Average mass units were used for calculation of molecular weights, based on proposed composition as follows: Hex, 162.15; HexNAc, 203.20; Fuc, 146.14; Hep, 192.17; KDO, 220.18; PEA, 123.05; H2O, 18.02.
Observed fragment ion corresponds to anhydro-KDO-containing glycoform.
Most abundant glycoform in LPS HP0479 mutants of strains SS1 and O:3 (based on the fragment ion intensity).
Most abundant glycoform in LPS HP0479 mutant of strain PJ1 (based on the fragment intensity).
Glycoforms present in LPS HP0479 mutant of strain PJ1.
Glycoforms present in LPS HP0479 mutants of strains SS1, 26695, and O:3.
Most abundant glycoform in LPS HP0479 mutant of strain 26695 (based on the fragment ion intensity).
FIG. 6.
LPS structures of H. pylori strains 26695, SS1, O:3, and PJ1 and corresponding HP0479 mutants. (A) LPS structures from strains SS1, 26695, O:3, and PJ1. (B) HP0479 mutant LPS structure.
Structural analysis of H. pylori LPS from the complemented 26695::HP0479-HP0480cam strain.
SDS-PAGE and Western blot analyses of the complemented 26695::HP0479-HP480cam strain showed complete restoration of the O chain (Fig. 3) although only minor restoration of LeX reactivity. Comparison of the sugar ratios of LPS from the restored strain with those obtained for the parental 26695 strain confirmed the restoration of the O chain, although as is commonly seen in isolates cultured on solid agar, the chain appears to be shortened. Additionally, restoration of dd-Hep in the linking region and α-1,6-glucan moiety (Table 1) was observed. FAB-MS analysis of the permethylated LPS of the 26695 parent and complemented 26695 strains (Fig. 4A and C) showed several A-type primary and secondary glycosyl oxonium ions, consistent with the presence of LeY determinants at m/z 812 and m/z 606 and other blood group-related residues at m/z 682 [Fuc, GlcNAc, Hep]+. Consistent with the immunoblot data, only a negligible amount of LeX epitope at m/z 432 was detected. Furthermore, methylation analysis of the purified LPS from the complemented HP0479-HP0480::cam 26695 strain confirmed the presence of smooth-type LPS and showed the presence of terminal and 3-linked Fuc; terminal, 3-linked, and 6-linked Glc; 2-linked Gal; significant amounts of 2,7-linked dd-Hep; 2-, 3-, and 6-linked dd-Hep; and 3-, 4-, and 3,4-linked GlcNAc. Consistent with sugar analysis, somewhat lower ratios of 2-, 6-, and 7-linked dd-Hep and 4-linked and 3,4-linked d-GlcNAc were obtained compared with the linkage analysis data for the parental 26695 strain, consistent with reduction in the O-chain length of the complemented 26695::HP0479-HP480cam strain. The amount of 6-linked Glc remained unchanged, suggesting complete restoration of the α-1,6-glucan moiety. In addition, significantly less 4-linked Glc was present, a contaminant previously found to be produced by some strains of H. pylori and associated with β-1,4-glucan (23).
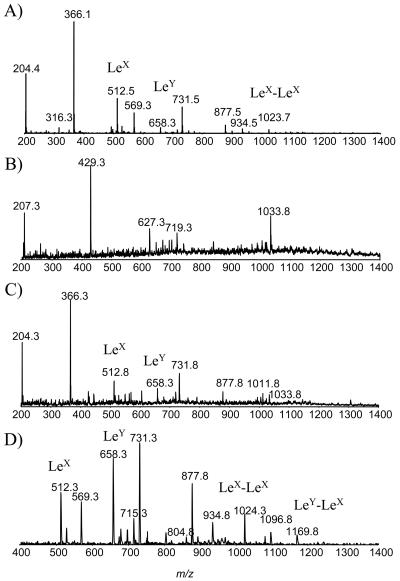
To confirm the complete restoration of the O-chain polysaccharide, delipidated LPS from the H. pylori HP0479-HP0480::cam strain was subjected to an IS-CID-MS analysis (27). This technique allows in-source fragmentation of the O-chain polysaccharide, thus confirming its sequence. The resulting IS-CID-MS spectrum was consistent with the presence of both LeY and LeX determinants and fragment ions corresponding to single repeating units of LeX (m/z 512.3) and LeY (m/z 658.3), and double repeating units of LeY-LeX at m/z 1169.4 and LeX-LeX at m/z 1023 were observed (Fig. 7C and D). When delipidated H. pylori 26695 LPS was subjected to IS-CID-MS analysis, similar results were obtained, although the intensity of fragment ions at m/z 1023.8, corresponding to LeX-LeX disaccharide, was significantly higher than the intensity of fragment ions at m/z 1169.4, corresponding to LeY-LeX (Fig. 7A). With the exception of terminal LeX structures, the complementation with a wild-type copy of HP0479 had completely restored a parental O-chain phenotype. It is well known that subculture of Helicobacter on agar media can lead to a reduction in O-chain length, the consequence of which, at least in this case, appears to be preferential capping by LeY. IS-CID-MS analysis of the 26695::HP0479kan mutant LPS confirmed the absence of the Le antigen-containing O-chain polysaccharide (Fig. 7B).
FIG. 7.
CE-IS-CID-MS (m/z 200 to1400) analysis of delipidated H. pylori LPS, using an orifice voltage of 200 V. (A) Extracted mass spectrum for strain 26695; (B) extracted mass spectrum for 26695::HP0479kan mutant strain; (C) extracted mass spectrum for the complemented 26695::HP0479-HP0480 strain; (D) extracted spectrum in the precursor ion scan for m/z 204. The collision energy was ramped from 40 to 60 V for the scan range of m/z 400 to 1400, with nitrogen as the collision gas.
Mouse colonization experiments.
The effects of the HP0479 mutation on colonization were investigated using H. pylori strains SS1 and PJ1. Experiments were performed in duplicate. The SS1 strain has been shown to consistently colonize mice and has been universally used as a mouse colonization model for H. pylori (7, 11, 26, 29). The clinical isolate PJ1 has also been shown to consistently colonize mice stomachs, although at reduced rates compared to SS1 (32). In all cases, the parental strains colonized the mice as expected, while with the HP0479 mutant strains colonization was abolished (Table 3).
TABLE 3.
Viable counts of H. pylori recovered from mice at 4 weeks postinoculation
| Inoculum strain | Log10 CFU (mean ± SD from 5 mice) |
|---|---|
| SS1 | 5.82 ± 0.12 |
| SS1::HP0479kan | BDLa |
| PJ1 | 4.79 ± 0.572 |
| PJ1::HP0479kan | BDL |
BDL, below detectable limits.
DISCUSSION
We have cloned an α-1,2-d-glycero-d-manno-heptosyltransferase (dd-heptosyltransferase) gene, HP0479, from H. pylori 26695. Structural analysis of a number of H. pylori HP0479kan mutants from both typeable and nontypeable strains confirmed that the HP0479kan mutant LPS was truncated at DDHepI, which connects the outer core region oligosaccharide and the O chain, leaving the core backbone oligosaccharide intact and indicating that the HP0479 gene product likely adds the critical branching dd-heptose (DDHepII) to the LPS. This structural element of the H. pylori LPS is conserved among all strains studied so far (2, 33). To our knowledge, this is the first reported dd-heptosyltransferase cloned from H. pylori and only the second dd-heptosyltransferase gene to be characterized thus far. The dd-heptosyltransferase gene lbgB from Haemophilus ducreyi has been identified by Tn916 transposon mutagenesis, cloned, and characterized (12, 15, 53). The lbgB gene encodes an α-1,6-d-glycero-d-manno-heptosyltransferase that adds dd-heptose to glucose in the H. ducreyi lipooligosaccharide.
The genetics of LPS assembly in H. pylori has not been well characterized. The completion of the genome sequence of two strains (1, 51) facilitated the annotation of 22 putative LPS glycosyltransferase genes based on homologies to LPS genes from other organisms, but only 4 of these have been functionally characterized. These include three fucosyltransferases which are involved in expression of Lewis antigens (31, 41, 55). In addition, Logan and coworkers (29) used functional genomics to identify and conclusively show that the HP0826 gene encoded the β-1,4-galactosyltransferase which was critical in O-chain backbone biosynthesis. Mutagenesis of this gene in H. pylori SS1, a strain able to colonize the murine stomach, resulted in significantly reduced colonization levels. Endo and coworkers (10) cloned and expressed the homologous β-1,4-galactosyltransferase gene from H. pylori NCTC11637, giving further evidence for its function.
To date none of the dd-heptosyltransferases involved in the biosynthesis of the core oligosaccharide or the addition of dd-heptose to the dd-heptan-linking region have been functionally identified, although the waaC and waaF genes, which add LDHepI and LDHepII, respectively, have been identified based on sequence homology to analogous genes in other organisms. Even with the availability of genome sequences, identification of new heptosyltransferases by homology searches is difficult, since very few examples of organisms that incorporate dd-heptose into their LPS exist and the nucleotide or amino acid sequences for only two dd-heptosyltransferases are available. Structural data on the LPSs from several strains of H. pylori (33) indicate that there may potentially be at least seven different dd-heptosyltransferases responsible for addition of heptose residues to the core of H. pylori LPS. Unlike in other gram-negative bacteria (C. jejuni, Escherichia coli, and Salmonella spp.), the genes responsible for the assembly of the LPS in H. pylori are not clustered together, compounding the problem of gene identification.
Functional characterization of this dd-heptosyltransferase has not been described due to a lack of the appropriate synthetic donor-acceptor molecules for in vitro assays and the difficulty in producing the necessary truncated H. pylori LPS molecule to use as acceptor. Consequently, it is necessary to rely on structural analysis of LPSs from isogenic mutants for indication of function. While this approach may fail to address the issue of secondary effects on LPS biosynthesis, in the current study mutational analysis of isogenic mutants from a number of different strains of Helicobacter which produce unique outer core structures, as well as complementation experiments, gives strength to the functional assignment. In all isogenic mutants generated, the identical truncated structure was observed, pointing to a direct role for HP0479 as a α-1,2-dd-heptosyltransferase. In addition, structural analysis of LPS has proven to be a reliable method for the functional characterization of glycosyltransferases involved in LPS assembly from other bacterial species (19, 21, 46). The absence of DDHepII in the side chain, which serves as a link between the O chain and the core oligosaccharide, resulted in the loss of O chain and dd-heptoglycan (for serotype O:3). In addition, all mutant LPSs also lacked a 3-linked Glc in the backbone oligosaccharide, an observation which was also made for the LPSs from a number of parental strains (32) and is likely not a direct effect of this mutation. CE-MS analysis of the delipidated LPS confirmed the presence of glycoforms containing [HexNAc,Fuc] or [Fuc] capping at position O-2 of DDHepI in approximately half of all possible structural variants, while the other major glycoform contained the backbone oligosaccharide structure HexHexHepHepHep(PE)KDO (Table 2) as confirmed by methylation and CE-MS analyses. In all cases the O chain and α-1,6-glucan were lost as indicated by methylation analysis, and the mutants of 26695, O:3, and SS1 no longer reacted with LeX- or LeY-specific antisera. These results confirm that the function of the HP0479 gene product was the addition of a first branching dd-heptose of the core oligosaccharide.
The HP0479 activity was restored by the reintroduction of the wild-type gene back into the chromosome of strain 26695 by “antibiotic cassette swapping.” This method of reintroducing a wild-type gene into the chromosome was preferable over plasmid complementation methods in that the correct copy number and location of the gene are restored. The mutant strain into which a wild-type copy of HP0479 was introduced via insertion into the chromosome resulted in the restoration of the parental LPS phenotype as evidenced by migration on SDS-PAGE and reactivity with anti-LeY antibodies. Interestingly, while all structural features of the parental 26695 LPS were restored, only a negligible amount of LeX antigen could be detected by immunoblotting and FAB-MS in the mutant LPS. However, both methods are based on the detection of the outer extremities of LPS molecule and do not give any information on internal sequences. In order to overcome this problem, we have applied a recently developed IS-CID-MS method for the direct analysis of polysaccharides (27). CE-MS analysis of delipidated LPS from the restored H. pylori 26695 strain confirmed the complete restoration of LeY and internal LeX residues. The apparent lack of LeX expression as observed by immunoblotting and FAB-MS was shown to actually represent only loss of capping LeX structures. The more thorough IS-CID-MS analysis revealed that both LeX and LeY structures are indeed restored.
The role of Le antigens in colonization is currently unclear. Earlier work by Takata and coworkers (49) using C3H/HeJ mice indicated that Le antigen expression was not necessary for colonization, while Suresh and coworkers (47) observed colonization of mice by a strain of H. pylori that did not express detectable Le antigens. Previous studies showed that mutant strains unable to express O chain (Le antigen) but which retained an outer core structure displayed only a moderate reduction in colonization (approximately 18%) (29), while deeper truncation of the outer core of LPS (HP0159) showed an even more marked reduction (48%) in colonization (S. M. Logan and coworkers, unpublished data). In contrast, Martin and coworkers (31) reported that a LeX/Y double fucT mutant of H. pylori, strain 4187E-KO379/651, showed markedly reduced colonization compared with the parent strain in mouse colonization studies using HSD/ICR mice. Structural studies on LPS from this mutant indicated that while Le antigens were no longer present, H type 1 and H type 1-LacNAc-LacNAc structures were still present, although data on the length of the O chain in this particular mutant were not reported. Our current data clearly demonstrate that an extended core structure is necessary for colonization in the mouse model even in the absence of Le epitopes. The HP0479 mutation was introduced into two mouse-colonizing strains of H. pylori, SS1 and clinical isolate PJ1. Both parent strains colonize mice, with strain PJ1 colonizing to a lesser extent (32). Wild-type SS1 expresses LeY, while strain PJ1 lacks Le antigens and instead produces a long dd-heptoglycan (32). The HP0479 mutation led to phenotypically similar truncated LPS structures, and colonization ability was abolished for both mutant strains.
In summary, we have found that the HP0479 gene from H. pylori 26695 likely encodes an α-1,2-d-glycero-d-manno-heptosyltransferase, and this is the first dd-heptosyltransferase from H. pylori to be characterized. HP0479 appears to add DDHepII to the core backbone oligosaccharide. DDHepII is a critical residue providing the link between the core oligosaccharide backbone and the O chain through the dd-heptan region. Our data provides evidence that the length of the LPS may be a significant factor contributing to colonization regardless of Le antigen status. Hynes and coworkers (20) made similar observations, showing that there was a relationship between the length of the LPS and the ability of H. pylori to colonize mice. A minimal length may be required for colonization, either for LPS to interact with the environment or for survival of the organism in the stomach. This study has generated, through insertional mutagenesis, a conserved deeply truncated LPS structure which offers potential as a conserved target of H. pylori.
Acknowledgments
We thank Tom Devecseri for assistance with photography and Sonia Leclerc and Anna Cunningham for DNA sequencing and oligonucleotide primer biosynthesis.
REFERENCES
- 1.Alm, R. A., L. S. L. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. deJonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jiang, D. E. Taylor, G. F. Vovis, and T. Trust. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176-180. [DOI] [PubMed] [Google Scholar]
- 2.Altman, E., N. Smirnova, J. Li, A. Aubry, and S. M. Logan. 2003. Occurrence of a nontypable Helicobacter pylori strain lacking Lewis blood group O antigens and dd-heptoglycan: evidence for the role of the core α1,6-glucan chain in colonization. Glycobiology 13:777-783. [DOI] [PubMed] [Google Scholar]
- 3.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brisson, J. R., E. Crawford, D. Uhrin, N. H. Khieu, M. B. Perry, W. B. Severn, and J. C. Richards,. 2002. The core oligosaccharide component from Mannheimia (Pasteurella) haemolytica serotype A1 lipopolysaccharide contains l-glycero-d-manno- and d-glycero-d-manno-heptoses: analysis of the structure and conformation by high-resolution NMR spectroscopy. Can. J. Chem. 80:949-963. [Google Scholar]
- 5.Campbell, J. A., G. J. Davies, V. Bulone, and B. Henrissat. 1997. A classification of nucleotide-diphospho-sugar glycosyltransferases based on amino acid sequence similarities. Biochem. J. 326:929-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciucanu, I., and F. Kerek. 1984. A simple and rapid method for the permethylation of carbohydrates. Carbohydr. Res. 131:209-217. [Google Scholar]
- 7.Conlan, J. W., R. Kuolee, A. Webb, and M. B. Perry. 1999. Immunosuppression by a corticosteroid fails to exacerbate Helicobacter pylori infection in a mouse model of gastric colonisation. Can. J. Microbiol. 45:975-980. [PubMed] [Google Scholar]
- 8.Coutinho, P. M., E. Deleury, G. J. Davies, and B. Henrissat. 2003. An evolving hierarchical family classification for glycosyltransferases. J. Mol. Biol. 328:307-317. [DOI] [PubMed] [Google Scholar]
- 9.Dubois, M., K. A. Gilles, J. K. Hamilton, P. A. Rebers, and F. Smith. 1956. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28:350-356. [Google Scholar]
- 10.Endo, T., S. Koizumi, K. Tabata, and A. Ozaki. 2000. Cloning and expression of β1,4-galactosyltransferase gene from Helicobacter pylori. Glycobiology 10:809-813. [DOI] [PubMed] [Google Scholar]
- 11.Ferrero, R. L., J.-M. Thiberge, M. Huerre, and A. Labigne. 1998. Immune response of specific-pathogen-free mice to chronic Helicobacter pylori (strain SS1) infection. Infect. Immun. 66:1349-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filiatrault, M. J., B. W. Gibson, B. Schilling, S. Sun, R. S. Munson, Jr., and A. A. Campagnari. 2000. Construction and characterization of Haemophilus ducreyi lipooligosaccharide (LOS) mutants defective in expression of heptosyltransferase III and β1,4-glucosyltransferase: identification of LOS glycoforms containing lactosamine repeats. Infect. Immun. 68:3352-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galarneau, C., S. Rioux, and M. Jacques. 2001. Core oligosaccharide mutants of Actinobacillus pleuropneumoniae serotype 1 obtained by mini-Tn10 mutagenesis. Unpublished sequence submitted to GenBank.
- 14.Gerwig, G. J., J. P. Kamerling, and J. F. G. Vliegenthart. 1979. Determination of the absolute configuration of the monosaccharides in complex carbohydrates by capillary G.L.C. Carbohydr. Res. 77:1-7. [DOI] [PubMed] [Google Scholar]
- 15.Gibson, B. W., A. A. Campagnari, W. Melaugh, N. J. Phillips, M. A. Apicella, S. Grass, J. Wang, K. L. Palmer, and R. S. Munson, Jr. 1997. Characterization of a transposon Tn 916-generated mutant of Haemophilus ducreyi 35000 defective in lipooligosaccharide synthesis. J. Bacteriol. 79:5062-5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haas, R., T. F. Meyer, and J. P. M. Van Puten. 1993. Aflagellated mutants of Helicobacter pylori generated by genetic transformation of naturally competent strains using transposon shuttle mutagenesis. Mol. Microbiol. 8:753-760. [DOI] [PubMed] [Google Scholar]
- 17.Hitchcock, P. J., and T. M. Brown. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 154:269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holst, O. 1999. Chemical structure of the core region of lipopolysaccharides. p. 115-154. In H. Brade, S. M. Opal, S. N. Vogel, and D. C. Morrison. (ed.), Endotoxin in health and disease. Marcel Dekker, Inc., New York, N.Y.
- 19.Hood, D. W., M. E. Deadman, T. Allen, H. Masoud, A. Martin, J.-R. Brisson, R. Fleischmann, J. C. Richards, and E. R. Moxon. 1996. Use of the complete genome sequence information of Haemophilus influenzae strain Rd to investigate lipopolysaccharide biosynthesis. Mol. Microbiol. 22:951-965. [DOI] [PubMed] [Google Scholar]
- 20.Hynes, S. O., H. Sjunnesson, E. Sturegard, T. Wadstrom, and A. P. Moran. 1999. A relationship between the expression of high molecular weight lipopolysaccharides by Helicobacter pylori and colonising ability in mice, p. 148. In H. L. T. Mobley et al. (ed.), Abstracts of the 10th International Workshop on Campylobacter, Helicobacter, and Related Organisms. University of Maryland Press, College Park, Maryland.
- 21.Kaniuk, N. A., M. Monteiro, C. T. Parker, and C. Whitfield. 2002. Molecular diversity of the genetic loci responsible for lipopolysaccharide core oligosaccharide assembly within the genus Salmonella. Mol. Microbiol. 46:1305-1318. [DOI] [PubMed] [Google Scholar]
- 22.Kidd, M., K. Miu, L. H. Tang, G. I. Pérez-Pérez, M. J. Blaser, A. Sandor, and I. M. Modlin. 1997. Helicobacter pylori lipopolysaccharide stimulates histamine release and DNA synthesis in rat enterochromaffin-like cells. Gastroenterology 113:1110-1117. [DOI] [PubMed] [Google Scholar]
- 23.Knirel, Y. A., N. A. Kocharova, S. O. Hynes, G. Widmalm, L. P. Andersen, P.-E. Jansson, and A. P. Moran. 1999. Structural studies on lipopolysaccharides of serologically nontypable strains of Helicobacter pylori, AF1 and 007, expressing Lewis antigenic determinants. Eur. J. Biochem. 266:123-131. [DOI] [PubMed] [Google Scholar]
- 24.Labigne-Roussel, A., P. Courcoux, and L. Tompkins. 1988. Gene disruption and replacement as a feasible approach for mutagenesis of Campylobacter jejuni. J. Bacteriol. 170:1704-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 26.Lee, A., J. O'Rourke, M. Corazon de Ungria, B. Robertson, G. Daskaolopoulos, and M. F. Dixon. 1997. A standardised mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 112:1386-1397. [DOI] [PubMed] [Google Scholar]
- 27.Li, J., Z. Wang, and E. Altman. 2005. In-source fragmentation and analysis of polysaccharides by capillary electrophoresis/mass spectrometry. Rapid Commun. Mass Spectrom. 19:1305-1314. [DOI] [PubMed] [Google Scholar]
- 28.Logan, S. M., and T. J. Trust. 1984. Structural and antigenic heterogeneity of lipopolysaccharides of Campylobacter jejuni and Campylobacter coli. Infect. Immun. 45:210-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Logan, S. M., J. W. Conlan, M. A. Monteiro, W. W. Wakarchuk, and E. Altman. 2000. Functional genomics of Helicobacter pylori: identification of a β-1,4 galactosyltransferase and generation of mutants with altered lipopolysaccharide. Mol. Microbiol. 35:1156-1167. [DOI] [PubMed] [Google Scholar]
- 30.Marionneau, S., A. Cailleau-Thomas, J. Rocher, B. Le Moullac-Vaidye, N. Ruvoën, M. Clement, and J. Le Pendu. 2001. ABH and Lewis histo-blood group antigens, a model for the meaning of oligosaccharide diversity in the face of a changing world. Biochimie 83:565-573. [DOI] [PubMed] [Google Scholar]
- 31.Martin, S. L., M. R. Edbrooke, T. C. Hodgman, D. H. van den Eijnden, and M. I. Bird. 1997. Lewis X biosynthesis I Helicobacter pylori: molecular cloning of an α(1-3)-fucosyltransferase gene. J. Biol. Chem. 272:21349-21356. [DOI] [PubMed] [Google Scholar]
- 32.Monteiro, M. A., F. St. Michael, D. A. Rasko, D. E. Taylor, J. W. Conlan, K. H. Chan, S. M. Logan, B. J. Appelmelk, and M. B. Perry. 2001. Helicobacter pylori from asymptomatic hosts expressing heptoglycan but lacking Lewis O-chains: Lewis blood-group O-chains may play a role in Helicobacter pylori induced pathology. Biochem. Cell Biol. 79:449-459. [PubMed] [Google Scholar]
- 33.Monteiro, M. A. 2002. Helicobacter pylori: a wolf in sheep's clothing: the glycotype families of Helicobacter pylori lipopolysaccharides expressing histo-blood groups: structure, biosynthesis, and role in pathogenesis. Adv. Carbohydr. Chem. Biochem. 57:99-158. [DOI] [PubMed] [Google Scholar]
- 34.Moran, A. P. 1995. Cell surface characteristics of Helicobacter pylori. FEMS Immunol. Med. Microbiol. 10:271-280. [DOI] [PubMed] [Google Scholar]
- 35.Myers, E. W., and W. Miller. 1988. Optimal alignments in linear space. Comput. Appl. Biosci. 4:11-17. [DOI] [PubMed] [Google Scholar]
- 36.Okumura, T., E. Shoji, N. Takahashi, H. Wakebe, K. Imagawa, M. Kikuchi, and Y. Kohgo. 1998. Delayed gastric emptying by Helicobacter pylori lipopolysaccharide in conscious rats. Dig. Dis. Sci. 43:90-94. [DOI] [PubMed] [Google Scholar]
- 37.Ootsubo, C., T. Okumura, N. Takahashi, H. Wakebe, K. Imagawa, M. Kikuchi, and Y. Kohgo. 1997. Helicobacter pylori lipopolysaccharide inhibits acid secretion in pylorus-ligated conscious rats. Biochem. Biophys. Res. Commun. 236:532-537. [DOI] [PubMed] [Google Scholar]
- 38.Piotrowski, J., E. Piotrowski, D. Skrodzka, A. Slomiany, and B. L. Slomiany. 1997. Induction of acute gastritis and epithelial apoptosis by Helicobacter pylori lipopolysaccharide. Scan. J. Gastroenterol. 32:203-211. [DOI] [PubMed] [Google Scholar]
- 39.Piotrowski, J., D. Skrodzka, A. Slomiany, and B. L. Slomiany. 1997. Reversal of gastric somatostatin receptor inhibition by Helicobacter pylori lipopolysaccharide with ebrotidine and sulglycotide. Gen. Pharmacol. 28:705-708. [DOI] [PubMed] [Google Scholar]
- 40.Raetz, C. R. H. 1990. Biochemistry of endotoxins. Annu. Rev. Biochem. 59:129-170. [DOI] [PubMed] [Google Scholar]
- 41.Rasko, D. A., G. Wang, M. M. Palcic, and D. E. Taylor. 2000. Cloning and characterization of the α(3/4) fucosyltransferase of Helicobacter pylori. J. Biol. Chem. 275:4988-4994. [DOI] [PubMed] [Google Scholar]
- 42.Sakagami, T., J. Vella, M. F. Dixon, J. O'Rourke, R. Radcliffe, and P. Sutton. 1997. The endotoxin of Helicobacter pylori is a modulator of host-dependent gastritis. Infect. Immun. 65:3462-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 44.Senchenkova, S. N., G. V. Zatonsky, S. O. Hynes, G. Widmalm, L. P. Andersen, Y. A. Knirel, P. E. Jansson, and A. P. Moran. 2001. Structure of a d-glycero-d-manno-heptan from the lipopolysaccharide of Helicobacter pylori. Carbohydr. Res. 331:219-224. [DOI] [PubMed] [Google Scholar]
- 45.St. Michael, F., J.-R. Brisson, S. Larocque, M. Monteiro, J. Li, M. Jacques, M. B. Perry, and A. D. Cox. 2004. Structural analysis of the lipopolysaccharide derived core oligosaccharides of Actinobacillus pleuropneumoniae serotypes 2, 5a and the genome strain 5b. Carbohydr. Res. 339:1973-1984. [DOI] [PubMed] [Google Scholar]
- 46.Sun, S., N. K. Scheffler, B. W. Gibson, J. Wang, and R. S. Munson, Jr. 2002. Identification and characterization of the N-acetylglucosamine glycosyltransferase gene of Haemophilus ducreyi. Infect. Immun. 70:5887-5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suresh, M. R., M. B. Fanta, J. Kriangkum, Q. Jiang, and D. E. Taylor. 2000. Colonization and immune responses in mice to Helicobacter pylori expressing different Lewis antigens. J. Pharmacol. Pharmaceut. Sci. 3:234-241. [PubMed] [Google Scholar]
- 48.Süsskind, M., L. Brade, H. Brade, and O. Holst. 1998. Identification of a novel heptoglycan of α1-2 linked d-glycero-d-manno-heptopyranose. J. Biol. Chem. 273:7006-7017. [DOI] [PubMed] [Google Scholar]
- 49.Takata, T., E. El-Omar, M. Camorlinga, S. A. Thompson, Y. Minohara, P. B. Ernst, and M. J. Blazer. 2002. Helicobacter pylori does not require Lewis X or Lewis Y expression to colonize C3H/HeJ mice. Infect. Immun. 70:3073-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thibault, P., J. Li, A. Martin, J. C. Richards, D. W. Hood, and E. R. Moxon. 1998. Electrophoretic and mass spectrometric strategies for the identification of lipopolysaccharides and immunodeterminants in pathogenic strains of Haemophilus influenzae; application to clinical isolates, p. 439-462. In A. L. Burlingame et al. (ed.), Mass spectrometry in biology and medicine. Humana Press, Totowa, N.J.
- 51.Tomb, J.-F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zou, E. F. Kirkness, S. Peterson, B. Lotus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, E. Hickey, D. E. Berg, J. D. Gocayne, T. R. Utterback, J. D. Peterson, J. M. Kelley, M. D. Cotton, J. M. Weidman, C. Fujii, C. Bowman, L. Watthey, E. Wallin, W. S. Haynes, M. Borodovsky, P. D. Karp, H. O. Smith, C. M. Fraiser, and J. C. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 52.Tsai, C. M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharide in polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]
- 53.Tullius, M. V., N. J. Phillips, N. K. Scheffler, N. M. Samuels, R. S. Munson, Jr., E. J. Hansen, M. Stevens-Riley, A. A. Campagnari, and B. W. Gibson. 2002. The lbgAB gene cluster of Haemophilus ducreyi encodes a β-1,4-galactosyltransferase and an α-1,6-dd-heptosyltransferase involved in lipooligosaccharide biosynthesis. Infect. Immun. 70:2853-2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vinogradov, E. V., Z. Sidorczyk, and Y. A. Knirel. 2002. Structure of the core part of the lipopolysaccharides from Proteus penneri strains 7, 8, 14, 15, and 21. Carbohydr. Res. 337:643-649. [DOI] [PubMed] [Google Scholar]
- 55.Wang, G., P. G. Boulton, N. W. Chan, M. M. Palcic, and D. E. Taylor. 1999. Novel Helicobacter pylori α1,2-fucosyltransferase, a key enzyme in the synthesis of Lewis antigens. Microbiology 145:3245-3253. [DOI] [PubMed] [Google Scholar]
- 56.Westphal, O., and K. Jann. 1965. Bacterial lipopolysaccharides. Extraction with phenol-water and further applications of the procedure. Methods Carbohydr. Chem. 5:83-91. [Google Scholar]
- 57.Wugeditsch, T., N. E. Zachara, M. Puchberger, P. Kosma, A. A. Gooley, and P. Messner. 1999. Structural heterogeneity in the core oligosaccharide of the S-layer glycoprotein from Aneurinibacillus thermoaerophilus DSM 10155. Glycobiology 9:787-795. [DOI] [PubMed] [Google Scholar]
- 58.Yao, R., R. A. Alm, T. J. Trust, and P. Guerry. 1993. Construction of new Campylobacter cloning vectors and a new mutational cat cassette. Gene 130:127-130. [DOI] [PubMed] [Google Scholar]
- 59.York, W. S., A. G. Darvill, M. McNeil, T. T. Stevenson, and P. Albersheim. 1985. Isolation and characterization of plant cell walls and cell-wall components. Methods Enzymol. 118:3-40. [Google Scholar]