Abstract
Several genetic systems that allow the use of iron-protoporphyrin IX (heme) have been described for the pathogenic bacterium Neisseria meningitidis. However, many questions about the process of heme acquisition and utilization remain to be answered. To isolate and analyze unidentified genes that play a role in heme iron uptake and utilization, a Himar1 transposon mutant library was screened in N. meningitidis serogroup A strain IR4162. One locus identified by transposon mutagenesis conferred protection against heme toxicity. A mutant with a deletion in a gene termed ght (gene of hydrophobic agent tolerance) within this locus was susceptible to heme and other hydrophobic agents compared to the parental strain. Transcriptional analysis indicated that ght is cotranscribed with an upstream open reading frame NMA2149. Uncharacterized orthologues of ght were identified in many other gram-negative bacteria. We present genetic evidence for the importance of ght in resistance to hydrophobic agents and its potential role in interaction with other hydrophobic agent resistance mechanisms within N. meningitidis.
The success of a microorganism within its host often depends on that microbe's ability to scavenge iron. Host proteins, such as hemoglobin and myoglobin, contain iron in the form of iron-protoporphyrin IX (e.g., heme). It has been established that iron contained in heme can serve as the source of iron in a wide range of bacteria. Heme also performs a crucial role in many metabolic processes within the cell (20, 29, 33, 35). However, the internal presence of heme, or excess iron resulting from its degradation, can have detrimental effects because heme can also facilitate oxidative damage within the bacteria. The bacterial response to the detrimental effects of heme can be readily observed in stringent iron regulation of the expression of genes involved in heme and/or iron uptake (7, 9, 28). Heme has been shown to be toxic at low concentrations for Staphylococcus aureus, Streptococcus faecalis, and Bacillus cereus (15). Thus, bacteria must take up and utilize heme by mechanisms that also allow for protection against the toxic side effects caused by heme.
Heme uptake and utilization has been studied extensively in the meningococci. Two phase-variable TonB-dependent hemoglobin uptake systems have been described previously, the bipartite receptor, HpuAB, and the one-component receptor, HmbR (18, 30). In addition, a cytoplasmic heme oxygenase, HemO, has also been described, which allows for the extraction of iron from heme and subsequently provides a measure of protection against the toxicity of heme itself (37). However, no inner membrane transport system similar to those of other heme iron-utilizing bacteria has been thus far identified in meningococci. Moreover, the utilization of heme without a protein ligand and in a TonB-independent manner is known to be possible, but the mechanism for this process has yet to be elucidated (32). A recent study on the growth of Neisseria gonorrhoeae under iron restriction has shown that spontaneous genetic changes can result in bacterial growth by the use of heme, dissociated from hemoglobin, as the sole iron source. This process occurs in an HpuAB-independent manner (3). Two classes of mutants were identified that could use heme through this process. One class was identified as a specific point mutation in the pilQ locus, the gene coding for the type IV pilus secretin (8), while the other class remains unidentified. The point mutation in pilQ is predicted to allow for increased entrance of heme, as well other hydrophobic agents (HAs), including antibiotics, through the mutated secretin. This study indicated that the passage of heme, itself a hydrophobic compound, and other HAs across the outer membrane may be possible through channel-like proteins similar to PilQ.
Toxicity of HAs and mechanisms of HA resistance (HAr) have been extensively studied in the gonococci (2, 10, 11, 26, 36). The gonococcal Mtr efflux system is a member of the resistance-nodulation-division family of energy-dependent efflux pumps. It has been shown to be involved in the efflux of hydrophobic drugs, dyes, detergents, antibacterial peptides, and protoporphyrin IX/metalloporphyrins. The expression of the Mtr system has been found to be positively regulated by the AraC-like activator MtrA and negatively controlled by the repressor MtrR; mutations in mtrR result in high levels of HAr. HA toxicity in the meningococci has been only recently studied. Two recent genome sequencing projects have identified the Mtr counterparts in Neisseria meningitidis, and a recent study of mtr regulation suggests that they are not under the control of MtrA and MtrR but are regulated by integration host factor in a transcriptional and posttranscriptional manner involving a 155- to 159-bp Correia element that is inserted upstream of the mtrCDE operon (21, 24, 34).
In this study, a transposon library was screened to identify additional genes involved in uptake and utilization of heme iron in N. meningitidis. We identified a gene (ght) that confers protection to meningococci against heme toxicity. We report that ght is important in the protection of meningococci against the toxicity of not only heme but other HAs as well through a process independent of PilQ or the MtrC-MtrD-MtrE efflux system. We also show that homologues of ght can be identified throughout the genomes of many gram-negative organisms and that these homologues retain discrete conserved regions of unknown functional importance. (A preliminary account of these results was presented at the 14th International Pathogenic Neisseria Conference held in Milwaukee, Wis., 5 to 10 September, 2004.)
MATERIALS AND METHODS
Bacterial strains and media.
The N. meningitidis, N. gonorrhoeae, and Escherichia coli strains and plasmids used in these experiments are listed in Table 1. Neisseria strains were routinely cultured at 37°C under 5% CO2 (vol/vol) atmosphere on GC medium base agar (Difco Laboratories, Detroit, MI) or in GC broth (1.5% Proteose peptone no. 3 [Difco], 0.4% K2HPO4 [Sigma-Aldrich, St. Louis, MO], 0.1% KH2PO4 [Sigma-Aldrich], 0.5% NaCl [Sigma-Aldrich]) with Kellogg's supplements I and II. In some cases, meningococci were grown in brain heart infusion broth (Difco) with 0.043% NaHCO3 (wt/vol). N. meningitidis transconjugants were selected on modified Thayer-Martin II media (BBL), in which 100 μl of a 1.25-mg/ml chloramphenicol solution was spread on the plate and allowed to absorb. E. coli was grown at 37°C in LB (Luria-Bertani broth). Where indicated, the following antibiotics were used with meningococci: kanamycin at 50 μg/ml, erythromycin at 3 μg/ml, streptomycin at 750 μg/ml, chloramphenicol at 5 μg/ml, rifampin at 3 μg/ml, and tetracycline at 2 μg/ml. Work with E. coli used kanamycin at 50 μg/ml, erythromycin at 300 μg/ml, chloramphenicol at 30 μg/ml, and tetracycline at 10 μg/ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| Neisseria spp. | ||
| KH14 | mtrD::aphA-3 derivative of FA19 (gonococcus) | 10 |
| 276D | ght::Himar 1 derivative of IR4162 | This study |
| IR4048 | N. meningitidis serogroup A clinical isolate | Laboratory collection |
| IR4162 | hpuB::ermC derivative of IR4048 | 1 |
| IR5571 | ght::aphA-3 gloA+ derivative of IR4048 | This study |
| IR5627 | ght::Himar1 retransformation derivative of IR4162 | This study |
| IR5575 | spontaneous Strr derivative of IR4048 | This study |
| IR5579 | gloA::aphA-3 derivative of IR4048 | This study |
| IR5648 | PilQ::cm N. meningitidis serogroup B | 34a |
| IR5694 | ght::ermC-rpsL derivative of IR5575 | This study |
| IR5700 | Δght derivative of IR5694 | This study |
| IR5721 | mtrD::aphA-3 derivative of IR5575 | This study |
| IR5725 | mtrD::aphA-3 derivative of IR5700 | This study |
| IR5737 | IR4048 containing unaltered pHT128 | This study |
| IR5738 | IR4048 containing pAWR3088 | This study |
| IR5744 | ght::aphA-3 gloA+ derivative of IR5738 | This study |
| IR5746 | ght::aphA-3 gloA+ derivative of IR5737 | This study |
| IR5749 | pilQ::Cmr derivative of IR4048 | This study |
| IR5751 | pilQ::Cmr derivative of IR5700 | This study |
| IR5757 | IR5694 containing pAWR3088 | This study |
| IR5759 | NMA2149::aphA-3 derivative of IR5757 | This study |
| IR5762 | IR5757 harboring pAWR3088 | This study |
| E. coli | ||
| DH5α | Cloning strain | Stratagene |
| TOP 10 | Cloning strain | Invitrogen |
| Plasmids | ||
| pCR 2.1-TOPO | Cloning vector, Ampr, Kanr | Invitrogen |
| pBS II SK− | Cloning vector, Ampr | Fermentas |
| pUC4-KSAC | Cloning vector, Kanr | Pharmacia |
| pUC18K | Cloning vector, nonpolar Kanr | 19 |
| pFLOB4300 | pUC18 but ermC rpsL cassette | 13 |
| pHT128 | Cmr, TetrN. meningitidis cloning vector | 33a |
| pRK2013 | Mobilization helper plasmid | 17 |
| pWMZ1591a | 3-kb ermC-lacIOP cassette cloned upstream of hemO | 37 |
| pAWR2090 | 2.1-kb fragment with ght cloned into pCR 2.1-TOPO | This study |
| pAWR2092 | 2.1-kb fragment with ght cloned into pBluescriptII-SK- | This study |
| pAWR3019 | 0.6-kb 5′ gloA gene fragment cloned into pCR 2.1-TOPO | This study |
| pAWR3020 | 0.4-kb 3′ gloA gene fragment cloned into pCR 2.1-TOPO | This study |
| pAWR3033 | 1.0-kb gloA fragment with internal Nsil site cloned into pCR 2.1-TOPO | This study |
| pAWR3034 | 1.0-kb gloA fragment with internal Nsil site cloned into pBS II SK- | This study |
| pAWR3035 | Like pAWR3034, but aphA-3 cassette cloned into Nsil site of gloA | This study |
| pAWR3038 | Like pAWR2090, but nonpolar aphA-3 cloned into ght Nrul site | This study |
| pAWR3055 | 0.3-kb 5′ ght deletion fragment in pCR 2.1-TOPO | This study |
| pAWR3057 | 0.7-kb 3′ ght deletion fragment in pCR 2.1-TOPO | This study |
| pAWR3063 | 1-kb ght deletion fragment in pCR 2.1-TOPO | This study |
| pAWR3068 | PCR of ermC rpsL from pFLOB4300 with Smal ends in pCR 2.1-TOPO | This study |
| pAWR3070 | Like pAWR2090, but with ermC-rpsL in ght NruI site | This study |
| pAWR3075 | ght with ribosome binding site in pCR 2.1-TOPO | This study |
| pAWR3081 | 3-kb ermC-lacIOPOP NotI fragment from pWMZ1581a in pAWR3075 | This study |
| pAWR3087 | 3-kb ght-lacIOPOP PCR fragment with ClaI ends in pCR 2.1-TOPO | This study |
| pAWR3088 | 3-kb ght-lacIOPOP cloned into the ClaI site of pHT128 | This study |
| pAWR3090 | 0.5-kb 5′ NMA2149 fragment in pCR 2.1-TOPO | This study |
| pAWR3091 | 0.4-kb 3′ NMA2149 fragment in pCR 2.1-TOPO | This study |
| pAWR3092 | 0.9-kb NMA2149 fragment in pCR 2.1-TOPO | This study |
| pAWR3093 | Like pAWR3092, but with nonpolar aphA-3 clones in SmaI site | This study |
Transposon mutant library screening.
A Himar1 transposon mutant library created previously in N. meningitidis strain IR4162 (hpuB::ermC derivative of clinical strain IR4048) was screened for heme and hemoglobin utilization mutants (1). Ten thousand kanamycin-resistant mutants were screened individually for their heme iron utilization profile. Screening by a GCB-Desferal plate assay involved plating a suspension of bacteria on GC agar plates containing only supplement I (glucose supplement) of the Kellogg's supplements and 50 μM deferroxamine mesylate (Sigma-Aldrich) and placing filter disks (6-mm Whatman 3MM paper) impregnated with test compounds (10 μl of 5-mg/ml stock solution of hemin chloride [Porphyrin Products Inc., Logan, Utah] in 100 mM NaOH and a 10-mg/ml stock solution of human hemoglobin [Sigma-Aldrich] in sterile, deionized, distilled H2O). Confluent zones of growth were measured around the hemin chloride disks, and the number of colonies around the hemoglobin disk was assessed. A mutant (276D) hypersusceptible to heme was identified by this process. DNA from mutant 276D was then used to transform parental strain IR4162 to establish linkage between the Himar1-associated kanamycin resistance and heme toxicity phenotypes. Genetic transformation of N. meningitidis strains and chromosomal DNA isolation were carried out as described by Richardson and Stojiljkovic (23).
Himar1 transposon mapping was carried out by ligation-mediated PCR as previously described (22). Ligation-mediated PCR products were cloned into pCR2.1-TOPO and sequenced. For Southern blot hybridization analysis of the Himar1 insertions, chromosomal DNA from transposon mutant 276D and transformant strain IR5627 was digested with ClaI and probed with the 1.3-kb PstI fragment of pUC4-KSAC containing the aphA-3 gene. The probe, prepared by restriction digest and purified by agarose gel separation, was labeled using a digoxigenin DNA labeling and detection kit according to the manufacturer's specifications (Roche, Basel, Switzerland).
Construction of insertional mutations in N. meningitidis.
Plasmid DNA preparation, restriction endonuclease analysis, and ligations were carried out by standard methods (25). Inactivation of ght was accomplished by insertion of the nonpolar aphA-3 kanamycin resistance cassette (19) into the gene. This was carried out by PCR amplifying the region of the genome containing ght from IR4162 genomic DNA using primers 5′NMA2148 (5′-CATGAAACTTATCTATACCGTCATC-3′) and 3′NMA2148 (5′-CCTTTCGCAGATCAAGGATGC-3′), which included the neisserial uptake sequence required for efficient transformation. The resulting 2.1-kb PCR product was cloned into pCR2.1-TOPO, creating pAWR2090. Plasmid pAWR2090 was then linearized using NruI. An 850-bp SmaI fragment carrying the nonpolar aphA-3 cassette from pUC18K was then ligated to the linearized pAWR2090. The resulting plasmid pAWR3038 was then linearized with NotI and transformed into strain IR4048, and kanamycin-resistant colonies were selected. Insertion was verified in the resulting transformant (strain IR5571) by PCR. The heme hypersensitivity phenotype was verified by the disk assay on GC-deferroxamine mesylate plates as stated above.
The gene directly downstream of ght, gloA, was inactivated by insertion of the aphA-3 cassette. This was carried out by PCR amplifying the region of the genome containing gloA from IR4048 genomic DNA using two sets of primers: set 1, 5′XhoI-gloA (5′-CCCTCGAGCGGAAGGCGACGTAC-3′) and 3′gloA-NsiI (5′-CATGCATAGTATGGAGTAAGCGCATT-3′); set 2, 5′gloA-NsiI (5′-CATGCATGCTCCGCGTGGGCAATCTC-3′) and 3′XbaI-GloA (5′-CGAGATCTATGCCGCGCAGGGAAAA-3′). PCR assays with both set 1 and set 2 primers produced fragments of ∼0.6 and ∼0.4 kb, respectively, which were cloned into pCR2.1-TOPO, creating pAWR3019 and pAWR3020. Both plasmids were linearized by NsiI, ligated together, and used as the template for PCR amplification to obtain a 1-kb fragment, which was then cloned into pCR2.1-TOPO to create plasmid pAWR3033. The 1-kb fragment from pAWR3033 was then excised with EcoRI and cloned into the EcoRI site in pBluescript II SK(−), forming plasmid pAWR3034. That plasmid was linearized by cutting at the engineered NsiI site and cloning in a 1.3-kb aphA-3 PstI fragment from pUC4-KSAC, creating plasmid pAWR3035. pAWR3035 was then linearized with NotI and transformed into strain IR4048, and a Kanr colony was selected, creating strain IR5579. Insertion was verified by PCR.
The gene directly upstream of ght, NMA2149, was inactivated by insertion of a nonpolar aphA-3 cassette. This was carried out by PCR amplifying the region of the genome containing NMA2149 from IR4048 genomic DNA using two sets of primers: set 1, 5′NMA2149-NUS (5′-GCCGTCTGAAGGTCTGCTCATGATGTAAAGC-3′) and NMA2149-SmaI (5′-CCCGGGCAAACATTCCGAACACGATGCCG-3′); set 2, 5′NMA2149-SmaI (5′-CCCGGGACGGTTGTTGTCGTTACGTGGCG-3′) and 3′NMA2149-NUS (5′-GCCGTCTGAAGGGTGAGGTTCAAATCATACG-3′). PCR assays from both set 1 and set 2 primers produced fragments of ∼0.5 and ∼0.4 kb, respectively, which were cloned into pCR2.1-TOPO, creating pAWR3090 and pAWR3091. Both plasmids were linearized by SmaI, ligated together, and used as the template for PCR amplification to obtain a 0.9-kb fragment, which was then cloned into pCR2.1-TOPO to create plasmid pAWR3092. That plasmid was linearized by cutting at the engineered SmaI site and cloning in a 0.85-kb nonpolar aphA-3 SmaI fragment from pUC18K, creating plasmid pAWR3093. pAWR3093 was then linearized with NotI and transformed into strain IR5694, and a Kanr Strr colony was selected, creating strain IR5759. Insertion was verified by PCR.
A deletion of ght was constructed by PCR amplification of the upstream and downstream regions of ght from IR4048 genomic DNA using two sets of primers: set 1, 5′NMA2148 (5′-CATGAAACTTATCTATACCGTCATC-3′) and 3′gohTdel-KpnI (5′-GGTACCCATATCGGCTTTCTCAAGG-3′); set 2, 3′NMA2148 (5′-CCTTTCGCAGATCAAGGATGC-3′) and 5′gohTdel-KpnI (5′-GGTACCAACAAACAAATGGCAGACGTTTA-3′). Both PCR products (∼300 bp and ∼700 bp) were cloned into pCR2.1-TOPO, creating pAWR303055 and pAWR3057. Plasmid DNA from both plasmids were digested with KpnI and treated with phenol-chloroform, and the DNA was ethanol precipitated. The plasmid DNA was then mixed and ligated together prior to PCR amplification with primers 5′gohST PE and 5′XhoI-gloA to generate a ∼1.4-kb PCR product containing the deletion of ght. The fragment containing the deletion of ght was then cloned into pCR2.1-TOPO, creating plasmid pAWR3063. Next, pAWR3063 was linearized with NotI and used to transform strain IR5694 for resistance to streptomycin. Strain IR5575, used as the host strain for deletion of ght, was created by first isolating a spontaneous streptomycin-resistant (Strr) mutant of parent strain IR4048 by plating 1 × 1010 cells on GC media supplemented with streptomycin (750 μg/μl). Next, the rpsL-ermC construct from pFLOB4300 (13) was PCR amplified using primers M13(−21) Forward-SmaI (5′-TCCCCCGGGTGTAAAACGACGGCCAGT-3′) and M13 Reverse-SmaI (5′-TCCCCCGGGCAGGAAACAGCTATGACC-3′) and cloned into pCR2.1-TOPO, creating pAWR3068. The rpsL-ermC construct was then excised with SmaI from pAWR3068 and cloned into the NruI site in pAWR2092, creating pAWR3070. This plasmid was linearized with NotI and transformed into IR3070, and erythromycin-resistant colonies were selected. Isolates were then patched onto GC agar with streptomycin to confirm the presence of the dominant streptomycin-sensitive cassette. The resulting Ermr Strs strain, IR5694, was used for the creation of ght deletion knock-in strain IR5700. Deletions were verified by DNA sequencing and PCR.
An insertional mutation in the mtrD gene of N. meningitidis was accomplished by transforming strains IR5575 (spontaneous Strr parent strain) and IR5700 (Δght) with chromosomal DNA from N. gonorrhoeae strain KH14 (mtrD::Kanr), creating strains IR5721 and IR5725, respectively, with selection for Kanr transformants.
An insertional mutant in the pilQ locus was constructed by transforming strain IR4048 and IR5700 with genomic DNA prepared from strain IR5648 (pilQ::Cmr [34a]), creating strains IR5749 and IR5751, with selection for Cmr transformants.
Complementation analysis.
Complementation of ght was accomplished by the construction of an inducible ght on a plasmid utilizing an ermC lacIq lacOP lacOP construct previously described (37). ght was PCR amplified from meningococcal strain IR4048 chromosomal DNA using primers 5′NMA2148com (5′-CGTCGACAGCCTTGAGAAAGCCGA-3′) and 3′ NcoINMA2148ex (5′-GCCCATGGAGTAAGCGCATTTTTG-3′) and cloned into pCR2.1-TOPO, creating pAWR3075. The ermC lacIq lacOP lacOP construct was liberated from pWMZ1591a by digestion with NotI and cloned into the NotI site in pAWR3075 upstream of ght and its native ribosome binding site, creating pAWR3078. The ght-lacIq lacOP lacOP construct was PCR amplified from plasmid pAWR3078 with primers 3′NMA2148ex-ClaI (5′-ATCGATGGAGTAAGCGCATTTTTG-3′) and 3′lacIq-ClaI (5′-ATCGATTCACTGCCCGCTTTCCAGTC-3′) and cloned into pCR2.1-TOPO, creating pAWR3087. The ∼3.0-kb ght-lacIq lacOP lacOP insertion was excised from pAWR3087 with ClaI and ligated into ClaI-digested pHT128 (33a), creating pAWR3088. The complementing plasmid pAWR3088 and unaltered pHT128 were conjugated into N. meningitidis strain IR4048 by a triparental mating procedure. Briefly, suspensions were made from the donor and helper strains (with pRK2013) (17) in LB broth and from the recipient strain in GC broth from overnight plate growth. Fifty microliters of the donor suspension, 50 μl of the helper suspension, and 25 μl of the recipient suspension were added to the surface of a GC agar plate, gently mixed, and incubated overnight at 37°C and 5% CO2 overnight. A suspension of overnight growth was made in GC broth and plated on modified Thayer-Martin II plates supplemented with chloramphenicol to select for meningococcal transconjugants. Transconjugants IR5737 (containing unaltered pHT128) and IR5738 (containing pAWR3088) were transformed with chromosomal DNA from IR5571 to create ght::aphA-3 strains IR5746 and IR5744, respectively. Insertions were verified by PCR.
For an insertional mutant in NMA2149 which also expresses ght extrachromosomally, plasmid pAWR3088 was mated into strain IR5694 in the same manner it was transferred above, creating strain IR5757. That strain was then transformed with linearized pAWR3093, and a Kanr Strr colony was selected, creating strain IR5762. Insertions were verified by PCR.
Determination of MBCs and MICs.
The minimum bactericidal concentrations (MBCs) of metalloporphyrin compounds (heme, PPIX, IX, Ga-PPIX, and Zn-PPIX) were determined as described previously (12, 31), while the MICs of other antimicrobial compounds were determined as described by Sparling et al. (27).
RT-PCR and PE experiments.
RNA preparation for reverse transcriptase PCR (RT-PCR) was carried out by extracting RNA from an overnight culture of strain IR4048 using the Rneasy Midi Kit (QIAGEN, Hilden, Germany) according to the manufacturer's specifications. Contaminating DNA was digested by adding the RNase-free DNase set (QIAGEN). RT-PCR was carried out using the SuperScript one-step RT-PCR with platinum Taq kit (Invitrogen) according to the manufacturer's specifications. Primer 3′RTgohT (5′-GGCTCCGGTTGTATCGGGAGAG-3′), which anneals within ght, was used for the reverse transcriptase reaction. Primers 3′RTgohT and 5′NcoI-2148ex (5′-GGCCATGGACAATGAATTGTGGAT-3′) were used for the PCR of only the ght portion of the transcript. Primers 3′RTgohT and 5′2149-BglII-2 (5′-GGAGATCTTTTCCTACCTGCCGGGG-3′) were used for the PCR of the region spanning both NMA2149 and ght. Primer extension (PE) products were produced using the SuperScript one-step RT-PCR with platinum Taq kit, but the PCR amplification step was omitted. Primer 3′2149-BglII-1 (5′-GGAGATCTAACGGCATCCGTATTA-3′), which anneals within the NMA2149 transcript, was used for the PE reaction on 25 μg of total RNA. The transcription start point (TSP) was determined by electrophoresis of the PE product on a 6% DNA sequencing gel using 6% Gene-Page Plus 7 M urea denaturing acrylamide blend (Amresco, Inc., Solon, Ohio) according to manufacturer's specifications. DNA sequencing reactions were carried out using SequiTherm EXCELII DNA sequencing kit according to the manufacturer's specifications (Epicenter, Madison, Wisconsin).
Analysis of sequence information.
Putative proteins with similarity to Ght were identified though the Basic Local Alignment Search Tool program's protein-protein BLAST (BLASTP) searches at the National Center for Biotechnology Information (Bethesda, MD) website (http://www.ncbi.nlm.nih.gov/BLAST/) and at The Institute for Genomic Research (Rockville, MD) website (http://tigrblast.tigr.org/cmr-blast/). Protein alignments, percent identity, and divergence were calculated using the MegAlign program of the Lasergene software suite of sequence analysis tools, version 5.5 for Macintosh (DNASTAR, Madison, WI).
RESULTS
Identification of ght by transposon mutagenesis.
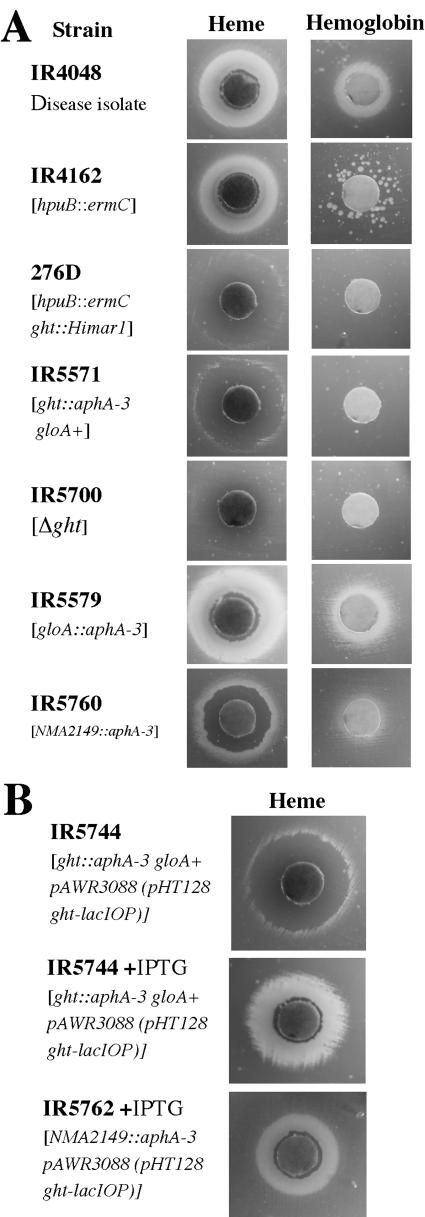
An initial screen of 10,000 individual mutants in a Himar1 transposon library of strain IR4162 (hpuB::ermC) was undertaken to identify candidate open reading frames (ORFs) whose gene products participate in heme and hemoglobin uptake and utilization in N. meningitidis. Mutants were screened by a GC-deferroxamine mesylate (a ferric iron chelator) plate assay (37) to assess the ability of each mutant to use heme and hemoglobin as sole sources of iron. As verification of the ability of this screen to identify genes that participate in heme and hemoglobin uptake, a mutation in the previously characterized heme iron uptake gene, hmbR, was identified in our screen. Moreover, we also identified mutants (data not presented) with mutations in exbB and exbD that are important in TonB-mediated energy delivery required for certain iron uptake and utilization processes (33). One mutant, 276D, showed a heme toxicity phenotype in that it was unable to utilize heme close to the filter disk and only a faint zone of growth a few millimeters away from the disk was evident (Fig. 1A). Hemoglobin utilization was also impaired in strain 276D. Only a few HmbR phase-on colonies of strain 276D were seen compared with the parental stain IR4162 (Fig. 1A).
FIG. 1.
Growth phenotypes of various mutants of N. meningitidis by disk assay on iron restriction plates. (A) Phenotypes of mutants grown in the presence of heme and hemoglobin are shown. (B) Complementation of ght. The phenotypes of ght strains carrying complementing plasmids with and without IPTG grown in the presence of heme are shown.
Southern blot hybridization analysis revealed a single transposon insertion in strain 276D (data not presented). To verify linkage between the heme toxicity phenotype and the transposon insertion (containing the Kanr marker), the parental strain, IR4162, was transformed with 276D chromosomal DNA. Ten Kanr colonies were selected and tested for the heme toxicity phenotype. All 10 Kanr transformants exhibited the heme toxicity phenotype displayed by donor strain 276D (data not presented), indicating linkage between the transposon and the heme toxicity phenotype.
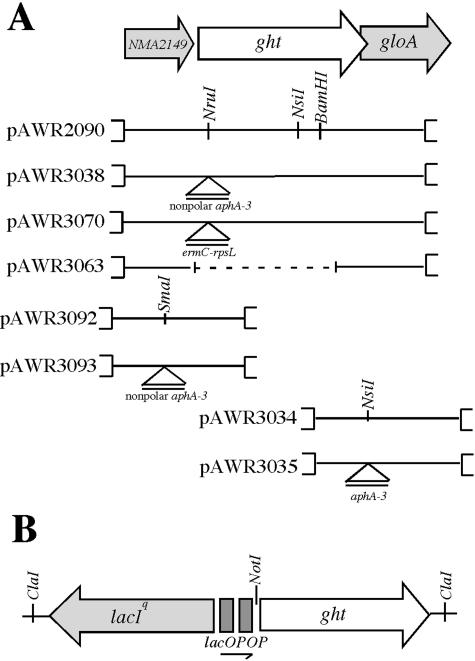
Ligation-mediated PCR was performed to map the insertion of the Himar1 transposon as described previously (22). Sequencing of PCR products and comparison to the genome sequence of the serogroup A N. meningitidis strain Z2491 revealed insertion of the transposon at nucleic acid residue 465 (data not presented) of ORF NMA2148, which was annotated by Parkhill et al. as encoding as a putative periplasmic hypothetical protein (21). It was predicted to encode a 389-amino-acid polypeptide with a predicted molecular mass of 44.5 kDa (pI 7.46). Directly upstream of NMA2148, a small ORF of 315 bp, predicted to encode a 104-amino-acid hypothetical protein with a molecular mass of 11.4 kDa (pI of 10.7), was identified. Overlapping the 3′ end of NMA2148 is gloA (Fig. 2), a glyoxalase I gene described previously (14). One additional transposon mutant (strain 15F) displayed an identical phenotype to 276D, which was subsequently determined to have a transposon insertion in NMA2148.
FIG. 2.
Schematic representation of some plasmids frequently used in this study. (A) Visual representation of N. meningitidis ght locus and surrounding open reading frames. Plasmids used in gene replacements are shown below. Labeled triangles indicate the positions of antibiotic resistant cassettes used to generate insertion mutations in individual genes. The coding region of ght overlaps gloA, a glyoxylase I gene, by 34 bp. The dotted line in pAWR3070 indicates a deleted segment. (B) Schematic representation of the lacIOPOP-ght construct in pAWR3088, the inducible ght-complementing plasmid.
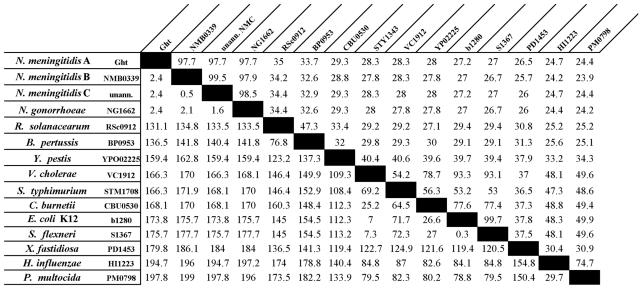
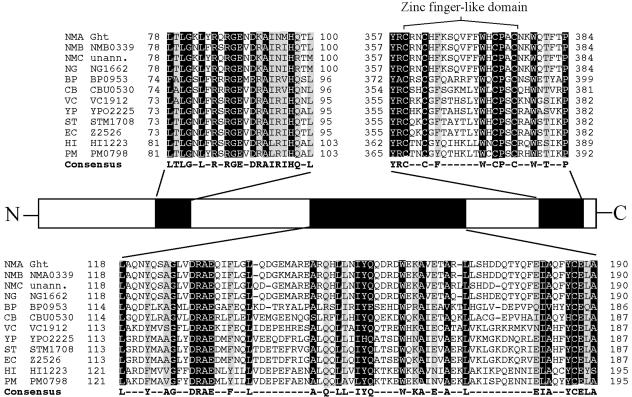
Analysis of the genome sequences of the serogroup B and C N. meningitidis genomes and the N. gonorrhoeae FA1090 genome sequence (www.genome.ou.edu) revealed that other meningococci and gonococci contain homologues of NMA2148 with 97.7% identity (Fig. 3). Interestingly, NMA2148 appears to have homologues in a diverse range of gram-negative bacteria; analysis of genetic databases at the National Center for Biotechnology Information and The Institute for Genomic Research identified more than 40 homologues among sequenced genomes; a selected few are listed in Fig. 3. These bacterial species include notable pathogens such as E. coli O157:H7, Vibrio cholerae, Bordetella pertussis, Pasteurella multocida, Haemophilus influenzae, Salmonella enterica serovar Typhimurium, and Coxiella burnetii. Several highly conserved domains appear to be present in the NMA2148-encoded ORF and its homologues; however, only two appear to belong to characterized domain families (Fig. 4). The first characterized domain is a C-terminal zinc-finger motif with a CX2CX10CX2C amino acid residue arrangement and is completely conserved among all homologues. Additionally, many ght homologues contain a tetratricopeptide repeat (TPR), suggesting a region involved in protein-protein interaction (6). The predicted NMA2148 product does contain a hydrophobic N-terminal region and periplasmic localization sequence (data not presented) that would indicate a periplasmic localization for the protein. Because of the lack of similarity to any described proteins and the hypersensitivity to heme and other HAs (see below) in the transposon mutant, ORF NMA2148 was named ght (gene of hydrophobic agent tolerance); the genomic organization of ght and flanking genes is shown in Fig. 2.
FIG. 3.
Amino acid comparison between N. meningitidis serogroup A Ght and a some of its homologues: Neisseria meningitidis serogroup A strain Z2491, Neisseria meningitidis serogroup B strain MC58, Neisseria meningitidis serogroup C strain FAM18, Neisseria gonorrhoeae strain FA1090, Ralstonia solanacearum strain GMI1000, Bordetella pertussis strain Tohama I, Yersinia pestis CO92, Vibrio cholerae O1 biovar eltor strain N16961, Salmonella enterica serovar Typhimurium strain LT2, Coxiella burnetii strain RSA 493, Escherichia coli K-12 strain MG1655, Shigella flexneri 2a stain 2457T, Xylella fastidiosa Pierce's disease strain, Haemophilus influenzae strain RD KW20, and Pasteurella multocida strain PM70. Percent identity is shown in the upper right, while divergence is shown in the lower left. Comparison was done using available sequences from The Institute for Genomic Research and the National Center for Biotechnology Information and utilizing MegAlign in the Lasergene software suite of molecular biology tools (DNAstar). unann., unannotated genome.
FIG. 4.
Amino acid sequence comparison of the predicted N. meningitidis group A Ght protein with homologues from 12 human pathogens. Amino acid sequences were aligned by the ClustalW method using MegAlign in the Lasergene software suite (DNAstar). Residues conserved in 11 to 12 species are highlighted in black with white writing; residues conserved in 9 to 10 species are highlighted in gray with black writing. The consensus sequence of 9 to 12 matches is found below the alignment. NMA, Neisseria meningitidis group A Z2491; NMB, Neisseria meningitidis group B MC58; NMC, Neisseria meningitidis group C FAM18; NG, Neisseria gonorrhoeae FA1090; BP, Bordetella pertussis Tohama I; CB, Coxiella burnetii RSA 493; VC, Vibrio cholerae O1 biovar El Tor N16961; YP, Yersinia pestis CO92; ST, Salmonella enterica serovar Typhimurium LT2 SGSC1412; EC, Escherichia coli O157:H7 EDL933; HI, Haemophilus influenzae RD KW20; PM, Pasteurella multocida PM70; unann., unannotated genome.
Transcriptional characterization of ght.
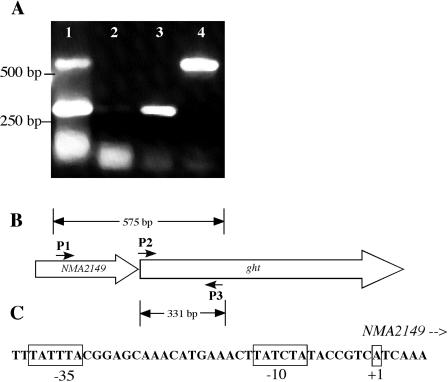
The predicted stop codon of the ORF upstream of ght, NMA2149, is separated from ght by only 10 bases. RT-PCR analysis determined that an mRNA was transcribed from the NMA2149 region that spanned both NMA2149 and ght, indicating an operon structure for these determinants (Fig. 5A and B). Primer extension experiments were conducted to map the start site of ght transcription. A TSP was detected near the 5′ end of NMA2149 (Fig. 5C). It was farther downstream than the TSP predicted by Parkhill et al. (21), lying in the predicted translational coding region of NMA2149. From sequence analysis, the actual translational start of NMA2149 may be as much as 168 bp downstream of the predicted start codon (the next ATG codon), making for a much smaller protein encoded by a truncated NMA2149. Analysis of the DNA sequence upstream of the mapped TSP revealed near consensus −10 and −35 hexamers that were separated by 18 nucleotides; the sequence of this promoter used for NMA2149-ght transcription is depicted in Fig. 5C.
FIG. 5.
Transcriptional characterization of ght region. (A) Reverse transcriptase PCR of the NMA2149 ght region. Lane 1, PCR using primers 5′2149-BglII-2 (P1), 5′NcoI-NMA2148ex (P2), and 3′RTgohT(P3) from IR4048 genomic DNA. Lane 2, PCR (minus RT) using the same primers as in lane 1 and IR4048 genomic RNA as a control for detecting DNA contamination. Lane 3, RT-PCR using primers 5′NcoI-NMA2148ex and 3′RTgohT. Lane 4, RT-PCR using primers 5′2149-BglII-2 and 3′RTgohT. (B) Representation of reverse transcriptase PCR products and their correspondence to the NMA2149 ght region. (C) Schematic of proposed −10, −35, and transcriptional start site upstream of NMA2149 as identified by primer extension.
Phenotypic characterization of ght mutants.
The GCB-deferroxamine mesylate plate assay was employed to test the ability of mutants in ght to use heme and heme-containing compounds as sources of iron. IR4048, a serogroup A clinical meningococcal isolate, is fully capable of utilizing heme and hemoglobin as sole iron sources (1), as illustrated by the large growth zones around both disks (Fig. 1A). Strain IR4162 (IR4048 hpuB::ermC), which was the parent strain in the Himar1 transposon mutagenesis, utilizes heme as well as IR4048 but uses hemoglobin less efficiently (1). Thus, utilization of hemoglobin was relegated to those rare single colonies that originated from phase-off HmbR bacteria switching to phase-on, due to the mutation of hpuB. Interruption of ght by a nonpolar aphA-3 cassette drastically altered the heme iron uptake in that the confluent heme zone disappeared and was replaced by a zone of inhibition with a very faint growth zone. In contrast, there was little visible growth around the hemoglobin disk, a characteristic exhibited by the original transposon mutant 276D (Fig. 1A). The heme growth zones could be restored by complementation with a extrachromosomal copy of ght on the broad-host-range vector pHT128 (Fig. 1B).
To assure that the interruption of ght in strain IR5571 did not have a polar effect on the downstream glyoxylase gene gloA, a mutation of this gene was constructed. An isogenic gloA mutation did not affect heme or hemoglobin utilization (Fig. 1A). Additionally, a deletion of ght, except for the first 8 and last 35 nucleotides in the coding sequence, preserved the heme toxicity in the mutant strain (Fig. 2A). gloA expression in the ght deletion was verified by reverse transcriptase PCR (data not presented). Taken together, the heme toxicity property exhibited by mutant strain 276D is due to the mutation in ght and not due to altered expression of gloA.
To discern whether NMA2149, the ORF directly upstream of ght, plays a role in heme tolerance, a nonpolar mutation of NMA2149 was constructed. Insertional mutants of NMA2149 produced an intermediate heme toxicity phenotype (Fig. 1A); hemoglobin utilization was also slightly affected. However, when ght alone was expressed from a plasmid in a NMA2149 isogenic mutant, the resulting strain exhibited a normal heme utilization phenotype (Fig. 1B). Hemoglobin utilization was also unaffected (data not presented). This indicated that the mutation in NMA2149 still impacted ght expression. Based on these observations, we believe that the NMA2149 gene product plays little, if any, direct role in heme tolerance in meningococci.
HA susceptibility and growth analysis of a ght mutant.
Bactericidal assays were carried out to better quantify the toxicity of heme against ght mutant strains and to assess whether any other metalloporphyrins or the heme precursor protoporphyrin IX (PPIX) displayed enhanced levels of toxicity. Heme was approximately 30-fold more toxic against the ght mutant strain (IR5511) compared to its parent strain (IR4162) (Table 2). However, both strains were equally sensitive to Zn-PPIX (zinc-protoporphyrin IX) and Ga-PPIX (gallium-protoporphryin IX). Unexpectedly, the mutant strain IR5571, but not parental strain IR4048, was very sensitive to PPIX, which lacks a metal ligand.
TABLE 2.
MBCs of porphyrins for IR4048 (parent strain) and IR5571 (ght::aphA gloA+)a
| Strain | MBC (mg/liter) of:
|
|||
|---|---|---|---|---|
| Heme | PPIX | Ga-PPIX | Zn-PPIX | |
| IR4048 | 62 | >500 | 32 | <2 |
| IR5571 | 2-4 | 2-4 | 32 | <2 |
PPIX, protoporphyrin IX; Ga-PPIX, gallium protoporphyrin; Zn-PPIX, zinc protoporphyrin.
Hypersensitivity to low levels of PPIX in gonococci has been reported recently. One study indicated that two classes of mutants could utilize heme from hemoglobin for growth in the absence of a functional hemoglobin receptor (3). One class of mutants was determined to be a point mutation in the pilus secretion gene, pilQ, while the second was outside pilQ at an unidentified site. The similarity of the phenotype in the ght mutant strain IR5571 to that of the previously described pilQ mutant (3) led us to test whether mutations in ght altered levels of meningococcal HA toxicity. We found that a meningococcal ght deletion mutant (IR5700) had increased susceptibility to the HAs erythromycin, rifampin, and Triton X-100 (Table 3). We did not detect hypersensitivity to ampicillin (Table 3) and other hydrophilic antibiotics (data not presented). To eliminate direct involvement of Ght with PilQ-mediated HA hypersensitivity, we constructed an isogenic pilQ mutant and a double mutant, having insertional mutations in both pilQ and ght. The strains were then compared to the isogenic ght mutant and the wild-type strain for levels of HA susceptibility. A pilQ mutant had no effect on HA sensitivity compared to the wild-type strain, and a double mutant bearing mutated pilQ and ght genes displayed levels of HA susceptibility identical to those of a strain bearing the ght mutation alone, indicating that the loss of Ght results in HA hypersensitivity of meningococci independent of PilQ (Table 3).
TABLE 3.
Effect of mtrD and pilQ mutations on meningococcal strains with and without a deletion of ght
| Strain | Relevant genotype | Source | MIC (mg/liter) ofa:
|
||||
|---|---|---|---|---|---|---|---|
| Heme | ERY | AMP | TX100 | RIF | |||
| IR4048 | Parent strain | Clinical isolate | 125 | 2.0 | 0.125 | 125 | 0.125 |
| IR5700 | Δght | Δght IR5575 | 15 | 1.0 | 0.125 | 30 | 0.0075 |
| IR5721 | mtrD::Kanr | mtrD::kanr IR4048 | 125 | 0.125 | 0.125 | 60 | <0.003 |
| IR5725 | Δght mtrD::Kanr | mtrD::kanr IR5700 | 30 | 0.125 | 0.125 | 30 | 0.0075 |
| IR5749 | pilQ::Cmr | pilQ::cmr IR4048 | 125 | 2.0 | 0.125 | 125 | 0.06 |
| IR5751 | Δght pilQ::Cmr | pilQ::cmr IR5700 | 15 | 1.0 | 0.125 | 30 | 0.003 |
ERY, erythromycin; AMP, ampicillin; TX100, Triton X-100; RIF, rifampin. Results are mean values from three evaluations.
The gonococcal MtrC-MtrD-MtrE efflux system, which has orthologues in the meningococcal genomes (21, 24, 34), also functions in HA tolerance and was recently found to recognize PPIX and certain metalloporphyrins (2). This led us to test HA tolerance in meningococcal ght and mtrD (the cytoplasmic membrane transporter of the mtr efflux pump) (11, 24) mutants and a strain bearing a ght-mtrD double mutation (Table 3). As expected, an mtrD mutation increased meningococcal susceptibility to Triton X-100, erythromycin, and rifampin but not to heme, indicating that ght acts independently of the mtr system in meningococcal resistance to heme.
DISCUSSION
We initiated this transposon mutagenesis study to identify additional genes involved in the uptake and utilization of heme iron in the meningococci. By screening the Himar1 mutant library, we identified ght, which when disrupted by the insertion of the transposon in a serogroup A meningococcus isolate, rendered the mutant highly susceptible to the toxic effects of heme iron. In addition to its hypersensitivity to heme, a ght mutant was also found to be highly susceptible to PPIX, the apoporphyrin (non iron-loaded) moiety that is the precursor to heme in heme biosynthesis. This meningococcus isolate has not been previously shown to acquire PPIX specifically. Moreover, the meningococcus isolate has the necessary prerequisite genes for making its own PPIX (21, 34). However, susceptibility to both heme and PPIX is not unprecedented in the pathogenic neisseriae. As mentioned previously, two classes of mutants have been isolated in Neisseria gonorrhoeae that were more susceptible to both heme and PPIX. In addition to these porphyrin sensitivities, both classes of mutants were hypersensitive to other HAs as well (3). Subsequently, tests similar to those carried out by Chen et al. (3) demonstrated that ght mutants are also hypersensitive to HAs such as Triton X-100 and rifampin in addition to heme and PPIX.
ght was found to be cotranscribed with the upstream ORF, NMA2149, which is not surprising, since NMA2149 and ght are separated by only 10 bp, and a TSP for this operon was mapped to the 5′ region of NMA2149. The TSP is downstream of the start site predicted by Parkhill et al. (21). Although an interruption of NMA2149 with a nonpolar cassette appeared to decrease heme resistance, the phenotype could be complemented by the expression of ght. It is likely that the interruption of NMA2149 effects downstream expression of ght in trans, despite efforts to avoid disruption. This evidence suggests that NMA2149 plays no major role in HA tolerance, as mediated through ght. It is possible that NMA2149 may be required for optimal function of ght.
It is reasonable to assume that gloA, the locus directly downstream of ght, is also cotranscribed with NMA2149 and ght, since the 5′ gloA coding sequence overlaps the 3′ ght coding sequence by 37 bp. It is also for this reason that, when a deletion of ght was constructed, specific nucleotides were retained to conserve the start codon of ght and the 5′ portion of gloA that overlaps ght in the event of translational coupling. Nevertheless, it was shown that a gloA mutation does not impact susceptibility to HAs.
Initial efforts to characterize ght concentrated only on its potential ability to participate in specific uptake and utilization of heme. Heme uptake in the meningococci involves outer membrane proteins that function to extract heme from heme-containing proteins and transport it into the periplasm where it is further relayed across the cytoplasmic membrane by an unidentified system. Once in the cytoplasm, the heme is degraded by a heme oxygenase to extract elemental iron; in the case of N. meningitidis, that heme oxygenase is HemO (37). The identity of the neisserial periplasmic transport system for heme has not been identified and was one of the targets of our mutagenesis. Initial studies attempted to prove a specific interaction between heme and Ght. We hypothesized that Ght may be functioning like a periplasmic binding protein of an ABC transporter system which has not been described for meningococci but has been assumed to exist (32). However, no such evidence was found from experiments that used heme-agarose affinity chromatography or spectrophotometric analysis (data not presented); these methods have been used previously to show direct interaction between neisserial proteins and heme (16). Moreover, Ght does not share similarity with other periplasmic heme binding proteins or any other described heme-binding proteins.
The similar hypersensitivity to certain HAs of strains bearing ght mutations, a point mutation in pilQ, and a yet unidentified mutation in the gonococci (3) is notable. Gonococcal strains bearing either the pilQ point mutation or the unidentified mutation could grow in the presence of hemoglobin as the sole source of iron despite lacking a functional HpuA or HpuB (3). This growth phenomenon could be abrogated by the addition of human serum albumin, indicating it was heme dissociated from hemoglobin that was the source of growth stimulation (3). The point mutation in the pilQ locus studied by Chen et al. (3) is presumed to allow increased entrance of hydrophobic compounds by alteration of PilQ structure, thus allowing passage of HAs through the altered secretin. PilQ had been thought to function by passively allowing only the extension and retraction of pilus structure; however, recent observations about the structure of PilQ suggest a more active role in pilus extension/retraction (4, 5). It has also been observed that wild-type PilQ may allow the passage of hydrophobic compounds across the outer membrane (J. Folster and W. Shafer, unpublished). Similar to both the pilQ point mutant and the uncharacterized mutant in N. gonorrhoeae, a ght mutant is also hypersensitive to Triton X-100 and rifampin and slightly sensitive to erythromycin. It is reasonable to hypothesize that ght may also play a role in stabilization of the pilus apparatus, specifically PilQ in the outer membrane. However, piliation was found to be normal in a ght mutant (data not presented), and a mutation of pilQ was unable to rescue normal tolerance to HAs in a ght mutant, indicating that the increased HA susceptibility is not mediated by passage of HAs through the PilQ secretin. Efforts to transfer the nonpolar ght::Kanr construct from the meningococci to the gonococci to better understand HA hypersensitivity and ght's effects on piliation proved unsuccessful (data not presented), suggesting that ght may be an essential gene in the gonococci. Moreover, a meningococcal strain harboring mutations in HpuB and HmbR is not able to grow in the presence of hemoglobin as a sole iron source under the same conditions described by Chen et al. for their gonococcal mutants (3).
The function of Ght in relation to heme toxicity is independent of the meningococcal MtrC-MtrD-MtrE efflux system. In contrast to recent work with the gonococci (2), our data have shown that heme resistance in meningococci is not related to MtrC-MtrD-MtrE efflux activity. Ght is also unrelated to any currently described efflux system, but it is possible that Ght represents an integral part of a new class of efflux pump that is able to export HAs such as heme.
Maintenance of the integrity of the bacterial outer membrane is crucial to survival. The presence of ght orthologues in only gram-negative, but not gram-positive, bacteria and its proposed periplasmic localization indicate a ubiquitous role in gram-negative bacteria's membrane physiology. Ght may aid in sustaining the permeability of the outer membrane, specifically against the passage of chemically hydrophobic compounds independent of the PilQ secretin. This may be accomplished in one of two ways. Ght could maintain the integrity of the outer membrane itself; the proper formation and stability of the lipid bilayer is the first line of defense in providing a barrier that is permeable to nutrients but keeps out toxic compounds. The other way by which membrane integrity can be maintained is through protein complexes that span the outer membrane similar to PilQ. This may be accomplished by directly interacting with membrane-spanning pores or indirectly, like proper folding of outer membrane protein components. Whatever the mechanism is by which Ght protects against the toxicity of hydrophobic agents, it may only be elucidated by isolating other interacting members that aid in the function of Ght and by defining its cellular location. Discovery of Ght's mechanism of function may help in understanding how exogenous compounds are inhibited from penetrating the membrane defenses and how a potentially toxic molecule like heme is manipulated to benefit its bacterial consumer.
Acknowledgments
We thank Gordon Churchward for critical review of the manuscript. We also thank Jadranka Bozja for assistance with the MBC assays, Asiya Gusa for help with the primer extension analysis, and L. Pucko for help with manuscript preparation.
This work was supported by NIH grants AI47870 (to I.S.), AI45883 (to R. C. Compans, Emory University), and AI21150 (to W.M.S.). W.M.S. is the recipient of a Senior Research Career Scientist Award from the VA Medical Research Service.
REFERENCES
- 1.Alexander, H. L., A. W. Rasmussen, and I. Stojiljkovic. 2004. Identification of Neisseria meningitidis genetic loci involved in the modulation of phase variation frequencies. Infect. Immun. 72:6743-6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bozja, J., K. Yi, W. M. Shafer, and I. Stojiljkovic. 2004. Porphyrin-based compounds exert antibacterial action against the sexually transmitted pathogens Neisseria gonorrhoeae and Haemophilus ducreyi. Int. J. Antimicrob. Agents 24:578-584. [DOI] [PubMed] [Google Scholar]
- 3.Chen, C. J., D. M. Tobiason, C. E. Thomas, W. M. Shafer, H. S. Seifert, and P. F. Sparling. 2004. A mutant form of the Neisseria gonorrhoeae pilus secretin protein PilQ allows increased entry of heme and antimicrobial compounds. J. Bacteriol. 186:730-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins, R. F., R. C. Ford, A. Kitmitto, R. O. Olsen, T. Tonjum, and J. P. Derrick. 2003. Three-dimensional structure of the Neisseria meningitidis secretin PilQ determined from negative-stain transmission electron microscopy. J. Bacteriol. 185:2611-2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins, R. F., S. A. Frye, A. Kitmitto, R. C. Ford, T. Tonjum, and J. P. Derrick. 2004. Structure of the Neisseria meningitidis outer membrane PilQ secretin complex at 12 A resolution. J. Biol. Chem. 279:39750-39756. [DOI] [PubMed] [Google Scholar]
- 6.D'Andrea, L. D., and L. Regan. 2003. TPR proteins: the versatile helix. Trends Biochem. Sci. 28:655-662. [DOI] [PubMed] [Google Scholar]
- 7.Delany, I., R. Rappuoli, and V. Scarlato. 2004. Fur functions as an activator and as a repressor of putative virulence genes in Neisseria meningitidis. Mol. Microbiol. 52:1081-1090. [DOI] [PubMed] [Google Scholar]
- 8.Drake, S. L., and M. Koomey. 1995. The product of the pilQ gene is essential for the biogenesis of type IV pili in Neisseria gonorrhoeae. Mol. Microbiol. 18:975-986. [DOI] [PubMed] [Google Scholar]
- 9.Grifantini, R., S. Sebastian, E. Frigimelica, M. Draghi, E. Bartolini, A. Muzzi, R. Rappuoli, G. Grandi, and C. A. Genco. 2003. Identification of iron-activated and -repressed Fur-dependent genes by transcriptome analysis of Neisseria meningitidis group B. Proc. Natl. Acad. Sci. USA 100:9542-9547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagman, K. E., C. E. Lucas, J. T. Balthazar, L. Snyder, M. Nilles, R. C. Judd, and W. M. Shafer. 1997. The MtrD protein of Neisseria gonorrhoeae is a member of the resistance/nodulation/division protein family constituting part of an efflux system. Microbiology 143:2117-2125. [DOI] [PubMed] [Google Scholar]
- 11.Hagman, K. E., W. Pan, B. G. Spratt, J. T. Balthazar, R. C. Judd, and W. M. Shafer. 1995. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology 141:611-622. [DOI] [PubMed] [Google Scholar]
- 12.Hindler, J. A., B. L. Howard, and J. F. Keiser. 1994. Antimicrobial agents and antimicrobial susceptibility testing, 2nd ed. Mosby Year Book, St. Louis, Mo.
- 13.Johnston, D. M., and J. G. Cannon. 1999. Construction of mutant strains of Neisseria gonorrhoeae lacking new antibiotic resistance markers using a two gene cassette with positive and negative selection. Gene 236:179-184. [DOI] [PubMed] [Google Scholar]
- 14.Kizil, G., K. Wilks, D. Wells, and D. A. Ala'Aldeen. 2000. Detection and characterisation of the genes encoding glyoxalase I and II from Neisseria meningitidis. J. Med. Microbiol. 49:669-673. [DOI] [PubMed] [Google Scholar]
- 15.Ladan, H., Y. Nitzan, and Z. Malik. 1993. The antibacterial activity of haemin compared with cobalt, zinc and magnesium protoporphyrin and its effect on potassium loss and ultrastructure of Staphylococcus aureus. FEMS Microbiol. Lett. 112:173-177. [DOI] [PubMed] [Google Scholar]
- 16.Lee, B. C. 1994. Isolation and characterization of the haemin-binding proteins from Neisseria meningitidis. Microbiology 140:1473-1480. [DOI] [PubMed] [Google Scholar]
- 17.Leong, S. A., G. S. Ditta, and D. R. Helinski. 1982. Heme biosynthesis in Rhizobium. Identification of a cloned gene coding for delta-aminolevulinic acid synthetase from Rhizobium meliloti. J. Biol. Chem. 257:8724-8730. [PubMed] [Google Scholar]
- 18.Lewis, L. A., E. Gray, Y. P. Wang, B. A. Roe, and D. W. Dyer. 1997. Molecular characterization of hpuAB, the haemoglobin-haptoglobin-utilization operon of Neisseria meningitidis. Mol. Microbiol. 23:737-749. [DOI] [PubMed] [Google Scholar]
- 19.Menard, R., P. J. Sansonetti, and C. Parsot. 1993. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 175:5899-5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mey, A. R., and S. M. Payne. 2001. Haem utilization in Vibrio cholerae involves multiple TonB-dependent haem receptors. Mol. Microbiol. 42:835-849. [DOI] [PubMed] [Google Scholar]
- 21.Parkhill, J., M. Achtman, K. D. James, S. D. Bentley, C. Churcher, S. R. Klee, G. Morelli, D. Basham, D. Brown, T. Chillingworth, R. M. Davies, P. Davis, K. Devlin, T. Feltwell, N. Hamlin, S. Holroyd, K. Jagels, S. Leather, S. Moule, K. Mungall, M. A. Quail, M. A. Rajandream, K. M. Rutherford, M. Simmonds, J. Skelton, S. Whitehead, B. G. Spratt, and B. G. Barrell. 2000. Complete DNA sequence of a serogroup A strain of Neisseria meningitidis Z2491. Nature 404:502-506. [DOI] [PubMed] [Google Scholar]
- 22.Pelicic, V., S. Morelle, D. Lampe, and X. Nassif. 2000. Mutagenesis of Neisseria meningitidis by in vitro transposition of Himar1 mariner. J. Bacteriol. 182:5391-5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson, A. R., and I. Stojiljkovic. 1999. HmbR, a hemoglobin-binding outer membrane protein of Neisseria meningitidis, undergoes phase variation. J. Bacteriol. 181:2067-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rouquette-Loughlin, C. E., J. T. Balthazar, S. A. Hill, and W. M. Shafer. 2004. Modulation of the mtrCDE-encoded efflux pump gene complex of Neisseria meningitidis due to a Correia element insertion sequence. Mol. Microbiol. 54:731-741. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor, Cold Spring Harbor, N.Y.
- 26.Shafer, W. M., X. Qu, A. J. Waring, and R. I. Lehrer. 1998. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc. Natl. Acad. Sci. USA 95:1829-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sparling, P. F., F. A. Sarubbi, Jr., and E. Blackman. 1975. Inheritance of low-level resistance to penicillin, tetracycline, and chloramphenicol in Neisseria gonorrhoeae. J. Bacteriol. 124:740-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stojiljkovic, I., A. J. Baumler, and K. Hantke. 1994. Fur regulon in gram-negative bacteria. Identification and characterization of new iron-regulated Escherichia coli genes by a fur titration assay. J. Mol. Biol. 236:531-545. [DOI] [PubMed] [Google Scholar]
- 29.Stojiljkovic, I., and K. Hantke. 1994. Transport of haemin across the cytoplasmic membrane through a haemin-specific periplasmic binding-protein-dependent transport system in Yersinia enterocolitica. Mol. Microbiol. 13:719-732. [DOI] [PubMed] [Google Scholar]
- 30.Stojiljkovic, I., V. Hwa, L. de Saint Martin, P. O'Gaora, X. Nassif, F. Heffron, and M. So. 1995. The Neisseria meningitidis haemoglobin receptor: its role in iron utilization and virulence. Mol. Microbiol. 15:531-541. [DOI] [PubMed] [Google Scholar]
- 31.Stojiljkovic, I., V. Kumar, and N. Srinivasan. 1999. Non-iron metalloporphyrins: potent antibacterial compounds that exploit haem/Hb uptake systems of pathogenic bacteria. Mol. Microbiol. 31:429-442. [DOI] [PubMed] [Google Scholar]
- 32.Stojiljkovic, I., and D. Perkins-Balding. 2002. Processing of heme and heme-containing proteins by bacteria. DNA Cell Biol. 21:281-295. [DOI] [PubMed] [Google Scholar]
- 33.Stojiljkovic, I., and N. Srinivasan. 1997. Neisseria meningitidis tonB, exbB, and exbD genes: Ton-dependent utilization of protein-bound iron in neisseriae. J. Bacteriol. 179:805-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33a.Takahashi H., and H. Watanabe. 2002. A broad-host-range vector of incompatibility group Q can work as a plasmid vector in Neisseria meningitidis: a new genetical tool. Microbiology 148(Pt. 1):229-36. [DOI] [PubMed] [Google Scholar]
- 34.Tettelin, H., N. J. Saunders, J. Heidelberg, A. C. Jeffries, K. E. Nelson, J. A. Eisen, K. A. Ketchum, D. W. Hood, J. F. Peden, R. J. Dodson, W. C. Nelson, M. L. Gwinn, R. DeBoy, J. D. Peterson, E. K. Hickey, D. H. Haft, S. L. Salzberg, O. White, R. D. Fleischmann, B. A. Dougherty, T. Mason, A. Ciecko, D. S. Parksey, E. Blair, H. Cittone, E. B. Clark, M. D. Cotton, T. R. Utterback, H. Khouri, H. Qin, J. Vamathevan, J. Gill, V. Scarlato, V. Masignani, M. Pizza, G. Grandi, L. Sun, H. O. Smith, C. M. Fraser, E. R. Moxon, R. Rappuoli, and J. C. Venter. 2000. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287:1809-1815. [DOI] [PubMed] [Google Scholar]
- 34a.Tonjum, T., D. A. Caugant, S. A. Dunham, and M. Koomey. 1998. Structure and function of repetitive sequence elements associated with a highly polymorphic domain of the Neisseria meningitidis PilQ protein. Mol. Microbiol. 29:111-124. [DOI] [PubMed] [Google Scholar]
- 35.Vanderpool, C. K., and S. K. Armstrong. 2001. The Bordetella bhu locus is required for heme iron utilization. J. Bacteriol. 183:4278-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veal, W. L., R. A. Nicholas, and W. M. Shafer. 2002. Overexpression of the MtrC-MtrD-MtrE efflux pump due to an mtrR mutation is required for chromosomally mediated penicillin resistance in Neisseria gonorrhoeae. J. Bacteriol. 184:5619-5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu, W., D. J. Hunt, A. R. Richardson, and I. Stojiljkovic. 2000. Use of heme compounds as iron sources by pathogenic neisseriae requires the product of the hemO gene. J. Bacteriol. 182:439-447. [DOI] [PMC free article] [PubMed] [Google Scholar]