Abstract
Members of the H-NS family of proteins play a relevant role as modulators of gene expression in gram-negative bacteria. Interaction of these proteins with members of the Hha/YmoA family of proteins has been previously reported. It has been hypothesized that the latter proteins are functionally equivalent to the N-terminal domain of H-NS-like proteins. In this report we test this assumption by replacing the N-terminal domain of Escherichia coli H-NS by Hha. It has been possible to obtain a functional protein that can compensate for some of the hns-induced phenotypes. These results highlight the relevance of H-NS-Hha interactions to generate heterooligomeric complexes that modulate gene expression in gram-negative bacteria.
The H-NS protein of Escherichia coli was described almost 30 years ago as a chromosome-associated protein. Since then it has been thoroughly studied as an architectural protein playing an important role in the global regulation of gene expression. H-NS protein and homologues (the H-NS family of proteins) are widespread in enterobacteria and other genera of gram-negative bacteria (38). Best characterized in different enteric bacteria such as E. coli or Salmonella enterica serovar Typhimurium, the H-NS protein is a relevant example of a global modulator exerting its effects in response to different environmental signals (for a recent review, see reference 8). The expression of approximately 5% of the genes of E. coli has been found to be directly or indirectly altered in hns mutant strains (13).
One of the outstanding features of H-NS is the ability to generate higher-order homomeric and heteromeric complexes. H-NS oligomerization depends upon the N-terminal domain of the protein, extending up to residue 65 (1, 9). Generation of dimers, trimers, and tetramers has been reported (5, 36). H-NS oligomerization appears as a process necessary for transcriptional repression (30). H-NS is able not only to generate homodimers and homooligomers but also to interact with other proteins. Generation of heterodimers and heterooligomers with the H-NS paralogue StpA is a well-documented process (15, 16, 39). Interaction of H-NS with StpA protects the latter protein from Lon-mediated proteolysis (16). It has also been reported that StpA can act as a molecular adapter for some species of truncated H-NS proteins to repress the bgl operon (11).
Members of the H-NS family also interact with members of the Hha/YmoA family (26, 27, 29). These small proteins (Mr, about 8 kDa) were initially described in E. coli (Hha) and Yersinia enterocolitica (YmoA) as thermomodulators of the expression of virulence factors (6, 23, 25). Both were independently considered new nucleoid-associated proteins that modulate gene expression (4, 6, 21). Since then, many other members of the Hha/YmoA family have been identified, both in the chromosomes of gram-negative bacteria and in conjugative plasmids (19). Interaction of Hha and H-NS was first evidenced when the biological role of Hha as a modulator of the expression of the operon encoding the E. coli toxin α-hemolysin (Hly) was assessed. Rather than showing affinity and specificity for DNA sequences, Hha showed high H-NS binding affinity and specificity (27). Further work demonstrated that, in fact, an Hha-H-NS complex modulates the expression of the hly operon (20). Recent studies have extended the Hha-H-NS interaction to other members of both families: YmoA interacts with Y. enterocolitica H-NS (26), and Hha and its E. coli paralogue YdgT interact with StpA. Interaction of Hha/YdgT with StpA prevents proteolytic degradation of this latter protein (29).
A mutational analysis of Hha focused to identify domains of the protein showed that almost all the protein sequence corresponds to a unique protein-binding domain. A comparison of the amino acid sequences corresponding to the Hha family and the N-terminal end of the H-NS family showed the existence of conserved regions (26). These results suggested that Hha-like proteins might have evolved to be functionally equivalent to the amino-terminal oligomerization domain of H-NS and, hence, interact with full-length H-NS proteins to generate heterodimers and heterooligomers that modulate gene expression in gram-negative bacteria (26). We show in this report that the replacement of the N-terminal domain of H-NS by Hha sequences generates a functional chimeric protein that, when expressed in hns mutants, restores different hns mutant phenotypes.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Bacterial strains and plasmids used are listed in Table 1. The different strains were grown either in Luria-Bertani medium or in minimal medium M63 supplemented with serine (40 mg/liter) and prepared as described previously (22). Antibiotics, when required, were used at the following concentrations: ampicillin, 50 μg/ml; tetracycline, 12.5 μg/ml; kanamycin, 25 μg/ml; and chloramphenicol, 50 μg/ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| E. coli strains | ||
| 5K | F−hsdR hsdM thr thi rpsL leu lacZ | 17 |
| BL21(DE3) Δhns | BL21(DE3) Δhns::Km | 42 |
| BSN26 | MC4100 trp::Tn10 | 14 |
| BSN27 | MC4100 trp::Tn10 Δhns | 14 |
| BSN29 | MC4100 trp::Tn10 Δhns stpA60::Kmr | 14 |
| HB101 | F−hsdS20 (rB− mB−) recA13 ara-14 proA2 lacY1 galK2 rpsL20 xyl-5 mtl-1 supE44 λ− | 2 |
| Plasmids | ||
| pUC19 | oriColE1, Apr | 40 |
| pUCHha | pUC19 + hha | This study |
| pUCHhaHNS | pUC19 + hha, +hns | This study |
| pUCHhaHnsHyb | pUC19 + phns-hha-hns | This study |
| pUC-HhaHnsHyb2 | pUC19 + Hha-Hns-Hyb2 | This study |
| pUC-HhaHnsHyb30 | pUC19 + Hha-Hns-Hyb30 | This study |
| pUC-HhaHnsHyb45 | pUC19 + Hha-Hns-Hyb45 | This study |
| pHly152 | hlyR+hlyC+hlyA+hlyB+hlyD+ | 28 |
| pDFY167 | orip15A, Kmr, bglG::lacZ | 3 |
| pLG338-30 | oripSC101; Apr | 7 |
| pLGHhaHnsHyb2 | pLG388-30 + Hha-Hns-Hyb2 | This study |
| pET15b | oriPMB1, promoter T7, Apr | 37 |
| pETHisHhaHnsHyb2 | pET15b + His-tagged-Hha-Hns-Hyb2 | This study |
Genetic and molecular procedures.
Isolation of plasmids, restriction digestion, ligation of DNA, and transformation were carried out by standard methods. PCR amplification and sequencing of DNA were done according to standard methodology. All of the oligonucleotides used are listed in Table 2.
TABLE 2.
Oligonucleotides used in this study
| Oligonucleotide | Sequence |
|---|---|
| HhaNde | 5′ CCATAGGTAGACATATGTCCG 3′ |
| HhaBam | 5′ CGGTTATGGATCCGAAAGCG 3′ |
| HNSBProx | 5′ GAATTTAAGGATCCATTATTACC 3′ |
| HNSBDist | 5′ CCGGATCCTAAAAAATCCCGC 3′ |
| pHNSNProx | 5′ AACTAATACATATGACTGAAAGG 3′ |
| pHNSNDist | 5′ GCTTCGCTCATATGAGTAATCTC 3′ |
| HibBProx | 5′ CTGAAAGGTCGGGATCCTACG 3′ |
| HNSBam2 | 5′ GCCGCTGGCGGGATCCTAAG 3′ |
| HisHha | 5′ GCATTCGCCATATGGACCATCACCATCACCATATGTCCGAAAAACC 3′ |
| HhaBamMet | 5′ AACCATAGGTGGATCCATGTCCG 3′ |
| RPISHha | 5′ CTCATTGAGCAGATCGACG 3′ |
| RPISHNS | 5′ CGGTTGCTGATGTGACCG 3′ |
| HlyR0 | 5′ GGGGAATTCCAAGCGAAGTCCA 3′ |
| HlyBam | 5′ GTTTTGGGATCCACCCTGATGG 3′ |
| HlyP | 5′ GTCATGCGTGGCGACATTGA 3′ |
| HlyS | 5′ CAGACCACACCTGGAAAAAC 3′ |
To construct plasmid pUCHhaHnsHyb, the hha gene was first PCR amplified using the oligonucleotides HhaNde, which adds an NdeI site, and HhaBam, which adds a BamHI site. The NdeI-BamHI PCR fragment (237 bp) was cloned into pUC19, rendering plasmid pUCHha. The hns gene was then PCR amplified using the oligonucleotides HNSBProx and HNSBDist, which add BamHI sites. The BamHI PCR fragment (508 bp) was cloned into the BamHI site of pUCHha, resulting in plasmid pUCHhaHNS. Finally, the promoter region of the hns gene (from position −273 to −3) was amplified using the oligonucleotides pHNSNProx and pHNSNDist, which add NdeI sites. The NdeI PCR fragment (270 bp) was cloned into the NdeI site of pUCHhaHNS. This plasmid was called pUCHhaHnsHYb.
Design of hybrid proteins based on a sequence-independent protein recombination method (35) was used to obtain plasmids pUCHhaHnsHyb2, pUCHhaHnsHyb30, and pUCHhaHnsHyb45. The hha and hns genes were amplified using the oligonucleotides RPISHha/HhaBam and HNSBProx/RPISHNS, respectively. Fragments were subsequently treated with Bal31 nuclease. Five-milliliter aliquots were collected every minute for 15 min, and the reaction was stopped by the addition of 40 mM EDTA. The resulting fragments were then Klenow filled and ligated. Finally, ligation products were amplified using the oligonucleotides HhaBamMet and HNSBDist and digested with MfeI and BamHI. This fragment was used to replace the MfeI-BamHI fragment of pUCHhaHnsHyb, obtaining plasmids pUCHhaHnsHyb2, pUCHhaHnsHyb30, and pUCHhaHnsHyb45.
To construct plasmid pLGHhaHnsHyb2, the sequence cloned into pUCHhaHnsHyb2 was PCR amplified using the oligonucleotides HibProx and HNSBam2, which add BamHI sites, and cloned into pLG388-30.
Plasmid pETHisHhaHnsHyb2 was obtained by cloning into pET15b a PCR fragment amplified from pUCHhaHnsHyb2 using the oligonucleotides His-Hha, which adds six codons of His residues and an NdeI site, and HNSBam2, which adds a BamHI site.
Measurement of hemolysin production.
Hemolysin production was assayed by measuring hemolytic activity as previously described (24).
Measurement of β-galactosidase activity.
β-Galactosidase activity was evaluated as previously described (24).
Overexpression of proteins by the T7 RNA polymerase system and purification of His-tagged proteins.
E. coli strain BL21(DE3) Δhns was used as a host for induction of the expression of protein Hha-Hns-Hyb2. Plasmid pETHisHhaHnsHyb2 was transformed into strain BL21(DE3) Δhns. Clear cellular extracts were obtained as described previously (27). His-tagged recombinant protein was purified by immobilized metal affinity chromatography by using Ni2+-nitrilotriacetic acid (NTA) technology (12) as described previously (27). His-Hha and H-NS-His were purified as previously described (26, 27).
Electrophoretic analysis of proteins.
Protein samples were analyzed in a tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis system (16.5%, 10%, and 4%) (31) and stained with Coomassie blue.
Gel retardation assays.
Gel retardation assays were performed as described previously (20). The fragments used were obtained by amplification of plasmid pHly152, using HlyR0 and HlyBam for the R0 fragment (2.7 kb) and HlyS and HlyP for the S-P fragment (1.1 kb).
RESULTS
Construction of a chimeric Hha-H-NS protein.
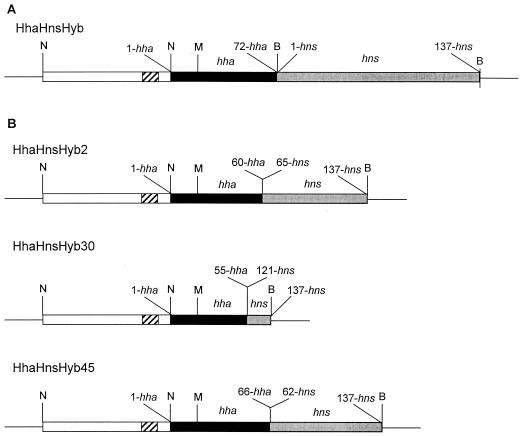
To obtain a hybrid Hha-Hns protein, we decided to generate random fusions between fragments corresponding to the N-terminal part of Hha and the C-terminal part of H-NS. As a preliminary step we generated a recombinant DNA fragment containing (i) the hns promoter, (ii) the hha gene (full-length), and (iii) the hns gene (full-length) (Fig. 1A) (see Materials and Methods for details). This DNA fragment, flanked by recognition sites for the enzymes NdeI and BamHI, was cloned into pUC19 (plasmid pUCHhaHnsHyb). The construction contained unique MfeI (inside hha) and BamHI (located at the 3′ end of hns) restriction sites. To obtain random fusions between hha and hns, both genes were PCR amplified and Bal31 digested. Upon Klenow filling and ligation, the ligation products were amplified with the oligonucleotides HhaBamMet and HNSBDist. Amplification products of the proper size (about 500 bp) were then MfeI-BamHI digested and cloned into plasmid pUCHhaHnsHyb. Upon transformation into strain E. coli 5K, inserts from 60 different clones were sequenced. Three of them contained an in-frame open reading frame (ORF) corresponding to an hha′-hns′ construct. The corresponding plasmids were termed pUCHhaHnsHyb2, pUCHhaHnsHyb30, and pUCHhaHnsHyb45. To avoid undesirable recombinational events, they were introduced into strain E. coli HB101 by transformation. Upon transformation, the inserts were sequenced again. Such clones were then used as a source of recombinant plasmids for subsequent work.
FIG. 1.
Hha-Hns hybrid constructions. The flanking amino acid residues either from hha and hns genes are indicated. (A) Insert from plasmid pUCHhaHnsHyb that contains the complete ORFs from hha and hns. (B) Structure of the different Hha-Hns-Hyb constructions obtained by using the sequence-independent protein recombination approach. White boxes, hns promoter region (from −273 to −3); striped boxes, −35 and −10 sequences of the hns promoter; black boxes, sequences corresponding to the hha gene; gray boxes, sequences corresponding to the hns gene; B, BamHI site; N, NdeI site; M, MfeI site.
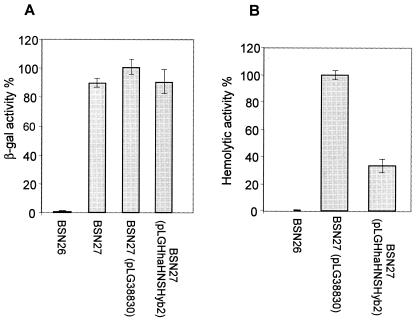
We next tested the ability of these plasmids to complement hns phenotypes. We decided to test expression of both hly and bgl operons (increased in hns mutants) in strains BSN26, BSN27(pUC19), BSN27(pUCHhaHnsHyb2), BSN27(pUCHhaHnsHyb30), and BSN27(pUCHhaHnsHyb45) (Fig. 2). To test hemolysin expression, plasmid pHly152 was introduced by conjugation. To test bgl expression, plasmid pDFY167(bglG::lacZ) was introduced by transformation. Plasmid pUCHhaHnsHyb45 did not complement, plasmid pUCHhaHnsHyb30 yielded intermediate levels of complementation, and plasmid pUCHhaHnsHyb2 significantly complemented both hns mutant phenotypes. Figure 3 shows the amino acid sequence of protein Hha-Hns-Hyb2, as well as details of the structural domains of both Hha and H-NS proteins that are present in the chimeric protein.
FIG. 2.
Effect of the different Hha-Hns-Hyb constructions on the expression of the bgl (A) or hly (B) operons in an hns genetic background. All strains carried either plasmid pDFY436 (bglG::lacZ) (A) or pHly152 (B). Activity of strain BSN27(pUC19), considered as 100%, was 1,500 units (A) or 1,200 units (B). Samples were collected at the exponential phase of growth (optical density at 600 nm, 0.4). The data shown are means ± standard deviations (error bars) of at least three independent experiments.
FIG. 3.
Amino acid sequence and structural domains of the proteins Hha, H-NS (N-terminal domain), and Hha-Hns-Hyb2 (N-terminal domain). Boxes indicate the α-helix domains of each protein. Boldface residues correspond to those preserved among members of both families of proteins. The flexible linkers that connect the N- and C-terminal domains in both H-NS and Hha-Hns-Hyb2 are shown.
Complementation of hns mutant phenotypes by protein Hha-Hns-Hyb2 depends upon gene dosage.
To further test the ability of protein Hha-Hns-Hyb2 to complement hns phenotypes, we decided to supply the protein in a low-copy-number system to mimic physiological expression levels. To do this, DNA from plasmid pUCHhaHnsHyb2 was amplified with the oligonucleotides HibBProx and HNSBam2 (thus yielding two BamHI restriction sites flanking the recombinant gene). The amplification product was then ligated into BamHI-digested pLG388-30. The recombinant plasmid was termed pLGHhaHnsHyb2. Plasmid pLGHhaHnsHyb2 was also tested for complementation of the hns mutation (Fig. 4). With respect to the deregulation of hemolysin expression that is apparent in hns mutants, a moderate effect was evident when plasmid pLGHhaHnsHyb2 was supplied in trans. In contrast, cells harboring this plasmid did not modify expression of the bgl operon when compared to plasmid-free hns cells.
FIG. 4.
Ability of the Hha-Hns-Hyb2 protein, supplied in a low-copy-number system, to complement hns phenotypes. (A) Expression of the bgl operon. (B) Expression of the hly operon. All strains carried either plasmid pDFY436 (A) or pHly152 (B). Activity of strain BSN27 (pLG388-30), considered as 100%, was 1,000 units (A) or 3,000 units (B). Samples were collected at the exponential phase of growth (optical density at 600 nm, 0.4). The data shown are means ± standard deviations (error bars) of at least three independent experiments.
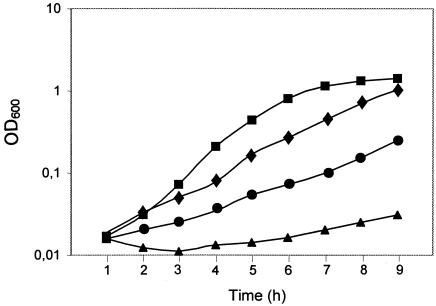
We further investigated whether protein Hha-Hns-Hyb2 was able to complement a global physiological effect of the hns mutation, i.e., the serine sensitivity of hns mutants (18). In vivo complementation of the serine susceptibility of hns mutants has been used to identify H-NS-like proteins in different gram-negative microorganisms (38). Strains BSN26, BSN27, BSN27 (pUCHhaHnsHYb2), and BSN27 (pLGHhaHnsHyb2) were grown in minimal medium supplemented with serine, and growth was monitored (Fig. 5). It could be shown that, when expressed in the high-copy-number vector pUC19, the chimeric protein partially alleviates the severe growth defects of strain BSN27 in this medium.
FIG. 5.
Serine sensitivity of strains BSN26 (squares), BSN27 (circles), BSN27 (pLGHhaHnsHyb2) (triangles), and BSN27 (pUCHhaHnsHyb2) (diamonds). Growth curves of control strains BSN27 (pUC19) and BSN27 (pLG388-30) (not shown) were similar to those of strains BSN27 and BSN27 (pLGHhaHnsHyb2), respectively. OD600, optical density at 600 nm.
Binding of Hha-Hns-Hyb2 to DNA sequences of the regulatory region of the hly operon.
We also tested Hha-Hns-Hyb2 for its ability to specifically bind to sequences that have been shown to be preferential binding sites for H-NS in the regulatory region of the hly operon (19). Previously, we obtained a purified preparation of Hha-Hns-Hyb2 by His-tagging this protein, overexpressing it in strain BL21(DE3) Δhns, and purifying it by using nickel-NTA agarose technology (Fig. 6A). We first tested the ability of Hha-Hns-Hyb2 to bind to the R0 fragment that corresponds to the hly regulatory region and includes specific binding sites for H-NS (20). The results obtained (Fig. 6B) showed that, when compared to H-NS, the hybrid protein must be supplied in higher concentrations to obtain similar low-migrating protein-DNA complexes. In spite of this, binding of Hha-Hns-Hyb2 protein to the R0 sequence showed specificity. When an additional DNA fragment that contains no specific sequences for H-NS is added to the reaction mixture (fragment S-P) (20), the R0 fragment is specifically retarded both by the H-NS and Hha-Hns-Hyb2 proteins (Fig. 6C). These results are consistent with the fact that the C-terminal domain of H-NS, responsible for protein-DNA interaction, is intact in the hybrid protein.
FIG. 6.
Interaction of Hha-Hns-Hyb2 with DNA. (A) Purification of His-Hha-Hns-Hyb2 by Ni2+-NTA agarose technology. Lane 1, crude extract; lanes 2 to 4, fractions obtained upon washing with buffer A; lanes 5 to 9, fractions eluted upon washing with increasing concentrations of imidazole (50 to 250 mM). Bands other than the 14.4-kDa band (arrow) correspond to truncated form (low band) or to multimeric forms (upper bands) of the protein. (B) Band shift assays with the R0 fragment (10 ng of DNA) and various concentrations of H-NS, Hha, or Hha-H-NS-Hyb2 proteins. (C) Competition gel retardation assay with fragments R0 and S-P (5 ng of each DNA fragment). Variable concentrations of H-NS or Hha-Hns-Hyb2 were added.
DISCUSSION
In this report we bring biochemical evidence supporting the hypothesis that, in fact, in spite of low similarity at the level of the amino acid sequence, Hha-like proteins can be functionally equivalent to the N-terminal domain of H-NS-like proteins and, hence, participate in the generation of heterooligomeric complexes that modulate gene expression in gram-negative bacteria. Substitution of the N-terminal domain of H-NS (amino acid residues 1 to 65) by the first 61 amino acid residues of Hha yields a chimeric protein that partially compensates for an H-NS defect in E. coli cells. Considering that the structure of both the N-terminal domain of H-NS (33) and full-length Hha are known (41), it is possible to predict the structure of the hybrid protein, which includes the first three α-helixes of Hha connected by the flexible linker to the C-terminal domain of H-NS (Fig. 3). It is apparent that not every substitution of the N-terminal end of H-NS by Hha yields proteins that can, at least partially, replace H-NS. From the 60 random fusions that were sequenced, only three corresponded to in-frame ORFs, and only the Hha-Hns-Hyb2 protein, when expressed at high level, can significantly alleviate hns mutant phenotypes. We assessed the ability of Hha-Hns-Hyb2 to compensate for H-NS loss by expressing the chimeric protein in both high-copy- and low-copy-number vectors. These results showed that a high level of expression is needed for the hybrid protein to efficiently compensate some of the phenotypes that exhibit hns mutants. This is not surprising and may correspond to the reduced efficiency of the chimeric protein to generate higher-order oligomeric complexes with itself and/or Hha. On the other hand, the presence of the intact H-NS C-terminal domain accounts for the fact that Hha-Hns-Hyb2 shows affinity and specificity for DNA sequences that are targets for H-NS.
Recent reports about the role of Hha in modulating the hilA promoter in S. enterica serovar Typhimurium and the esp operon of enterohemorrhagic E. coli O157:H7 have suggested that Hha specifically binds to its target sequences in hilA (10) and in esp (34). In both examples, authors purified the Hha protein used to test specific binding to DNA by using protein fusion protocols. In neither of the examples was it demonstrated that the purified Hha preparations were H-NS free. Thus, it cannot be ruled out that contaminating H-NS accounts for specific binding to the hilA and esp sequences. Because the molecular mass of Hha is about the half that of H-NS and because Hha tends to dimerize and oligomerize in solution, contamination by H-NS can only be detected by analyzing Hha preparations by Western blotting with H-NS-specific antibodies. Interestingly, for the hilA promoter different groups have identified Hha and H-NS as modulators, but no relationship between the proteins has been established (10, 32). Although it cannot be ruled out that Hha-like proteins may specifically bind to certain DNA targets, the results we present here strongly support the hypothesis that a main biological activity of Hha-like proteins is protein-binding activity and that members of this family of proteins specifically interact with members of the H-NS family of proteins to generate heterooligomeric complexes that efficiently modulate gene expression in gram-negative bacteria. Structural studies are currently being undertaken to better understand the nature of the heterooligomeric compounds formed by H-NS-like and Hha-like proteins.
Acknowledgments
This work was supported by grants from the Ministerio de Ciencia y Tecnología (BMC2001 3499) and from the Generalitat de Catalunya (2001 SGR 00100). Sonia Rodríguez was the recipient of an FI grant from the Generalitat de Catalunya.
REFERENCES
- 1.Bloch, V., Y. Yang, E. Margeat, A. Chavanieu, M. T. Augé, B. Robert, S. Arold, S. Rimsky, and M. Kochoyan. 2003. The H-NS dimerization domain defines a new fold contributing to DNA recognition. Nat. Struct. Biol. 10:212-218. [DOI] [PubMed] [Google Scholar]
- 2.Boyer, H. W., and D. Roulland-Dussoix. 1969. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J. Mol. Biol. 41:459-472. [DOI] [PubMed] [Google Scholar]
- 3.Caramel, A., and K. Schnetz. 1998. Lac and λ repressors relieve silencing of the Escherichia coli bgl promoter. Activation by alteration of a repressing nucleoprotein complex. J. Mol. Biol. 284:875-883. [DOI] [PubMed] [Google Scholar]
- 4.Carmona, M., C. Balsalobre, F. J. Muñoa, M. Mouriño, Y. Jubete, F. De la Cruz, and A. Juárez. 1993. Escherichia coli hha mutants, DNA supercoiling and expression of haemolysin genes from recombinant plasmid pANN202-312. Mol. Microbiol. 9:1011-1018. [DOI] [PubMed] [Google Scholar]
- 5.Ceschini, S., G. Lupidi, M. Coletta, C. L. Pon, E. Fioretti, and M. Angeletti. 2000. Multimeric self-assembly equilibria involving the histone-like protein H-NS, a thermodynamic study. J. Biol. Chem. 275:729-734. [DOI] [PubMed] [Google Scholar]
- 6.Cornelis, G., C. Sluiters, I. Delor, D. Gelb, K. Kaniga, C. Lambert de Rouvroit, M. P. Sory, J. C. Vanooteghem, and T. Michiels. 1991. ymoA, a Yersinia enterocolitica chromosomal gene modulating the expression of virulence functions. Mol. Microbiol. 5:1023-1034. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham, T. P., R. C. Montelaro, and K. E. Rushlow. 1993. Lentivirus envelope sequences and proviral genomes are stabilized in Escherichia coli when cloned in low-copy-number plasmid vectors. Gene 9:93-98. [DOI] [PubMed] [Google Scholar]
- 8.Dorman, C. J. 2004. H-NS: a universal regulator for a dynamic genome. Nat. Rev. Microbiol. 2:391-400. [DOI] [PubMed] [Google Scholar]
- 9.Esposito, D., A. Petrovic, R. Harris, S. Ono, J. F. Eccleston, A. Mbabaali, I. Haq, C. F. Higgins, J. C. Hinton, P. C. Driscoll, and J. E. Ladbury. 2002. H-NS oligomerization domain structure reveals the mechanism for high order self-association of the intact protein. J. Mol. Biol. 324:841-850. [DOI] [PubMed] [Google Scholar]
- 10.Fahlen, T. F., R. L. Wilson, J. D. Boddicker, and B. D. Jones. 2001. Hha is a negative modulator of transcription of hilA, the Salmonella enterica serovar Typhimurium invasion gene transcriptional activator. J. Bacteriol. 183:6620-6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Free, A., M. E. Porter, P. Deighan, and C. J. Dorman. 2001. Requirement for the molecular adapter function of StpA at the Escherichia coli bgl promoter depends upon the level of truncated H-NS protein. Mol. Microbiol. 42:903-917. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman, A., and R. G. Roeder. 1991. Purification of His-tagged proteins in non-denaturing conditions suggests a convenient method for protein interaction studies. Nucleic Acids Res. 19:6337-6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hommais, F., E. Krin, C. Laurent-Winter, O. Soutourina, A. Malpertuy, J. P. Le Caer, A. Danchin, and P. Bertin. 2001. Large-scale monitoring of pleiotropic regulation of gene expression by the prokaryotic nucleoid-associated protein, H-NS. Mol. Microbiol. 40:20-36. [DOI] [PubMed] [Google Scholar]
- 14.Johansson, J., B. Dagberg, E. Richet, and B. E. Uhlin. 1998. H-NS and StpA proteins stimulate expression of the maltose regulon in Escherichia coli. J. Bacteriol. 180:6117-6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johansson, J., S. Eriksson, B. Sondén, S. N. Wai, and B. E. Uhlin. 2001. Heteromeric interactions among nucleoid-associated bacterial proteins: localization of StpA-stabilizing regions in H-NS of Escherichia coli. J. Bacteriol. 183:2343-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansson, J., and B. E. Uhlin. 1999. Differential protease-mediated turnover of H-NS and StpA revealed by a mutation altering protein stability and stationary-phase survival of Escherichia coli. Proc. Natl. Acad. Sci. USA 96:10776-10781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juárez, A., C. Hughes, M. Vogel, and W. Goebel. 1984. Expression and regulation of the plasmid-encoded haemolysin determinant of Escherichia coli. Mol. Gen. Genet. 197:196-203. [DOI] [PubMed] [Google Scholar]
- 18.Lejeune, P., P. Bertin, C. Walon, K. Willemot, C. Colson, and A. Danchin. 1989. A locus involved in kanamycin, chloramphenicol and L-serine resistance is located in the bglY-galU region of the Escherichia coli K12 chromosome. Mol. Gen. Genet. 218:361-363. [DOI] [PubMed] [Google Scholar]
- 19.Madrid, C., J. M. Nieto, and A. Juárez. 2002. Role of the Hha/YmoA family of proteins in the thermoregulation of the expression of virulence factors. Int. J. Med. Microbiol. 291:425-432. [DOI] [PubMed] [Google Scholar]
- 20.Madrid, C., J. M. Nieto, S. Paytubí, F. Falconi, C. O. Gualerzi, C. O., and A. Juárez. 2002. Temperature- and H-NS-dependent regulation of a plasmid-encoded virulence operon expressing Escherichia coli hemolysin. J. Bacteriol. 184:5058-5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mikulskis, A. V., and G. Cornelis. 1994. A new class of proteins regulating gene expression in enterobacteria. Mol. Microbiol. 11:77-86. [DOI] [PubMed] [Google Scholar]
- 22.Miller, J. H. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 23.Mouriño, M., C. Madrid, C. Balsalobre, A. Prenafreta, F. J. Muñoa, J. Blanco, M. Blanco, J. E. Blanco, and A. Juárez. 1996. The Hha protein as a modulator of expression of virulence factors in Escherichia coli. Infect. Immun. 64:2881-2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mouriño, M., F. J. Muñoa, C. Balsalobre, P. Díaz, C. Madrid, and A. Juárez. 1994. Environmental regulation of the α-hemolysin expression in Escherichia coli. Microb. Pathog. 16:249-259. [DOI] [PubMed] [Google Scholar]
- 25.Nieto, J. M., M. Carmona, S. Bolland, Y. Jubete, F. de la Cruz, and A. Juárez. 1991. The hha gene modulates haemolysin expression in Escherichia coli. Mol. Microbiol. 5:1285-1293. [DOI] [PubMed] [Google Scholar]
- 26.Nieto, J. M., C. Madrid, E. Miquelay, J. L. Parra, S. Rodríguez, and A. Juárez. 2002. Evidence for direct protein-protein interaction between members of the enterobacterial Hha/YmoA and H-NS families of proteins. J. Bacteriol. 184:629-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nieto, J. M., C. Madrid, A. Prenafeta, E. Miquelay, C. Balsalobre, M. Carrascal, and A. Juárez. 2000. Expression of the hemolysin operon in Escherichia coli is modulated by a nucleoid-protein complex that includes the proteins Hha and H-NS. Mol. Gen. Genet. 263:349-358. [DOI] [PubMed] [Google Scholar]
- 28.Noegel, A., U. Rdest, and W. Goebel. 1981. Determination of the functions of hemolytic plasmid pHly152 of Escherichia coli. J. Bacteriol. 145:233-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paytubí, S., C. Madrid, N. Forns, J. M. Nieto, C. Balsalobre, B. E. Uhlin, and A. Juárez. 2004. YdgT, the Hha paralogue in Escherichia coli forms heteromeric complexes with H-NS and StpA. Mol. Microbiol. 54:251-263. [DOI] [PubMed] [Google Scholar]
- 30.Rimsky, S. 2004. Structure of the histone-like protein H-NS and its role in regulation and genome superstructure. Curr. Opin. Microbiol. 7:109-114. [DOI] [PubMed] [Google Scholar]
- 31.Schägger, H., and G. von Jagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 32.Schechter, L. M., S. Jain, S. Akbar, and C. A. Lee. 2003. The small nucleoid-binding proteins H-NS, HU, and Fis affect hilA expression in Salmonella enterica serovar Typhimurium. Infect. Immun. 71:5432-5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schröder, O., and R. Wagner. 2002. The bacterial regulatory protein H-NS. A versatile modulator of nucleic acid structures. Biol. Chem. 383:945-960. [DOI] [PubMed] [Google Scholar]
- 34.Sharma, V. K., and R. L. Zuerner. 2004. Role of hha and ler in transcriptional regulation of the esp operon of enterohemorrhagic Escherichia coli O157:H7. J. Bacteriol. 186:7290-7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sieber, V., C. A. Martinez, and F. H. Arnold. 2001. Libraries of hybrid proteins from distantly related sequences. Nat. Biotechnol. 19:456-460. [DOI] [PubMed] [Google Scholar]
- 36.Smyth, C. P., T. Lundbäck, D. Renzoni, G. Siligardi, R.Beavil, M. Layton, J. M. Sidebotham, J. D. C. Hinton, P. C. Driscoll, C. F. Higgins, and J. E.Ladbury. 2000. Oligomerization of the chromatin-structuring protein H-NS. Mol. Microbiol. 36:962-972. [DOI] [PubMed] [Google Scholar]
- 37.Studier, F. W., A. H. Rosengerg, J. J. Dunn, and J. W. Dubendorff. 1990. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 185:60-89. [DOI] [PubMed] [Google Scholar]
- 38.Tendeng, C., and P. Bertin. 2003. H-NS in gram-negative bacteria: a family of multifaceted proteins. Trends Microbiol. 11:511-518. [DOI] [PubMed] [Google Scholar]
- 39.Williams, R. M., S. Rimsky, and H. Buc. 1996. Probing the structure, function, and interactions of the Escherichia coli H-NS and StpA proteins by using dominant negative derivatives. J. Bacteriol. 178:4335-4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yanisch-Perron, C., J. Vieira, and M. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequencing of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 41.Yee, A., X. Chang, A. Pineda-Lucena, B. Wu, A. Semesi, B. Le, T. Ramelot, G. M. Lee, S. Bhattacharyya, P. Gutierrez, A. Denisov, C.-H. Lee, J. R. Cort, G. Kozlov, J. Liao, G. Finak, L. Chen, D. Wishart, W. Lee, L. P. McIntosh, K. Gehring, M. A. Kennedy, M. A. Edwards, and C. H. Arrowsmith. 2002. An NMR approach to structural proteomics. Proc. Natl. Acad. Sci. USA 99:1825-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang, A., S. Rimsky, M. E. Reaban, H. Buc, and M. Belfort. 1996. Escherichia coli protein analogs StpA and H-NS: regulatory loops, similar and disparate effects on nucleic acid dynamics. EMBO J. 15:1340-1349. [PMC free article] [PubMed] [Google Scholar]