Abstract
In response to iron limitation, Pseudomonas aeruginosa produces the fluorescent siderophore pyoverdine. Transcription of pyoverdine biosynthetic (pvd) genes is driven by the iron starvation sigma factor PvdS, which is negatively regulated by the Fur-Fe(II) holorepressor. We studied the effect of AlgQ, the Escherichia coli Rsd orthologue, on pyoverdine production by P. aeruginosa PAO1. AlgQ is a global regulatory protein which activates alginate, ppGpp, and inorganic polyphosphate synthesis through a cascade involving nucleoside diphosphate kinase (Ndk). AlgQ is also capable of interacting with region 4 of RpoD. In a reconstituted E. coli system, PvdS-dependent transcription from the pvdA promoter was doubled by the multicopy algQ gene. The P. aeruginosa ΔalgQ mutant exhibited a moderate but reproducible reduction in pyoverdine production compared with wild-type PAO1, as a result of a decline in transcription of pvd genes. PvdS expression was not affected by the algQ mutation. Single-copy algQ fully restored pyoverdine production and expression of pvd genes in the ΔalgQ mutant, while ndk did not. An increased intracellular concentration of RpoD mimicked the ΔalgQ phenotype, whereas PvdS overexpression suppressed the algQ mutation. E. coli rsd could partially substitute for algQ in transcriptional modulation of pvd genes. We propose that AlgQ acts as an anti-sigma factor for RpoD, eliciting core RNA polymerase recruitment by PvdS and transcription initiation at pvd promoters. AlgQ provides a link between the pyoverdine and alginate regulatory networks. These systems have similarities in responsiveness and physiological function: both depend on alternative sigma factors, respond to nutrient starvation, and act as virulence determinants for P. aeruginosa.
Iron is an essential nutrient for almost all forms of life (2), but only small amounts are bioavailable in aerobic environments at neutral pH. To fulfill their nutritional iron requirement, most unicellular organisms and some plants have developed the ability to produce and/or acquire from the environment iron-chelating molecules called siderophores (2).
Under iron-limiting conditions, the opportunistic bacterial pathogen Pseudomonas aeruginosa is capable of acquiring iron bound to a variety of exogenous and endogenous chelators (40). One of these chelators, pyoverdine, is the principal iron uptake option for P. aeruginosa both in natural environments and in infected hosts (reviewed in reference 61). Pyoverdine is a peptidic siderophore composed of a fluorescent chromophore (a quinoline derivative) linked to a variable amino acid arm (5). Pyoverdine has a high affinity for Fe(III) (Kf, ∼1032 M−1) and is capable of promoting P. aeruginosa infection and virulence in various animal models (reviewed in reference 61). A number of genes involved in the biosynthesis of pyoverdinePAO1 have been identified to date in the P. aeruginosa PAO1 chromosome and have been found to be clustered in the pvd locus, which also encompasses the fpvA gene encoding the ferripyoverdinePAO1 outer membrane receptor FpvA (43). PyoverdinePAO1 synthesis occurs in response to iron limitation (Fe concentration, <1 μM) and is shut off under iron-replete conditions to prevent potential iron overload and toxicity. Transcription of the pyoverdine biosynthesis and transport genes (i.e., pvd and fpvA) is repressed by binding of the dimeric Fur-Fe(II) holorepressor to the promoter-operator DNA regions (Fur boxes) of master regulatory genes, namely, pvdS, fpvI, and fpvR (44, 61). When there is an iron shortage, Fur repression is relieved, which gives free access to the σ70 (RpoD)-dependent RNA polymerase holoenzyme (RNAP) for transcription of pvdS (9, 37) and fpvI (3, 44), both of which encode extracytoplasmic function (ECF) sigma factors belonging to the iron starvation (IS) subgroup of the RpoD family (30, 63), as well as the fpvR gene, which encodes a cognate inner membrane-bound anti-sigma factor (27). PvdS recognizes a conserved DNA sequence called the IS box within target promoters and directs transcription of several genes, namely, the genes implicated in pyoverdine biogenesis (pvd genes) and in the expression of proteases and exotoxin A (40, 61). In contrast, FpvI controls only fpvA transcription (3, 44). The activity of both IS sigma factors, PvdS and FpvI, is under the control of a signaling mechanism that involves the FpvA receptor and the pyoverdinePAO1 molecule itself. In the absence of ferripyoverdinePAO1, the activity of both PvdS and FpvI is antagonized by FpvR (3). Binding of ferripyoverdinePAO1 to FpvA initiates a signal transduction cascade that involves FpvR and causes the activation of both PvdS and FpvI, which direct the transcription of target genes (3, 27). This sophisticated mechanism ensures that pyoverdinePAO1 is produced only when it is effective in delivering iron to the cell through productive engagement of FpvA (3).
We previously reported that the activity of pvdA, pvdD, and pvdE promoter fusions was significantly lower in the heterologous host Escherichia coli overexpressing PvdS than in the homologous PAO1 system, and we suggested that additional regulatory factors could be implicated in the positive control of the pvd gene system (30). Interestingly, reduced siderophore levels were observed in the stable alginate-producing P. aeruginosa 8830 strain carrying a mutation in the algQ gene, also known as algR2 (49, 60). Alginate is a critical virulence factor in chronic lung infections sustained by P. aeruginosa since it confers a typical mucoid phenotype which insulates bacteria from the host defenses (16, 18). The AlgQ protein positively regulates alginate synthesis, as well as the expression of enzymes related to nucleoside triphosphate (NTP) formation, namely, nucleoside diphosphate kinase (Ndk) and succinyl-coenzyme A synthetase. Hence, AlgQ modulates the levels of alginate, GTP, guanosine 3′,5′-bispyrophosphate (the ppGpp alarmone), and inorganic polyphosphate (polyP) (25, 48). Accordingly, the defect in synthesis of these metabolites in the algQ mutant 8830R2::Cm can be reversed by complementation with either the algQ or ndk gene in trans (25). AlgQ is 58% identical to the product of the pyoverdineWCS358 regulatory gene pfrA of Pseudomonas putida WCS358 (60). Cross-complementation studies with the algQ gene of P. aeruginosa and the pfrA gene of P. putida WCS358 first established the link between pyoverdine and alginate production (60). Indeed, it was demonstrated that PfrA could fully restore alginate production in a P. aeruginosa 8830 algQ mutant, while AlgQ could partially restore pyoverdineWCS358 synthesis in a P. putida WCS358 pfrA mutant (10, 60). More recently, production of alginate by P. aeruginosa PAO1 has been related to iron depletion, tightening the link between the iron starvation response and the regulation of alginate synthesis (24).
It is noteworthy that AlgQ also exhibits 55% similarity (87 of 157 amino acid residues) with the E. coli Rsd (regulator of sigma D) protein, which controls the level of functional RpoD subunits by forming a 1:1 complex with RpoD (20, 65). Like Rsd, AlgQ was shown to make contact with the C-terminal region of P. aeruginosa RpoD (12). On the basis of these findings, it has been hypothesized that AlgQ can also function as an anti-sigma factor for RpoD, increasing the amount of core RNAP (RNAPc) available for binding by alternative sigma factors (12). At the regulatory level, the algQ promoter shows basal expression during the exponential phase and significant activation during the stationary phase (25), recalling the growth phase-dependent expression of rsd in E. coli (20).
This work was undertaken to address the role of AlgQ in the regulation of pyoverdine biosynthesis by P. aeruginosa PAO1. We observed that pvdA transactivation in E. coli MC4100 carrying a multicopy pvdS gene was doubled by the presence of algQ in trans. We found that the reduced level of pyoverdinePAO1 in a P. aeruginosa PAO1 algQ null mutant correlated with diminished transcription of the pvdA, pvdD, and pvdE biosynthetic genes, while PvdS expression was not affected. No differences in either pyoverdinePAO1 production or expression of pvd biosynthetic genes were observed upon complementation of the P. aeruginosa PAO1 algQ null mutant with the ndk gene. Increasing the RpoD levels in wild-type P. aeruginosa mimicked the algQ mutation, while increasing the PvdS levels suppressed the algQ mutant phenotype. E. coli rsd could partially substitute for algQ in transcriptional modulation of pvd genes. Hence, we propose that AlgQ is the functional homolog of E. coli Rsd. According to our results, AlgQ modulates the expression of pvd genes by interacting with free RpoD subunits, enabling PvdS to compete effectively with RpoD for RNAPc binding, thereby favoring transcription initiation at pvd promoters.
MATERIALS AND METHODS
Strains, plasmids, and media.
The bacterial strains and plasmids used in this study are listed in Table 1. The P. aeruginosa PAO1 algQ null mutant, designated PAO1ΔalgQ, was a generous gift from M. Foglino (Centre National de la Recherche Scientifique, Marseille, France). This mutant has a deletion extending from bp 5916921 to bp 5917397 of the P. aeruginosa PAO1 chromosome, precisely matching the algQ coding sequence (data not shown). E. coli was routinely grown in LB medium or in M9 minimal medium (46). To reduce iron availability, the iron chelator 2,2′-dipyridyl was added to M9 minimal medium at a concentration of 150 μM. P. aeruginosa was grown in NYB or SM9 (64) or in cetrimide agar (Pseudosel; Target). DCAA was used as the low-iron medium for P. aeruginosa (62). Media were solidified with 1.2% agar N.1 (Unipath). Antibiotics were used in selective media at the following concentrations: tetracycline, 12.5 μg/ml for E. coli and 150 μg/ml for P. aeruginosa; chloramphenicol, 30 μg/ml for E. coli and 100 μg/ml for P. aeruginosa; ampicillin, 100 μg/ml for E. coli; carbenicillin, 500 μg/ml for P. aeruginosa; kanamycin, 25 μg/ml; nalidixic acid, 20 μg/ml; and streptomycin, 25 μg/ml for E. coli.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype and/or relevant characteristics | Source or reference |
|---|---|---|
| P. aeruginosa strains | ||
| PAO1 (ATCC 15692) | Prototroph | American Type Culture Collection |
| PAO1ΔalgQ | algQ null mutant | M. Foglino |
| E. coli strains | ||
| MC4100 | araD139 rpsL150 relA1 flbB5301 deoC1 pstF25 rbsR Δ(lacZYA-argF)U169, Strr | 7 |
| DH5αF′ | recA1 endA1 hsdR17 supE44 thi-1 gyrA96 relA1 Δ(lacZYA-argF)U169 [Φ80dlacZΔM15] Nalr | 31 |
| Plasmids | ||
| pRK2013 | Helper plasmid; ColE1 replicon, Kmr Mob+ Tra+ | 14 |
| pUCP18/19 | E. coli-Pseudomonas shuttle vector derived from pUC18/19; ColE1, pRO1600 replicon lacZα bla, Apr Cbr | 51 |
| pUCPalgQ | 896-bp PCR-generated fragment, encompassing the entire algQ gene and its promoter region XhoI/BamHI digested and the resulting 647-bp fragment ligated to the SalI-BamHI sites of pUCP19 | This study |
| pUCPndk | 708-bp PCR-generated fragment, encompassing the entire ndk gene and its promoter region, ligated to the EcoRI-BamHI sites of pUCP19 | This study |
| pUCPrpoD | 2.1-kb PCR-generated fragment, encompassing the entire rpoD gene and its promoter region, ligated to the EcoRI-PstI sites of pUCP19 | This study |
| pUCPpvdS | 700-bp XhoI-HindIII fragment from pQEpvdS (29), encompassing the entire pvdS gene and the PT5lacO control region, ligated to the SalI-HindIII sites of pUCP18 | This study |
| pPV226 | 1.1-kb BglII fragment of pPV5 ligated to pUCP18 | 29 |
| pJB785TT | Broad-host-range, low-copy-number vector (one copy/cell in P. aeruginosa; five to seven copies/cell in E. coli); RK2 replicon, luc bla Apr Cbr | 47 and references therein |
| pJBrsd | 848-bp XbaI-BamHI fragment, encompassing the entire rsd gene and its promoter region, ligated to pJB785TT | This study |
| pJBalgQ | 896-bp XbaI-BamHI fragment, encompassing the entire algQ gene and its promoter region, ligated to pJB785TT | This study |
| pMP190 | Broad-host-range, low-copy-number promoter-probe vector; IncQ replicon, lacZ Cmr Tra− | 53 |
| pMP220 | Broad-host-range, low-copy-number promoter-probe vector; IncP replicon, lacZ Tcr Tra− | 53 |
| pMP220::PpvdA | 300-bp SphI/BglII fragment, encompassing the entire pvdA promoter, ligated to pMP220 in the same orientation as the reporter lacZ gene (formerly designated pPV51) | 29 |
| pMP220::PpvdS | 587-bp BglII-EcoRI fragment, encompassing the entire pvdS promoter, ligated to pMP220 in the same orientation as the reporter lacZ gene | 1 |
| pMP190::PpvdD | Promoter of pvdD pyoverdine biosynthesis gene directionally cloned into pMP190 | 9 |
| pMP190::PpvdE | Promoter of pvdE pyoverdine biosynthesis gene directionally cloned into pMP190 | 9 |
| pACYC184 | p15A replicon, Cmr Tcr | 46 |
| pACYCalgQ | 647-bp PCR-generated fragment, encompassing the entire algQ gene and its promoter region, ligated to the SalI-BamHI sites of pACYC184 | This study |
| pACYCpfrA | 728-bp PCR-generated fragment, encompassing the pfrA gene and its putative promoter region, ligated to the SalI-BamHI sites of pACYC184 | This study |
| pACYCrsd | 848-bp PCR-generated fragment, encompassing the entire rsd gene and its promoter region, ligated to the SalI-BamHI sites of pACYC184 | This study |
| pBR322 | ColE1 replicon, Apr Tcr | 46 |
| pBRXB | 1.8-kb XhoI-BamHI fragment containing pvdS ligated to pBR322 | 9 |
| pASR1A | 9.3 kb KpnI fragment containing the P. putida WCS358 pfrA gene | 60 |
DNA manipulation and genetic techniques.
All procedures used for the handling of recombinant DNA have been described previously (46). Transfer of plasmids from E. coli to P. aeruginosa was performed by triparental mating with the helper plasmid pRK2013 (14). Recombinant plasmids carrying pfrA, algQ, rsd, or ndk were obtained by gene amplification and cloning, as indicated below. A 728-bp DNA fragment encompassing the entire pfrA gene and its putative promoter was obtained by PCR amplification with primers FWpfrA (5′-GGG GCT CGA GCC AGG GGG TAT TAA ACA CT-3′) and RVpfrA (5′-GGG GGA TCC TGA TCG CAC CCT ATA CG-3′), corresponding to nucleotides (nt) −171 to −190 and nt 520 to 537 relative to the ATG translation start codon of the pfrA gene, respectively, using pASR1A as the template (60). The XhoI and BamHI restriction sites (underlined) were included to make directional cloning of the amplicon into the SalI and BamHI restriction sites of pACYC184 possible, which yielded pACYCpfrA. An 896-bp DNA fragment encompassing the entire algQ gene and its promoter region (23) was obtained by PCR amplification with primers FWalgQ (5′-TAT CTC GGC TTC TCC ATC GT-3′) and RValgQ (5′-GGG GGA TCC TTC GAG ATC GAC CTG CTG-3′), corresponding to nt −321 to −301 and nt 556 to 575 relative to the ATG translation start codon of the algQ gene, respectively, using PAO1 genomic DNA as the template. The algQ amplicon was digested with BamHI (site underlined in RValgQ) and XhoI (249 bp upstream of the start codon) for directional cloning into the SalI and BamHI restriction sites of pACYC184 and pUCP19, yielding pACYCalgQ and pUCPalgQ, respectively. An 848-bp DNA fragment encompassing the entire rsd coding region and its indigenous promoter (21) was obtained by PCR amplification with primers FWrsd (5′-GCT CTA GAC TAA CCA AAC AGG TTC CCC-3′) and RVrsd (5′-TTC CGC GGA TCC CGA ATA AA-3′), corresponding to nt −264 to −238 and nt 565 to 584 relative to the rsd start codon, respectively, using E. coli MC4100 genomic DNA as the template. The rsd amplicon was digested with XbaI and BamHI (sites underlined in the primer sequences), cloned in pUCP19, and then excised by SalI-BamHI digestion and transferred into pACYC184, yielding pACYCrsd. The pACYC-derived constructs were individually used to transform E. coli MC4100 (7) carrying both pBRXB and the pMP220::PpvdA transcriptional fusion (9, 29). To express algQ and rsd genes from a low-copy-number vector, individual amplicons were ligated as XbaI-BamHI digests into the same sites of pJB785TT (47), yielding pJBalgQ and pJBrsd, respectively. A 708-bp DNA fragment encompassing the entire ndk gene and its promoter region (56) was obtained by PCR amplification with primers FWndk (5′-CCG GAA TTC GAG GAA CGG GAC TAG CC-3′) and RVndk (5′-CGC GGA TCC TCA CCC ACG CTC GAT CA-3′), corresponding to nt −260 to −241 and nt 429 to 445 relative to the ATG translation start codon of the ndk gene, respectively, using PAO1 genomic DNA as the template. The ndk amplicon was digested with EcoRI and BamHI (sites underlined) for directional cloning into pUCP19, yielding pUCPndk. A 2,085-bp DNA fragment encompassing the entire rpoD gene and its promoter was obtained by PCR amplification with primers FWrpoD (5′-CGG AAT TCA GAG AGC ACT ACA GTG TTG-3′) and RVrpoD (5′-GAA CTG CAG GCG GTC TCG CGG TGG A-3′), corresponding to nt −105 to −126 and nt 1941 to 1959 relative to the ATG translation start codon of the rpoD gene, respectively, using PAO1 genomic DNA as the template. EcoRI and PstI restriction sites (underlined) were included to make directional cloning of the amplicon into the same restriction sites of pUCP19 possible, yielding pUCPrpoD. The pvdS gene under the transcriptional control of the T5 promoter (PT5) was excised from vector pQEpvdS (29) by XhoI-HindIII digestion and ligated into the SalI-HindIII sites of pUCP18, yielding pUCPpvdS. Recombinant pUCP derivatives were individually introduced into P. aeruginosa PAO1 cells.
PyoverdinePAO1 determination and β-galactosidase assays.
P. aeruginosa strains were grown for 48 h at 37°C on cetrimide agar plates. Isolated colonies were suspended in saline, the A600 was adjusted to ∼1.0, and the preparations were diluted 1:50 in prewarmed DCAA. The cultures were grown at 37°C with vigorous aeration (250 rpm in a New Brunswick 25 orbital shaker). PyoverdinePAO1 was quantified by measuring the absorbance at 405 nm of culture supernatants diluted 1:2 in 100 mM Tris-HCl (pH 8.0), using a Perkin-Elmer LS50 spectrophotometer (64).
The pvdA, pvdD, pvdE, and pvdS promoters cloned upstream of the lacZ reporter gene in plasmids pMP220::PpvdA, pMP190::PpvdD, pMP190::PpvdE, and pMP220::PpvdS, respectively, have been described previously (1, 9, 29). For reporter gene activity measurements, P. aeruginosa strains harboring the pvd::lacZ transcriptional fusions were grown for 14 h at 37°C in DCAA supplemented with the appropriate antibiotics. The cultures were then diluted 1:100 in the same medium with or without 100 μM FeCl3 and subcultured for 4 to 6 h with shaking until the A600 was ∼0.4. E. coli MC4100 carrying both pMP220::PpvdA and pBRXB plus pACYCalgQ, pACYCpfrA, or pACYCrsd were grown for 18 h at 37°C in M9 minimal medium containing 100 μg/ml ampicillin, 10 μg/ml tetracycline, and 30 μg/ml chloramphenicol. The cultures were then diluted 1:100 in the same medium containing either 100 μM FeCl3 or 150 μM 2,2′-dipyridyl for an additional 12 h of growth at 37°C (final A600 in low-iron medium, ∼0.8; final A600 in high-iron medium, 1.2). The β-galactosidase (LacZ) activity was determined spectrophotometrically using o-nitrophenyl-β-d-galactopyranoside as the substrate. The activity was normalized to the A600 of the bacterial culture and was expressed in Miller units (36). The results of β-galactosidase assays were expressed as means of at least six independent experiments performed in duplicate. To overcome the experimental fluctuations resulting from variable traces of iron in different DCAA batches, β-galactosidase levels were also expressed as percentages relative to the level obtained for P. aeruginosa carrying the control vectors (pUCP19 or pJB785TT), which was considered 100%. Standard deviations were calculated for each raw data set and are reported below for mean percent values. The chi-square test was used to assess the statistical significance of differences between β-galactosidase activity values (with P ≤ 0.05).
Production of a mouse polyclonal antiserum against PvdS.
PvdS was purified as previously reported (30). Five BALB/c mice were immunized by intramuscular injection of 20 μg of protein dissolved in saline emulsified 1:1 in complete Freund's adjuvant (Sigma). The mice were given a booster immunization consisting of 10 μg of protein emulsified 1:1 with Freund's incomplete adjuvant (Sigma) 14 and 28 days later. On day 45 after the first immunization, the mice were bled, and the antibody titer in each serum was determined. Animal experiments were performed according to ethical guidelines for the conduct of animal research (D.L.vo 116/92).
SDS-polyacrylamide gel electrophoresis, immunoblotting, and densitometry.
Bacterial cultures were harvested by centrifugation and suspended in saline for protein content determination. The protein concentration was determined using a DC protein assay kit (Bio-Rad) with bovine serum albumin as the standard. Samples containing known amounts of protein were suspended in gel loading buffer (0.25 M Tris-HCl, 2% sodium dodecyl sulfate [SDS], 10% 2-mercaptoethanol, 20% glycerol), heated at 100°C for 5 min, and separated on a 0.1% SDS-15% polyacrylamide gel as described by Laemmli (26). After electrophoresis, gels were stained with Coomassie brilliant blue (46), or resolved proteins were electrotransferred onto a nitrocellulose filter (Hybond C extra; Amersham) using a semidry transfer unit (Hoefer Scientific Instruments) for 1 h at 150 mA. The filters were blocked with 2× TBST (100 mM Tris-HCl [pH 8.0], 1.0 M NaCl, 0.1% Tween 20) containing 1% bovine serum albumin, washed with 2× TBST, and incubated with polyclonal anti-PvdA (41) or anti-PvdS mouse antisera or with monoclonal anti-RpoD antibodies (Neoclone) diluted 1:100, 1:500, and 1:1,000 with 2× TBST, respectively. Proteins were detected by using secondary anti-mouse antibodies conjugated with either alkaline phosphatase (Promega) or horseradish peroxidase (Calbiochem). Final development was performed with the 5-bromo-4-chloro-3-indoylphosphate (BCIP) and nitroblue tetrazolium chloride reagents for colorimetric determinations (Promega) or with the Amersham ECL chemiluminescent reagents (Amersham Biosciences), followed by exposure to X-ray film (Kodak) for autoradiography. Densitometric measurements of band intensities were obtained by use of the Quantity One software and a Gel Doc 2000 charge-coupled device camera (Bio-Rad).
RNA purification and primer extension.
Total RNA for primer extension analysis (100 μg for each reaction) was extracted from P. aeruginosa and E. coli cultures grown in DCAA (A620, ∼0.6) and M9 minimal medium (A620, ∼0.8), respectively, as previously described (29). For primer extension analysis, 1 pmol of oligonucleotide RVPpa (5′-GGC GGT TGC AGT TGC CTG AGT CAT-3′; complementary to the coding strand of the pvdA gene from the eighth codon to the ATG translation start site) was end labeled with [γ-32P]ATP (Amersham Biosciences) and used for the reverse transcription reaction as described elsewhere (29). The unlabeled primer was used to sequence the DNA region upstream of the pvdA gene from plasmid pPV226, using a T7 sequencing kit (Pharmacia) and [α-32P]dATP. Primer extension products were run in parallel in the sequencing reactions to map the start sites of the transcripts.
In silico sequence and genome analysis.
Searches for P. aeruginosa PAO1 AlgQ homologues were performed by the BLASTP network service (National Center for Biotechnology Information; http://www.ncbi.nlm.nih.gov). Sequences were also retrieved from the Clusters of Orthologous Groups (COG) database (COG3160; http://www.ncbi.nlm.nih.gog/COG/new). Genomic contexts were obtained from http://www.pseudomonas.com and http://genome.jgi-psf.org/microbial/.
RESULTS
AlgQ, PfrA, and Rsd increase the PvdS-dependent activity of the pvdA promoter in the heterologous E. coli host.
To analyze the involvement of the algQ, pfrA, and rsd gene products in the transcriptional regulation of pyoverdine genes, we performed a heterologous expression assay with E. coli MC4100 carrying both the pMP220::PpvdA reporter gene system and plasmid pBRXB, which harbored the pvdS sigma factor gene under the control of its iron-regulated promoter (9, 29). Plasmids pACYCalgQ, pACYCpfrA, and pACYCrsd, containing the algQ, pfrA, and rsd coding sequences with their indigenous promoters, respectively, were individually introduced into E. coli MC4100(pMP220::PpvdA, pBRXB) for measurement of β-galactosidase activity under iron-depleted and iron-replete conditions (Table 2). Compared with the E. coli(pACYC184, pBRXB) control strain, the iron-regulated PpvdA activity was doubled by the presence of either algQ or rsd and was trebled by pfrA. In the absence of pBRXB (pvdS), no PpvdA activity was detected in E. coli MC4100, irrespective of the presence of transactivating genes. This indicates that the algQ, pfrA, and rsd gene products, in concert with PvdS, contribute to the positive control of pvdA expression in the heterologous E. coli system.
TABLE 2.
Effect of algQ, pfrA, and rsd on PpvdA::lacZ activity in E. coli MC4100 expressing the pvdS gene
| Plasmids | LacZ activitya
|
|
|---|---|---|
| M9 medium + 2,2′-dipyridylb | M9 medium + FeCl3b | |
| pMP220::PpvdA + pBR322 | 35 | 31 |
| pMP220::PpvdA + pBRXB | 256 | 27 |
| pMP220::PpvdA + pBRXB + pACYC184 | 214 | 29 |
| pMP220::PpvdA + pBR322 + pACYCalgQ | 32 | 33 |
| pMP220::PpvdA + pBR322 + pACYCpfrA | 35 | 30 |
| pMP220::PpvdA + pBR322 + pACYCrsd | 33 | 31 |
| pMP220::PpvdA + pBRXB + pACYCalgQ | 450 | 40 |
| pMP220::PpvdA + pBRXB + pACYCpfrA | 621 | 51 |
| pMP220::PpvdA + pBRXB + pACYCrsd | 419 | 48 |
β-Galactosidase (LacZ) activity is expressed in Miller units (36). The values are means of three independent determinations. The standard deviation is <13% of each value.
The M9 minimal medium was supplemented with 150 μM 2,2′-dipyridyl and with 100 μM FeCl3 to reduce and increase iron availability, respectively.
PyoverdinePAO1 production is reduced in a P. aeruginosa PAO1 algQ-defective background.
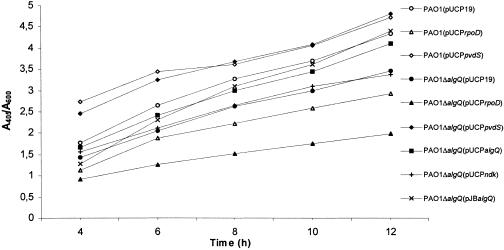
To investigate the effect of AlgQ on pyoverdinePAO1 production, we monitored pyoverdinePAO1 levels during the whole growth cycle of wild-type P. aeruginosa PAO1 strains [PAO1(pUCP19) and PAO1(pJB785TT)], of algQ null mutants [PAO1ΔalgQ(pUCP19) and PAO1ΔalgQ(pJB785TT)], and of algQ null mutants complemented with either a single copy or multiple copies of algQ [PAO1ΔalgQ(pUCPalgQ) and PAO1ΔalgQ(pJBalgQ), respectively]. The growth rates in low-iron medium (DCAA) were comparable for all strains tested throughout the experiment (data not shown). In spite of this, the ΔalgQ mutants reproducibly exhibited an ca. 20% reduction in pyoverdinePAO1 production compared with wild-type strain PAO1 carrying the control vectors (shown in Fig. 1 only for pUCP19). PyoverdinePAO1 production was totally restored upon complementation of the ΔalgQ mutation with a single copy of the algQ gene [PAO1ΔalgQ(pJBalgQ)] (Fig. 1). Introduction of either pUCPalgQ or pJBrsd into PAO1ΔalgQ increased pyoverdinePAO1 production to ca. 90% of the wild-type levels (Fig. 1 and data not shown). Comparatively, the multicopy plasmid pUCPalgQ was more effective in complementation during early growth, whereas the single-copy plasmid pJBalgQ had a delayed effect (Fig. 1). Since synthesis of alginate, GTP, ppGpp, and polyP is restored in a P. aeruginosa algQ mutant by complementation with the ndk gene (25), we measured pyoverdinePAO1 production in the P. aeruginosa PAO1 algQ null mutant harboring plasmid pUCPndk (Fig. 1). Comparable pyoverdinePAO1 levels were produced by PAO1ΔalgQ(pUCP19) and PAO1ΔalgQ(pUCPndk). Thus, AlgQ is required for optimal production of pyoverdinePAO1 in P. aeruginosa PAO1, but it cannot be replaced by Ndk for this function.
FIG. 1.
Effect of the algQ mutation on pyoverdine yields in iron-depleted cultures of P. aeruginosa PAO1 carrying a single copy of algQ and multiple copies of algQ, ndk, pvdS, or rpoD. Bacterial growth in DCAA was monitored by measuring the absorbance at 600 nm of the cultures at different times. At each time, pyoverdinePAO1 levels were determined by measuring the absorbance at 405 nm of cell-free culture supernatants diluted 1:2 in 100 mM Tris-HCl (pH 8.0). Data are expressed as relative fluorescence levels (A405/A600). All measurements were performed in duplicate and in multiple experimental trials with different batches of DCAA. The standard deviations are <8% of the values.
AlgQ modulates pvd gene expression at the transcriptional level.
To gain further insight into the role of AlgQ in the transcriptional control of pvd genes, the promoter activities of the pvdA::lacZ, pvdD::lacZ, pvdE::lacZ, and pvdS::lacZ transcriptional fusions were compared for wild-type strain PAO1 and the ΔalgQ mutant with or without the algQ, rsd, or ndk gene in trans under iron-deficient and iron-sufficient conditions. Under low-iron conditions, the β-galactosidase levels expressed by pvdA, pvdD, and pvdE transcriptional fusions were reduced by ca. 25 to 50% in the algQ mutant compared with wild-type strain PAO1, depending on the fusion (Table 3). Introduction of the single-copy pJBalgQ plasmid into PAO1ΔalgQ restored the wild-type activity of pvd promoters, while the multicopy construct pUCPalgQ had weaker effects, as also observed for the heterologous rsd gene in pJBrsd (Table 3). No significant difference in promoter activity was detected between the algQ null mutant carrying pUCP19 and the algQ null mutant carrying pUCPndk (Table 3). As expected, no promoter activity was observed in iron-rich cultures (Table 3). These results indicate that AlgQ positively affects the transcription of several coregulated genes of the pvd biosynthetic cluster in an Ndk-independent manner. Remarkably, the activities of the pvdS::lacZ transcriptional fusion were similar in algQ-deficient and -proficient backgrounds (Table 3).
TABLE 3.
Effect of the algQ mutation on the activities of different pvd::lacZ transcriptional fusions in P. aeruginosa PAO1 cells carrying rpoD, pvdS, algQ, ndk, and rsd genes in trans
| Strain | LacZ activitya
|
|||||||
|---|---|---|---|---|---|---|---|---|
| PpvdA::lacZ
|
PpvdD::lacZ
|
PpvdE::lacZ
|
PpvdS::lacZ
|
|||||
| −Fe(III) | +Fe(III) | −Fe(III) | +Fe(III) | −Fe(III) | +Fe(III) | −Fe(III) | +Fe(III) | |
| PAO1(pUCP19) | 7,973 | 45 | 6,402 | 52 | 5,504 | 52 | 4,180 | 58 |
| PAO1(pUCPrpoD) | 5,956 (75 ± 5)b | 28 | 3,559 (56 ± 4) | 31 | 3,242 (59 ± 4) | 29 | 4,431 (106 ± 4) | 51 |
| PAO1(pUCPpvdS) | 8,771 (110 ± 8) | 4,868 | 8,963 (140 ± 6) | 5,333 | 6,808 (123 ± 8) | 4,289 | 4,013 (96 ± 5) | 52 |
| PAO1ΔalgQ(pUCP19) | 5,980 (75 ± 8) | 25 | 4,049 (63 ± 4) | 29 | 3,821 (69 ± 8) | 26 | 4,270 (102 ± 4) | 62 |
| PAO1ΔalgQ(pUCPrpoD) | 3,572 (45 ± 6) | 33 | 2,681 (42 ± 3) | 27 | 2,708 (49 ± 5) | 32 | 4,598 (110 ± 7) | 47 |
| PAO1ΔalgQ(pUCPpvdS) | 8,124 (101 ± 4) | 4,471 | 8,771 (137 ± 8) | 4,959 | 6,671 (121 ± 7) | 4,032 | 3,929 (94 ± 6) | 54 |
| PAO1ΔalgQ(pUCPalgQ) | 7,554 (95 ± 5) | 32 | 5,013 (81 ± 14) | 28 | 4,933 (93 ± 3) | 35 | 4,318 (103 ± 8) | 43 |
| PAO1ΔalgQ(pUCPndk) | 6,177 (77 ± 7) | 31 | 4,081 (64 ± 4) | 30 | 3,928 (71 ± 7) | 33 | 4,265 (102 ± 6) | 55 |
| PAO1(pJB785TT) | 7,863 | 47 | 6,513 | 48 | 5,623 | 36 | 4,092 | 52 |
| PAO1ΔalgQ(pJB785TT) | 5,740 (73 ± 7) | 35 | 4,364 (67 ± 4) | 46 | 3,655 (65 ± 3) | 43 | 4,174 (102 ± 8) | 61 |
| PAO1ΔalgQ(pJBalgQ) | 7,627 (97 ± 5) | 36 | 6,448 (99 ± 3) | 43 | 5,848 (104 ± 6) | 52 | 3,969 (97 ± 5) | 54 |
| PAO1ΔalgQ(pJBrsd) | 6,684 (85 ± 6) | 48 | 5,275 (81 ± 4) | 37 | 4,554 (81 ± 5) | 49 | 3,928 (96 ± 4) | 53 |
β-Galactosidase activities were determined in lysates of P. aeruginosa PAO1 cultures grown for 6 h in DCAA [−Fe(III)] and in DCAA supplemented with 100 μM FeCl3 [+Fe(III)]. The values, expressed in Miller units (36), are the means of six independent determinations. The standard deviations are <15% of the means.
The values in parentheses (means ± standard deviations) are percentages based on the value obtained for P. aeruginosa PAO1 carrying the control vector (either pUCP19 or pJB785TT), which was considered 100%.
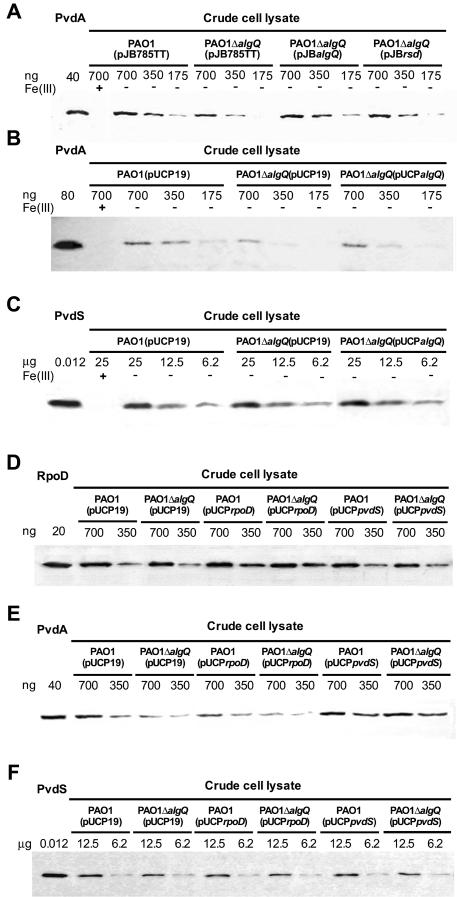
Next, we examined the effect of AlgQ on the expression levels of both PvdA and PvdS. Total proteins from iron-starved P. aeruginosa cell lysates were separated by SDS-polyacrylamide gel electrophoresis and probed with either anti-PvdA or anti-PvdS mouse polyclonal sera (41; this study). Quantitative estimation of band intensities in the Western blot assays demonstrated that PvdA expression by P. aeruginosa PAO1ΔalgQ was reduced by ca. 60% compared with the expression by wild-type strain PAO1 and was fully restored upon complementation with pJBalgQ (Fig. 2A). Also, in this case both pUCPalgQ and pJBrsd increased PvdA expression without reaching the wild-type level (Fig. 2A and B). Interestingly, PvdS levels were comparable in wild-type strain PAO1(pUCP19), PAO1ΔalgQ(pUCP19), and PAO1ΔalgQ(pUCPalgQ) (Fig. 2C). These results are in line with the observed regulation of pvdA::lacZ and pvdS::lacZ transcriptional fusions; in fact, they demonstrate that AlgQ affects the extent of PvdA expression without influencing PvdS levels. Because expression of PvdA and expression of PvdS were similar in PAO1ΔalgQ(pUCP19) and PAO1ΔalgQ(pUCPndk) (data not shown), the algQ-complementing activity of ndk was not investigated further.
FIG. 2.
Western blot analysis of PvdA, PvdS, and RpoD expression in P. aeruginosa PAO1 and PAO1ΔalgQ carrying different plasmids. Whole-cell lysates obtained from iron-poor (DCAA) [−Fe(III)] and iron-rich (DCAA plus 100 μM FeCl3) [+Fe(III)] cultures were probed with anti-PvdA (A, B, and E) or anti-PvdS (C and F) polyclonal mouse antisera and with commercial monoclonal anti-RpoD antibodies (D). The strains used and amounts of total proteins from each extract are indicated in each panel. The purified proteins (PvdA, 47.7 kDa; PvdS, 21.2 kDa; RpoD, 70.2 kDa) were used as positive controls. For details, see Materials and Methods.
Effect of AlgQ on the transcription initiation pattern at the pvdA promoter.
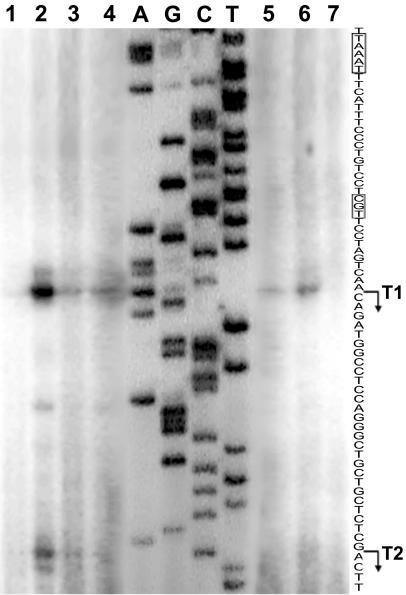
The following two transcription start sites were previously identified upstream of the pvdA gene: a major start site (T1) and a minor site (T2) located 68 and 43 nt upstream from the A of the pvdA start codon (29). To evaluate the effect of AlgQ on the transcription initiation pattern of pvdA, primer extension analysis of total RNA extracted from iron-deficient cells of wild-type strain PAO1(pUCP19) and its algQ null derivative PAO1ΔalgQ carrying either pUCP19 or pUCPalgQ was performed (Fig. 3). The transcription initiation profiles were similar in the wild-type and algQ-defective backgrounds, although the amount of a primer extension product(s) was smaller in the algQ mutant (Fig. 3, lanes 2 and 3, respectively). Introduction of pUCPalgQ into the algQ mutant improved the yield of a primer extension product(s) (Fig. 3, lane 4). As expected, no primer extension product(s) was detected with total RNA from iron-replete P. aeruginosa cells (Fig. 3, lane 1).
FIG. 3.
Effect of the algQ mutation on the initiation pattern of pvdA transcripts in P. aeruginosa PAO1 algQ-proficient and -defective backgrounds and in the heterologous host E. coli. The primer extension reaction was carried out with the 5′-end-labeled oligonucleotide RVPpa and equal amounts of total RNA isolated from P. aeruginosa and E. coli cells grown in DCAA and M9 medium, respectively. The single-stranded pvdA promoter sequence is shown on the right, and consensus motifs recognized by PvdS are enclosed in boxes. The previously identified 5′ ends of T1 and T2 transcripts (29) and the direction of transcription are indicated by bent arrows. Lanes A, G, C, and T, pvdA sequencing ladder generated from pPV226 with the same oligonucleotide (RVPpa): lane 1, primer extension analysis of total RNA extracted from P. aeruginosa PAO1(pUCP19) iron-rich cultures (DCAA plus 100 μM FeCl3); lanes 2 to 4, primer extension analysis with total RNA from P. aeruginosa PAO1(pUCP19) (lane 2), PAO1ΔalgQ(pUCP19) (lane 3), and PAO1ΔalgQ(pUCPalgQ) (lane 4); lanes 5 to 7, primer extension analysis with total RNA from E. coli MC4100(PpvdA::lacZ; pBRXB) carrying pACYCalgQ (lane 5), pACYCpfrA (lane 6), and pACYC184 (lane 7). Lane cutting and pasting were needed to visualize the sequencing ladder and primer extension products, which required different exposure times.
Primer extension analysis was also performed with total RNA extracted from iron-deficient cells of E. coli MC4100 carrying the pvdA::lacZ transcriptional fusion plus plasmid pBRXB and, alternatively, either pACYCalgQ or pACYCpfrA. In the heterologous E. coli system, pvdA transcription from the major T1 start point was enhanced by algQ and, to a greater extent, by pfrA (Fig. 3, lanes 5 and 6, respectively). Interestingly, no T2 product was detectable in E. coli, even after prolonged autoradiography (Fig. 3).
Multicopy rpoD mimics the algQ mutation in wild-type P. aeruginosa strain PAO1.
Because both AlgQ and Rsd bind RpoD (12), the effect of AlgQ on the expression of pvd genes could be explained by an anti-sigma factor mechanism. We hypothesized that AlgQ could increase the availability of free RNAPc and facilitate the formation of the PvdS-dependent RNAP holoenzyme, thereby allowing transcription of PvdS-dependent genes to occur more efficiently. To verify this hypothesis, we tested the effect of an overdose of RpoD on PvdS-dependent transcription of pvd genes. The multicopy plasmid pUCPrpoD, carrying the entire rpoD coding sequence and its own promoter (15), was introduced into wild-type and ΔalgQ P. aeruginosa strains. The presence of the multicopy rpoD gene increased the levels of RpoD by ca. 1.5-fold, as determined by Western blot analysis with monoclonal anti-RpoD antibodies (Fig. 2D). Interestingly, the pyoverdinePAO1 yields were diminished to the same extent in PAO1(pUCPrpoD) and PAO1ΔalgQ(pUCP19) and were further decreased in PAO1ΔalgQ(pUCPrpoD) (Fig. 1). Accordingly, expression of PpvdA::lacZ, PpvdD::lacZ, and PpvdE::lacZ transcriptional fusions was significantly reduced in both wild-type and ΔalgQ strains carrying pUCPrpoD (Table 3). The results of a comparison of PvdA expression in PAO1(pUCPrpoD) and PvdA expression in PAO1ΔalgQ(pUCPrpoD) mirrored the transcriptional response observed for the PpvdA::lacZ fusion (Fig. 2E and Table 3). Furthermore, transformation of the algQ mutant with pUCPpvdS, a multicopy plasmid driving constitutive pvdS expression, restored or even increased the pyoverdinePAO1 yields, PpvdA::lacZ, PpvdD::lacZ, and PpvdE::lacZ promoter activities, and PvdA expression levels (Fig. 1 and 2E and Table 3). Expression of the PpvdS::lacZ transcriptional fusion was not significantly affected by multicopy rpoD and pvdS in either wild-type or ΔalgQ strains (Table 3), and Western blot analysis of PAO1(pUCPrpoD) and PAO1ΔalgQ(pUCPrpoD) lysates failed to detect appreciable differences in the PvdS levels (Fig. 2F). Altogether, these results argue for an anti-RpoD mechanism in AlgQ transcriptional regulation of pvd biosynthetic genes.
DISCUSSION
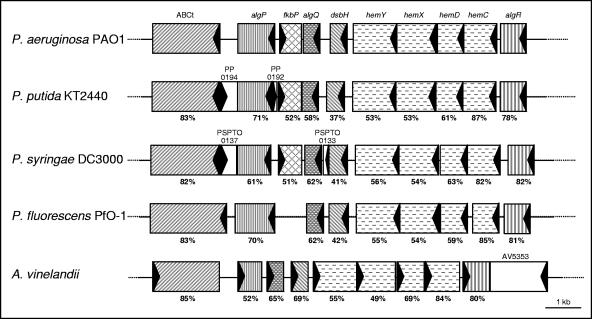
As a rule, proteins with relevant biological functions have been conserved during evolution. This is also true for AlgQ, whose homologues (Rsd-like proteins) can be retrieved from several γ-Proteobacteria (COG3160; http://www.ncbi.nlm.nih.gov/COG/new/). Comparative analysis of the algQ genomic region in P. aeruginosa PAO1, P. putida KT2440, Pseudomonas syringae pv. tomato strain DC3000, Pseudomonas fluorescens PfO-1, and Azotobacter vinelandii revealed remarkable conservation of the whole locus (Fig. 4), as previously documented for the pprA-pfrA-pprB locus of P. putida WCS358 (59). These features suggest that AlgQ homologues have an important function(s) in Pseudomonadaceae and, more generally, in γ-Proteobacteria.
FIG. 4.
Schematic diagrams of the genomic regions encompassing algQ homologues in P. aeruginosa PAO1, P. putida KT2440, P. syringae pv. tomato strain DC3000 (P. syringae DC3000), P. fluorescens PfO-1, and A. vinelandii. The designations of characterized and putative genes are indicated at the top. ABCt, ATP-binding transporter; algP, gene encoding the alginate regulatory protein; fkbP, gene encoding the peptidyl-prolyl cis-trans isomerase; algQ, gene encoding a regulatory protein; dsbH, gene encoding the DsbH family protein; hemY, gene encoding the HemY putative protein; hemX, gene encoding the uroporphyrin III C-methyltransferase; hemD, gene encoding the uroporphyrinogen III synthetase; hemC, gene encoding the porphobilinogen deaminase; algR, gene encoding a regulatory protein. Orthologues are indicated by the same pattern. The triangles indicate the direction of transcription. Annotation numbers of hypothetical open reading frames are indicated. The level of protein identity relative to P. aeruginosa PAO1 is indicated below each open reading frame.
AlgQ was originally identified as a positive transcriptional regulator of the alginate biosynthetic gene algD (23) and later was shown to act as a pleiotropic regulatory protein capable of modulating the expression of several P. aeruginosa virulence factors, including extracellular proteases, rhamnolipid, neuraminidase, and a siderophore(s) (6, 25, 49, 60). However, the molecular mechanism(s) underlying the AlgQ function(s) in P. aeruginosa PAO1 remains largely unresolved. Here, we combined quantitative pyoverdinePAO1 determination, pvd promoter-reporter gene assays, mRNA mapping, and immunoblot analysis of Pvd proteins to demonstrate that AlgQ finely modulates pyoverdinePAO1 production through positive control of PvdS activity. We used this experimental approach to circumvent the intrinsic drawback of large-scale transcriptome profiling, which suffers from overlooking minor differences in mRNA levels.
Previous investigations on the effect of AlgQ on siderophore production were performed with the algQ mutant of P. aeruginosa 8830 grown under ill-defined iron availability conditions (i.e., LB medium) (49). We noticed that both P. aeruginosa 8830 and its algQ mutant grow poorly under low-iron conditions (DCAA) compared with P. aeruginosa PAO1 (Ambrosi, unpublished results), while no differences between wild-type strain PAO1 and its isogenic ΔalgQ derivative were found. As shown for the E. coli K-12 rsd mutant (21), P. aeruginosa PAO1ΔalgQ apparently showed no distinct phenotype compared with the wild-type parental strain, as determined by growth kinetics, cell viability, and colony morphology in LB medium and DCAA. Thus, AlgQ is not essential for P. aeruginosa growth in laboratory medium under iron-depleted conditions.
The AlgQ-dependent stimulation of pyoverdinePAO1 synthesis in P. aeruginosa PAO1 was paralleled by increased transcription of the pvdA, pvdD, and pvdE biosynthetic genes. This could not be ascribed to increased expression of PvdS. In fact, the algQ mutation neither affected pvdS expression nor altered PvdS levels (Table 3 and Fig. 2C). Hence, AlgQ acts as positive regulator of pyoverdinePAO1 synthesis by acting at the posttranscriptional level on PvdS activity.
Experiments performed with the reconstituted E. coli system have demonstrated that PvdS-dependent transcription of pvdA is positively affected both by the Rsd-like proteins AlgQ and PfrA from Pseudomonas and by E. coli Rsd itself (Table 2). Related to this finding, AlgQ was previously shown to transactivate the AlgU-dependent algD promoter in E. coli cells grown under high-osmolarity conditions (23). Mapping of a pvdA mRNA(s) in the reconstituted E. coli system confirmed that transcription from the major T1 start point is enhanced by the presence of AlgQ and PfrA in trans. The absence of the minor T2 product and the inability of both AlgQ and PfrA to direct heterologous pvdA transcription at P. aeruginosa levels support our previous hypothesis (30) concerning the requirement of an as-yet-unidentified activating factor(s), besides PvdS and AlgQ, for full transcription at the pvdA promoter in P. aeruginosa.
By what mechanism does AlgQ act as a positive regulator of pyoverdine biosynthetic genes? First, we hypothesized that AlgQ could indirectly affect pyoverdinePAO1 yields through the up-regulation of Ndk, which enhances the formation of the ppGpp alarmone during nutritional starvation (25). We reasoned that the reduced levels of ppGpp in the P. aeruginosa algQ-defective background (25) could account for the diminished expression of the pvd biosynthetic genes. The ppGpp molecule bound to RNAPc has the potential (i) to alter promoter recognition and the kinetics of transcriptional initiation, either positively or negatively, (ii) to increase the competitiveness and association properties of alternative sigma factors for RNAPc (8, 22, 39), and (iii) to affect the induction profile of some genes controlled by alternative sigma factors (22, 58). However, an Ndk/ppGpp-dependent cascade of pvd promoter activation seems very unlikely, given that ndk did not suppress the algQ mutant phenotype at the level of pyoverdinePAO1 synthesis and pvd transcription. The results of an immunoblot analysis of PvdA and PvdS also excludes a role for Ndk in improving the translation efficiency of pvd messengers through activation of elongation factor Tu and increased GTP synthesis (38). An intriguing observation was the partial complementation of the PAO1ΔalgQ mutant by the multicopy algQ gene. Recovery of pyoverdine yields by complementation with low- and high-copy-number algQ exhibited a time-dependent profile (Fig. 1), suggesting that AlgQ must be present at appropriate concentrations at a given time to optimize expression of pvd genes. The reason for the dose-dependent effects of algQ on pvd gene expression is not known. However, evidence for incomplete complementation was also obtained in previous studies which showed that neither algQ nor ndk, under the control of the tac promoter, was able to fully restore alginate, ppGpp, and polyP synthesis in a P. aeruginosa algQ mutant (25, 56). Apart from this, it is evident that pyoverdine synthesis is modulated by the algQ gene but not by ndk.
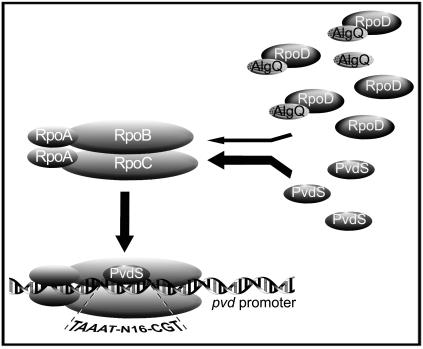
Because rsd partially substituted for algQ in transcriptional regulation of pvd genes, we entertained the alternative idea that AlgQ could increase pvd expression by acting as an anti-sigma factor for RpoD (12). We tested this hypothesis by introducing either rpoD or pvdS on a high-copy-number plasmid into PAO1 and PAO1ΔalgQ. As far as expression of pvd biosynthetic genes is concerned, multicopy rpoD mimicked and even exacerbated the ΔalgQ phenotype in wild-type and algQ mutant strains, respectively, without altering the levels of RNAPc (13). Accordingly, multicopy pvdS suppressed the ΔalgQ phenotype. A similar situation has been documented for the Rsd-dependent competition between RpoD and the alternative sigma factors RpoS and RpoH in E. coli (21). Based on the results described above, a model of AlgQ-dependent modulation of pvd expression was developed (Fig. 5). AlgQ could activate pvd genes by functioning as an anti-sigma factor of RpoD, consistent with its ability to interact with the RpoD subunit (12). The inhibition of an RpoD interaction with RNAPc would increase the opportunity for PvdS to bind RNAPc and to direct the RNAPc-PvdS holoenzyme to pvd promoters, thereby enhancing pvd gene expression. In E. coli, functional RNAPc is a limiting factor for the rate of transcription, and sigma subunits compete for a limited number of free RNAPc molecules (33, 52). Under iron-depleted conditions PvdS expression and activity increase rapidly, as measured by the rate of LacZ synthesis from both the PpvdS::lacZ and pvd::lacZ transcriptional fusions (Table 3) (29). While the increased intracellular level of PvdS is sufficient to direct pvd transcription, the positive effect of AlgQ on PvdS-RNAPc holoenzyme formation would ensure optimal transcription of pvd and possibly of the other PvdS-dependent genes, such as prpL, toxA, and aprA (40, 61). The lack of AlgQ modulation on pvdS expression is consistent with the general notion that genes whose products have a key role in cell survival under starvation conditions (e.g., iron-depleted conditions) are not affected by a reduction in RpoD-dependent RNAP levels (34).
FIG. 5.
Proposed model of AlgQ modulation of PvdS-dependent transcription. Under iron starvation conditions, the interaction between AlgQ and free RpoD subunits facilitates RNAPc recruitment by the IS sigma factor PvdS and hence transcription initiation at the pvd promoters. Canonical sequences recognized by PvdS are indicated.
In P. aeruginosa, transcription of the algD gene depends mainly on the alternative sigma factor AlgU, another member of the ECF subfamily of eubacterial sigma factors (11, 30, 32). AlgU activity is under negative control of the anti-sigma factor MucA, encoded by the mucA gene (35). However, it has been proposed that the responsiveness of algD to intracellular low iron levels (4, 17, 57) is AlgQ mediated (55). Given the minute amount of AlgU in stressed cells of P. aeruginosa PAO1 (50), the anti-RpoD activity of AlgQ could also enhance AlgU-dependent transcription.
Finally, it is also possible that AlgQ positively affects pyoverdine production via a direct effect on pvd promoters, as documented for rhlR and lasR promoters (28). Interestingly, it was recently shown that Rsd can also interact with RNAPc (19), likely directing RNAP to particular subsets of promoters. Whether this is also true for AlgQ remains to be proven.
Taken together, our data substantiate the connection between pyoverdinePAO1 production, alginate biosynthesis, and energy metabolism (24). Alginate is known for being produced under nutrient-limited conditions or as a consequence of impaired energy metabolism. In particular, the algQ promoter responds to nutrient starvation (namely, phosphate limitation) by increasing Ndk expression to compensate for the decrease in NTP and deoxynucleoside triphosphate (dNTP) synthesis under phosphate-deficient conditions (25). On the other hand, NTP and dNTP deficiency can also result from a diminished supply of ATP from aerobic respiration, a primary metabolic drawback of iron depletion (42, 45, 54). This is in keeping with the observed correlation between iron limitation, poor oxygen transfer, and alginate synthesis under controlled bioreactor conditions (24). AlgQ might therefore contribute to the maintenance of the intracellular pool of NTPs and dNTPs directly through Ndk and indirectly by promoting pyoverdine-dependent iron uptake and hence formation of iron-containing respiratory enzymes and ATP synthesis from aerobic respiration. Moreover, the anti-RpoD activity of AlgQ could orient RNAP toward promoters governed by the alternative sigma factors PvdS and AlgU, causing stimulation of pyoverdine and alginate synthesis to protect bacteria from stressful situations, such as those encountered in vivo. The moderate effect of AlgQ on PvdS- and AlgU-dependent transcription (12, 66) is consistent with the hypothesis that it is a protein that is capable of antagonizing the activity of the major vegetative sigma subunit.
In conclusion, AlgQ seems to play a part in many regulatory processes, resulting in modulation of the expression of several genes. As a global regulatory protein, AlgQ could participate in the transcriptional control of target genes within its regulon in concert with other factors, thereby allowing fine-tuning of gene expression in response to various environmental stimuli.
Acknowledgments
We are grateful to M. Foglino for supplying the P. aeruginosa PAO1 algQ null mutant used in this study. We thank A. Merante and D. Perroni for assistance with artwork and V. Pallottini for animal house work.
This work was supported by MIUR-PRIN grant 2004060270_005 and ISPESL contract B98/DIPIA/03.
REFERENCES
- 1.Ambrosi, C., L. Leoni, and P. Visca. 2002. Different responses of pyoverdine genes to autoinduction in Pseudomonas aeruginosa and the group Pseudomonas fluorescens-Pseudomonas putida. Appl. Environ. Microbiol. 68:4122-4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews, S. C., A. K. Robinson, and F. Rodriguez-Quinones. 2003. Bacterial iron homeostasis. FEMS Microbiol. Rev. 27:215-237. [DOI] [PubMed] [Google Scholar]
- 3.Beare, P. A., R. J. For, L. W. Martin, and I. L. Lamont. 2003. Siderophore-mediated cell signalling in Pseudomonas aeruginosa: divergent pathways regulate virulence factor production and siderophore receptor synthesis. Mol. Microbiol. 47:195-207. [DOI] [PubMed] [Google Scholar]
- 4.Boyce, M. J., and R. V. Miller. 1982. Selection of nonmucoid derivatives of mucoid Pseudomonas aeruginosa is strongly influenced by the level of iron in the culture medium. Infect. Immun. 37:695-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budzikiewicz, H. 1997. Siderophores of fluorescent pseudomonads. Z. Naturforsch. Sect. C 52:713-720. [PubMed] [Google Scholar]
- 6.Cacalano, G., M. Kays, L. Saiman, and A. Prince. 1992. Production of the Pseudomonas aeruginosa neuraminidase is increased under hyperosmolar conditions and is regulated by genes involved in alginate expression. J. Clin. Investig. 89:1866-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casabadan, M. J., and S. N. Cohen. 1980. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J. Mol. Biol. 138:179-207. [DOI] [PubMed] [Google Scholar]
- 8.Cashel, M., D. R. Gentry, V. J. Hernandez, and D. Vinella. 1996. The stringent response, p. 1458-1496. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. American Society for Microbiology, Washington, D.C.
- 9.Cunliffe, H. E., T. R. Merriman, and I. L. Lamont. 1995. Cloning and characterization of pvdS, a gene required for pyoverdine synthesis in Pseudomonas aeruginosa: PvdS is probably an alternative sigma factor. J. Bacteriol. 177:2744-2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darzins, A., and A. M. Chakrabarty. 1984. Cloning of genes controlling alginate biosynthesis from a mucoid cystic fibrosis isolate of Pseudomonas aeruginosa. J. Bacteriol. 159:9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeVries, C. A., and D. E. Ohman. 1994. Mucoid-to-nonmucoid conversion in alginate-producing Pseudomonas aeruginosa often results from spontaneous mutations in algT, encoding a putative alternative sigma factor, and shows evidence for autoregulation. J. Bacteriol. 176:6677-6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dove, S. L., and A. Hochschild. 2001. Bacterial two-hybrid analysis of interactions between region 4 of the σ70 subunit of RNA polymerase and the transcriptional regulators Rsd from Escherichia coli and AlgQ from Pseudomonas aeruginosa. J. Bacteriol. 183:6413-6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dykxhoorn, D. M., R. St. Pierre, and T. Linn. 1996. Synthesis of the β and β′ subunits of Escherichia coli RNA polymerase is autogenously regulated in vivo by both transcriptional and translational mechanisms. Mol. Microbiol. 29:483-493. [DOI] [PubMed] [Google Scholar]
- 14.Figurski, D. H., and D. R. Helinski. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 76:1648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujita, M., K. Tanaka, H. Takahashi, and A. Amemura. 1993. Organization and transcription of the principal sigma gene (rpoDA) of Pseudomonas aeruginosa PAO1: involvement of a sigma 32-like RNA polymerase in rpoDA gene expression. J. Bacteriol. 175:1069-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Govan, J. R. W., and V. Deretic. 1996. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 60:539-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haas, B., J. Kraut, J. Marks, S. C. Zanker, and D. Castignetti. 1991. Siderophore presence in sputa of cystic fibrosis patients. Infect. Immun. 59:3997-4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hassett, D. J., J. Cuppoletti, B. Trapnell, S. V. Lymar, J. J. Rowe, S. S. Yoon, G. M. Hilliard, K. Parvatiyar, M. C. Kamani, D. J. Wozniak, S. H. Hwang, T. R. McDermott, and U. A. Ochsner. 2002. Anaerobic metabolism and quorum sensing by Pseudomonas aeruginosa biofilms in chronically infected cystic fibrosis airways: rethinking antibiotic treatment strategies and drug targets. Adv. Drug Deliv. Rev. 54:1425-1443. [DOI] [PubMed] [Google Scholar]
- 19.Ilag, L. L., L. F. Westblade, C. Deshayes, A. Kolb, S. J. Busby, and C. V. Robinson. 2004. Mass spectrometry of Escherichia coli RNA polymerase: interactions of the core enzyme with sigma70 and Rsd protein. Structure 12:269-275. [DOI] [PubMed] [Google Scholar]
- 20.Jishage, M., and A. Ishihama. 1998. A stationary phase protein in Escherichia coli with binding activity to the major sigma subunit of RNA polymerase. Proc. Natl. Acad. Sci. USA 95:4953-4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jishage, M., and A. Ishihama. 1999. Transcriptional organization and in vivo role of the Escherichia coli rsd gene, encoding the regulator of RNA polymerase sigma D. J. Bacteriol. 181:3768-3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jishage, M., K. Kvint, V. Shingler, and T. Nyström. 2002. Regulation of sigma factor competition by the alarmone ppGpp. Genes Dev. 16:1260-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato, J., L. Chu, K. Kitano, J. D. DeVault, K. Kimbara, A. M. Chakrabarty, and T. K. Misra. 1989. Nucleotide sequence of a regulatory region controlling alginate synthesis in Pseudomonas aeruginosa: characterization of the algR2 gene. Gene 84:31-38. [DOI] [PubMed] [Google Scholar]
- 24.Kim, E. J., W. Sabra, and A. P. Zeng. 2003. Iron deficiency leads to inhibition of oxygen transfer and enhanced formation of virulence factors in cultures of Pseudomonas aeruginosa PAO1. Microbiology 149:2627-2634. [DOI] [PubMed] [Google Scholar]
- 25.Kim, H. Y., D. Schlictman, S. Shankar, Z. Xie, A. M. Chakrabarty, and A. Kornberg. 1998. Alginate, inorganic polyphosphate, GTP and ppGpp synthesis co-regulated in Pseudomonas aeruginosa: implications for stationary phase survival and synthesis of RNA/DNA precursors. Mol. Microbiol. 27:717-725. [DOI] [PubMed] [Google Scholar]
- 26.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227:680-685. [DOI] [PubMed] [Google Scholar]
- 27.Lamont, I. L., P. A. Beare, U. Ochsner, A. I. Vasil, and M. L. Vasil. 2002. Siderophore-mediated signalling regulates virulence factor production in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 99:7072-7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ledgham, F., C. Soscia, A. M. Chakrabarty, A. Lazdunski, and M. Foglino. 2003. Global regulation in Pseudomonas aeruginosa: the regulatory protein AlgR2 (AlgQ) acts as a modulator of quorum sensing. Res. Microbiol. 154:207-213. [DOI] [PubMed] [Google Scholar]
- 29.Leoni, L., A. Ciervo, N. Orsi, and P. Visca. 1996. Iron regulated transcription of the pvdA gene in Pseudomonas aeruginosa: effect of Fur and PvdS on promoter activity. J. Bacteriol. 178:2299-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leoni, L., N. Orsi, V. de Lorenzo, and P. Visca. 2000. Functional analysis of PvdS, an iron starvation sigma factor of Pseudomonas aeruginosa. J. Bacteriol. 182:1481-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liss, L. 1987. New M13 host: DH5 F′ competent cells. Focus 9:13. [Google Scholar]
- 32.Lonetto, M. A., K. L. Brown, K. E. Rudd, and M. J. Buttner. 1994. Analysis of the Streptomyces coelicolor sigE gene reveals the existence of a subfamily of eubacterial RNA polymerase sigma factors involved in the regulation of extracytoplasmic functions. Proc. Natl. Acad. Sci. USA 91:7573-7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maeda, H., N. Fujita, and A. Ishihama. 2000. Competition among seven Escherichia coli sigma subunits: relative binding affinities to the core RNA polymerase. Nucleic Acids Res. 28:3497-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magnusson, L. U., T. Nyström, and A. Farewell. 2003. Underproduction of sigma 70 mimics a stringent response. A proteome approach. J. Biol. Chem. 278:968-973. [DOI] [PubMed] [Google Scholar]
- 35.Mathee, K., C. J. McPherson, and D. E. Ohman. 1997. Posttranslational control of the algT (algU)-encoded sigma22 for expression of the alginate regulon in Pseudomonas aeruginosa and localization of its antagonist proteins MucA and MucB (AlgN). J. Bacteriol. 179:3711-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller, J. H. 1972. Experiments in molecular genetics, p. 252-255. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 37.Miyazaki, H., H. Kato, T. Nakazawa, and M. Tsuda. 1995. A positive regulatory gene, pvdS, for expression of pyoverdin biosynthetic genes in Pseudomonas aeruginosa PAO. Mol. Gen. Genet. 248:17-24. [DOI] [PubMed] [Google Scholar]
- 38.Mukhopadhyay, S., S. Shankar, W. Walden, and A. M. Chakrabarty. 1997. Complex formation of the elongation factor Tu from Pseudomonas aeruginosa with nucleoside diphosphate kinase modulates ribosomal GTP synthesis and peptide chain elongation. J. Biol. Chem. 272:17815-17820. [DOI] [PubMed] [Google Scholar]
- 39.Nyström, T. 2004. Growth versus maintenance: a trade-off dictated by RNA polymerase availability and sigma factor competition? Mol. Microbiol. 54:855-862. [DOI] [PubMed] [Google Scholar]
- 40.Poole, K., and G. A. McKay. 2003. Iron acquisition and its control in Pseudomonas aeruginosa: many roads lead to Rome. Front. Biosci. 8:d661-d686. [DOI] [PubMed] [Google Scholar]
- 41.Putignani, L., C. Ambrosi, P. Ascenzi, and P. Visca. 2004. Expression of l-ornithine Ndelta-oxygenase (PvdA) in fluorescent Pseudomonas species: an immunochemical and in silico study. Biochem. Biophys. Res. Commun. 313:245-257. [DOI] [PubMed] [Google Scholar]
- 42.Rainnie, D. J., and P. D. Bragg. 1973. The effect of iron deficiency on respiration and energy-coupling in Escherichia coli. J. Gen. Microbiol. 77:339-349. [DOI] [PubMed] [Google Scholar]
- 43.Ravel, J., and P. Cornelis. 2003. Genomics of pyoverdine-mediated iron uptake in pseudomonads. Trends Microbiol. 11:195-200. [DOI] [PubMed] [Google Scholar]
- 44.Redly, G. A., and K. Poole. 2003. Pyoverdine-mediated regulation of FpvA synthesis in Pseudomonas aeruginosa: involvement of a probable extracytoplasmic-function sigma factor, FpvI. J. Bacteriol. 185:1261-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roessler, P. G., and K. D. Nadler. 1982. Effects of iron deficiency on heme biosynthesis in Rhizobium japonicum. J. Bacteriol. 149:1021-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 47.Santos, P. M., I. Di Bartolo, J. M. Blatny, E. Zennaro, and S. Valla. 2001. New broad-host-range promoter probe vectors based on the plasmid RK2 replicon. FEMS Microbiol. Lett. 195:91-96. [DOI] [PubMed] [Google Scholar]
- 48.Schlictman, D., A. Kavanaugh-Black, S. Shankar, and A. M. Chakrabarty. 1994. Energy metabolism and alginate biosynthesis in Pseudomonas aeruginosa: role of the tricarboxylic acid cycle. J. Bacteriol. 176:6023-6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schlictman, D., M. Kubo, S. Shankar, and A. M. Chakrabarty. 1995. Regulation of nucleoside diphosphate kinase and secretable virulence factors in Pseudomonas aeruginosa: roles of algR2 and algH. J. Bacteriol. 177:2469-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schurr, M. J., H. Yu, J. C. Boucher, N. S. Hibler, and V. Deretic. 1995. Multiple promoters and induction by heat shock of the gene encoding the alternative sigma factor AlgU (sigma E) which controls mucoidy in cystic fibrosis isolates of Pseudomonas aeruginosa. J. Bacteriol. 177:5670-5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schweizer, H. P. 1991. Escherichia-Pseudomonas shuttle vectors derived from pUC18/19. Gene 97:109-112. [DOI] [PubMed] [Google Scholar]
- 52.Shepherd, N., P. Dennis, and H. Bremer. 2001. Cytoplasmic RNA polymerase in Escherichia coli. J. Bacteriol. 183:2527-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spaink, H. P., R. J. H. Okker, C. A. Wijffelman, E. Pees, and B. J. J. Lugtenberg. 1987. Promoters in the nodulation region of the Rhizobium leguminosarum Sym plasmid pRL1J1. Plant Mol. Biol. 9:27-39. [DOI] [PubMed] [Google Scholar]
- 54.Spiro, S., and J. R. Guest. 1991. Adaptive responses to oxygen limitation in Escherichia coli. Trends Biochem. Sci. 16:310-314. [DOI] [PubMed] [Google Scholar]
- 55.Storey, D. G., E. E. Ujack, I. Mitchell, and H. R. Rabin. 1997. Positive correlation of algD transcription to lasB and lasA transcription by populations of Pseudomonas aeruginosa in the lungs of patients with cystic fibrosis. Infect. Immun. 65:4061-4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sundin, G. W., S. Shankar, S. A. Chugani, B. A. Chopade, A. Kavanaugh-Black, and A. M. Chakrabarty. 1996. Nucleoside diphosphate kinase from Pseudomonas aeruginosa: characterization of the gene and its role in cellular growth and exopolysaccharide alginate synthesis. Mol. Microbiol. 20:965-979. [DOI] [PubMed] [Google Scholar]
- 57.Terry, J. M., S. Pina, and S. J. Mattingly. 1992. Role of energy metabolism in conversion of nonmucoid Pseudomonas aeruginosa to the mucoid phenotype. Infect. Immun. 59:471-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Venturi, V. 2003. Control of rpoS transcription in Escherichia coli and Pseudomonas: why so different? Mol. Microbiol. 49:1-9. [DOI] [PubMed] [Google Scholar]
- 59.Venturi, V., C. Ottewanger, M. Bracke, and P. J. Weisbeek. 1995. Iron regulation of siderophore biosynthesis and transport in Pseudomonas putida WCS358: involvement of a transcriptional activator and of the Fur protein. Mol. Microbiol. 15:1081-1093. [DOI] [PubMed] [Google Scholar]
- 60.Venturi, V., C. Ottevanger, J. Leong, and P. J. Weisbeek. 1993. Identification and characterization of a siderophore regulatory gene (pfrA) of Pseudomonas putida WCS358: homology to the alginate regulatory gene algQ of Pseudomonas aeruginosa. Mol. Microbiol. 10:63-73. [DOI] [PubMed] [Google Scholar]
- 61.Visca, P. 2004. Iron regulation and siderophore signalling in virulence by Pseudomonas aeruginosa, p. 69-123. In J. L. Ramos (ed.), Pseudomonas: virulence and gene regulation, vol. II. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 62.Visca, P., A. Ciervo, V. Sanfilippo, and N. Orsi. 1993. Iron-regulated salicylate synthesis by Pseudomonas spp. J. Gen. Microbiol. 139:1995-2001. [DOI] [PubMed] [Google Scholar]
- 63.Visca, P., L. Leoni, M. J. Wilson, and I. L. Lamont. 2002. Iron transport and regulation, cell signalling and genomics: lessons from Escherichia coli and Pseudomonas. Mol. Microbiol. 45:1177-1190. [DOI] [PubMed] [Google Scholar]
- 64.Visca, P., L. Serino, and N. Orsi. 1992. Isolation and characterization of Pseudomonas aeruginosa mutants blocked in the synthesis of pyoverdin. J. Bacteriol. 174:5727-5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Westblade, L. F., L. L. Ilag, A. K. Powell, A. Kolb, C. V. Robinson, and S. J. Busby. 2004. Studies of the Escherichia coli Rsd-sigma70 complex. J. Mol. Biol. 335:685-692. [DOI] [PubMed] [Google Scholar]
- 66.Wu, W., H. Badrane, S. Arora, H. V. Baker, and S. Jin. 2004. MucA-mediated coordination of type III secretion and alginate synthesis in Pseudomonas aeruginosa. J. Bacteriol. 186:7575-7585. [DOI] [PMC free article] [PubMed] [Google Scholar]