Abstract
Methanosarcina acetivorans C2A is able to convert several substrates to methane via at least four distinct methanogenic pathways. A common step in each of these pathways is the reduction of methyl-coenzyme M (CoM) to methane catalyzed by methyl-CoM reductase (MCR). Because this enzyme is used in each of the known pathways, the mcrBDCGA operon, which encodes MCR, is expected to be essential. To validate this prediction, a system for conditional gene inactivation was developed. A heterologous copy of the mcrBDCGA operon was placed under the control of the highly regulated mtaC1 promoter, which directs the expression of genes involved in methanol utilization, and recombined onto the M. acetivorans chromosome. This allowed for disruption of the endogenous mcr operon in the presence of methanol. Because the PmtaC1 promoter is transcribed only during growth on methanol, mcrBDCGA was rendered methanol dependent and the strain was unable to grow in trimethylamine media, strongly suggesting that mcrBDCGA is essential. Upon prolonged incubation, suppressed mutants which expressed mcrBDCGA constitutively could be selected. Expression analysis of PmtaC1::uidA gene fusions in several isolated suppressed mutants suggests that they carry trans-active mutations leading to deregulation of all genes under control of this promoter. Subsequently, proteome analysis of one such suppressed mutant revealed that all known proteins derived from mtaC1 promoter-dependent expression were constitutively expressed in this mutant. This genetic system can therefore be employed for the testing of essential genes and for the identification of genes under a common regulatory mechanism by making regulatory mutations phenotypically selectable.
Methanosarcina species are metabolically the most diverse of the methanogenic Archaea and can use H2 plus CO2, H2 plus methanol, CO, methanol, methylamines, methyl sulfides, and acetate as substrates for methanogenesis (references 32 and 39 and references therein). Use of these substrates encompasses substantially different methanogenic pathways. For several methanogens, it is known that enzymes necessary for the utilization of a particular growth substrate are highly synthesized during growth on that substrate, while genes encoding components involved in utilization of other substrates are transcribed only at basal levels (24, 28). However, little is known about the mechanism of this growth substrate-dependent regulation, which enables Methanosarcina species to be more metabolically flexible than other methanogens.
In Methanosarcina, utilization of methanol as a growth substrate proceeds by transfer of the methyl group to the corrinoid protein MtaC, which is catalyzed by the methanol-specific methyltransferase MtaB (34). Subsequently, the methyl group is transferred from methyl-MtaC to coenzyme M (CoM) by MtaA (15), generating methyl-CoM which is in turn reduced to methane by the methyl-CoM reductase (MCR) complex (8). In all Methanosarcina species analyzed to date, the genes for MtaC and MtaB are organized in a putative operon (34). Genomic analysis of Methanosarcina acetivorans C2A has revealed the presence of three copies of mtaCB (mtaCB1, mtaCB2, and mtaCB3) on the chromosome (10). Genetic analysis later showed that each operon is sufficient to allow growth on methanol and that mtaCB1 encodes the most active methanol methyltransferase system in M. acetivorans (29). It was further shown for Methanosarcina thermophila that the levels of MtaB1, MtaC1, MtaB2, and MtaC2 are regulated in response to the growth substrate, as larger amounts of these proteins are present when the organism is growing on methanol than when it is growing on acetate (7, 19).
The reduction of methyl-CoM to methane catalyzed by MCR is common to all known methanogenic pathways. MCR is a highly abundant protein complex, constituting up to 10% of the total protein in Methanobacterium thermautotrophicum (33). While some other methanogenic Archaea encode two homologs of MCR (mcrBDCGA) (12, 31), the genome of M. acetivorans C2A contains only one set of these genes (10). Because all methanogens investigated to date rely on methanogenesis for energy generation, the genes encoding MCR are expected to be essential for M. acetivorans C2A and therefore not subject to facile genetic manipulation.
A gene can be directly assessed by mutational analysis only if conditions exist under which the gene product is dispensable. Thus, simple loss-of-function mutations, such as gene deletions, cannot be made in genes that are essential under all growth conditions. If the gene in question appears to be essential under all physiological conditions, statistical evidence can be used to demonstrate its essentiality: i.e., the inability to make a mutation relative to the ability to make a similar mutation in a nonessential control gene can indicate that a locus is essential (38). Another possibility is to provide a (heterologous) complementing copy of the gene in question in trans. The ability to generate the mutation in the complemented strain when the same mutation cannot be obtained in the wild type provides compelling evidence that the gene in question is essential under the conditions tested (13). A nonstatistical approach utilizes conditional gene inactivation, such as temperature-sensitive mutations; however, isolation of such mutants is very time-consuming and laborious. An alternative approach is to put a complementing copy of the gene in question under the control of a tightly regulated promoter, followed by deletion of the wild-type allele under conditions that allow expression of the complementing copy. If the recombinant organism is unable to grow under nonexpressing conditions, then the gene in question is essential.
To validate the prediction that MCR is indispensable for M. acetivorans, and to establish a system to test potentially essential genes, we report here the development of a conditional gene inactivation system. By complementing an mcrBDCGA lesion with a heterologous mcrBDCGA copy under the control of the mtaC1 promoter, a strain with a methanol-dependent phenotype was created. In addition to its utility in the examination of essential genes, we also demonstrate the utility of the method for the isolation of regulatory mutants.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Escherichia coli cells were grown under standard conditions (41). Methanosarcina acetivorans strains, described in Table 1, were grown in high-salt (HS) medium as described previously (36). Solid medium contained 1.5% (wt/vol) Bacto agar (3). For the selection of Methanosarcina strains carrying the puromycin transacetylase gene (pac), puromycin (CalBiochem, San Diego, CA) was added from sterile, anaerobic stocks at a final concentration of 2 μg/ml. The purine analog 8-aza-2,6-diaminopurine (Sigma, St. Louis, MO) was added from sterile, anaerobic stocks at a final concentration of 20 μg/ml for selection against the hypoxanthine phosphoribosyl transferase gene (hpt). Growth of M. acetivorans was monitored at an optical density of 600 nm (OD600) using a Milton Roy Company Spectronic 21.
TABLE 1.
Plasmids and strains used in this study
| Plasmid or strain | Construction, description, and/or relevant genotypea | Reference or source |
|---|---|---|
| Plasmids | ||
| pBlueSK | General cloning vector | Stratagene |
| pBlueKS | General cloning vector | Stratagene |
| pPB41 | 1,400-bp mcrB fragment disrupted with the pac cassette in pBlueKS | This study |
| pPB61 | Subclone of M. barkeri genomic library into pMP42; contains genomic region of mcrBCDGA operon | This study |
| pMP42 | Vector for insertion into hpt locus | 30 |
| pAMG46 | Derivative of pMP42 with different cloning sites | This study |
| pMP38 | 1-kb PmtaC1 fragment in pBlueSK | This study |
| pMP45 | Source of uidA | 30 |
| pWM368 | Source of M. barkeri PmcrB | 30 |
| pMP58 | PmtaC1-uidA fusion in pMP42 | This study |
| pMR50 | PmcrB-uidA fusion in pAMG46 | This study |
| pMR29 | Derivative of pBlue; contains PmtaC1-uidA fusion and the pac cassette flanked by 1 kb of upstream and downstream regions of mcrBCDGA | This study |
| pMR05 | Derivative of pMP42; contains mcrBCDGA operon under control of PmtaC1 | This study |
| pJK41 | Cloning vector containing the pac cassette | 21 |
| pJK3 | Cloning vector analogous to pJK41 | 22 |
| Strains | ||
| DH5α | E. coli cloning host | 14 |
| C2A | M. acetivorans wild type | 37 |
| WWM12 | C2A Δhpt::PmtaC1-uidA | This study |
| M50 | C2A Δhpt::PmcrB-uidA | This study |
| J1 | C2A Δhpt::mcrBCDGA (pPB61) | This study |
| MR05 | C2A Δhpt::PmtaC1-mcrBCDGA (pMR05) | This study |
| J29 | J1 ΔmcrBCDGA::PmtaC1-uidA pac | This study |
| J41 | J1 mcrA::pac | This study |
| M29 | MR05 ΔmcrBCDGA::PmtaC1-uidA pac | This study |
| M29mut | Spontaneous mutant of M29 with constitutive β-glucuronidase expression | This study |
DNA sequences and maps of all plasmids are available upon request.
For some experiments, solid media without agar were desired, due to the ability of M. acetivorans to grow on endogenous substrates found in agar. To obtain single colonies without using agar, M. acetivorans was cultivated on nylon filter membranes. Four layers of sterile cellulose filter pads (47 mm; Millipore, Bedford, MA) were placed in tight-lid petri dishes (47 mm; Fisher, Pittsburgh, PA) soaked in 6.5 ml HS medium containing methanol or trimethylamine. M. acetivorans strains M29 and J29 were grown on methanol-containing medium and serially diluted in substrate-free medium. Ten milliliters of the dilutions were filtered through nylon membrane filters (pore size, 0.45 μm [Millipore]; 47 mm [Fisher]) and placed on top of the filter pads. Incubation was with the filter side up.
Molecular methods, plasmid constructions, and transformation.
Standard molecular methods were used for manipulation of plasmid DNA from E. coli (2). The plasmids used are presented in Table 1. All plasmids in this study are nonreplicating in Methanosarcina. Genomic DNA from M. acetivorans was isolated using a modified cetyltrimethylammonium bromide method (23). Insertion and deletion mutants of M. acetivorans were confirmed by Southern hybridization (30). DNA sequences of all cloning intermediates employing PCR were confirmed by sequencing at the W. M. Keck Center for Comparative and Functional Genomics, University of Illinois, using the BigDye Terminator Cycle Sequencing protocol (Applied Biosystems, Foster City, CA). E. coli was transformed by electroporation (9). Liposome-mediated transformation was used for Methanosarcina species as previously described (22) and modified (3).
Cloning of Methanosarcina barkeri mcrBDCGA.
The mcrBDCGA operon of M. barkeri was identified within a cosmid library of the M. barkeri Fusaro genome (43) by hybridization with an mcrB probe. The complete operon was subsequently cloned into a vector for insertion of DNA into the hpt locus of M. acetivorans C2A (pMP42) (30). The resulting plasmid, pPB61, contains the complete mcrBDCGA operon, including substantial upstream and downstream regions.
Two-dimensional polyacrylamide gel electrophoresis (2D PAGE).
Protein samples (50 μg per gel) were prepared according to recommendations of Kendrick Labs, Inc. (Madison, WI). Two-dimensional electrophoresis was performed according to the method of O'Farrell (26) by Kendrick Labs. Isoelectric focusing was carried out in a glass tube using 2% pH 4 to 6 ampholines (Amersham Biosciences, Piscataway, NJ) and 2% pH 4 to 8 ampholines (Gallard-Schlesinger Industries, Inc., Garden City, NY) for 20 h at 1,000 V. After equilibration for 10 min in buffer containing 10% glycerol, 50 mM dithiothreitol, 2.3% sodium dodecyl sulfate, and 63 mM Tris-HCl (pH 6.8), the tube gel was sealed to the top of a 10% polyacrylamide slab gel. Electrophoresis was carried out for 5 h at 25 mA. The proteins were stained with silver (25) and dried between sheets of cellophane.
Protein identification.
Silver-stained spots were excised from the gel, destained (11), and dehydrated. The dried gel pieces were soaked with 0.012 μg modified trypsin and 0.1 μg endoproteinase Lys-C in a minimum amount of 25 mM Tris (pH 8.5) and incubated overnight at 32°C. Peptide fragments were eluted with 50% acetonitrile-2% trifluoroacetic acid and dried. For matrix-assisted laser desorption ionization mass spectrometry analysis, a PerSeptive Voyager DE-RP spectrometer (Applied Biosystems, Foster City, CA) was used in the linear mode. Obtained peptide masses were compared to theoretical ones derived from the translated M. acetivorans C2A genome database using the MS-Digest program (6).
β-Glucuronidase activity.
For determination of β-glucuronidase activity, M. acetivorans strains carrying the uidA reporter gene were harvested by centrifugation at an OD600 of ∼0.5 and osmotically lysed by addition of 50 mM Tris-HCl buffer, pH 8.0, containing 1 mM dithiothreitol, 0.1 μg ml−1 DNase I, 0.1 μg ml−1 RNase A. The lysate was cleared by recentrifugation, and the specific activity of β-glucuronidase was determined as described previously (30). Protein concentration was determined by the method of Bradford (4) using bovine serum albumin as a standard.
RESULTS
Expression of uidA reporter gene fusions of PmcrB and PmtaC1.
In methylotrophic methanogens, neither cis- nor trans-active factors responsible for the regulation of the methanol-specific methyltransferase system in response to the growth substrate present have been identified to date. To define the promoter of the mtaCB1 operon of M. acetivorans to a first approximation, a reporter gene fusion was constructed in which approximately 1 kb of DNA preceding the mtaC1 coding region was fused to the reporter gene uidA such that their respective translation start codons were superimposed, thus allowing us to monitor the combined transcriptional and translational regulatory sequences of this gene. (However, it should be noted that additional control features, such as transcript stability, protein turnover, etc., are not monitored by such fusions.) As a control, a fusion of uidA and a 196-bp fragment from M. barkeri Fusaro known to comprise the mcrB promoter (PmcrB) (42) was also employed. The reporter gene fusion constructs were inserted into the hpt locus on the chromosome of M. acetivorans C2A by markerless exchange (30). The resulting reporter strains (WWM12 and M50) were tested for β-glucuronidase activity during exponential growth on various substrates (Table 2). For M50, which contains the uidA gene preceded by PmcrB from M. barkeri Fusaro, β-glucuronidase activity was high (approximately 1 U) in cells grown on methanol or mono-, di-, or trimethylamine, which indicates constitutive gene expression from the mcrB promoter. Acetate as the energy source resulted in a threefold increase of expression from PmcrB. On the other hand, β-glucuronidase activity was strictly methanol dependent in WWM12, which carries the PmtaC1-uidA fusion (Table 2). uidA expression was approximately 100- to 300-fold higher in cells grown on methanol than in cells grown on any of the other substrates tested. The results suggest that mtaCB1 expression is regulated on the transcriptional level and that the mtaC1 promoter (PmtaC1) is contained in the DNA fragment fused to uidA in WWM12. Specific β-glucuronidase activities in methanol-grown WWM12 and M50 differed by only about threefold, indicating that the transcription rates of the two promoters under this growth condition are roughly comparable.
TABLE 2.
β-Glucuronidase activity in M. acetivorans strains carrying PmtaC1 and PmcrB gene fusions grown under different conditions
| Strain | β-Glucuronidase activity in given growth substratea
|
||||
|---|---|---|---|---|---|
| Methanol | TMA | DMA | MMA | Acetate | |
| WWM12 (PmtaC1::uidA) | 315 ± 47 | 3.3 ± 0.3 | 1.14 ± 0.2 | 1.1 ± 0.1 | 3.57 ± 0.68 |
| M50 (PmcrB::uidA) | 904 ± 44 | 1,183 ± 47 | 1,093 ± 120 | 1,061 ± 15 | 3,286 ± 82 |
Values are given in mU (nmol × min−1 × mg−1) and are averages of at least nine independent experiments. Strains were pregrown for at least 30 generations on the respective substrate. DMA, dimethylamine; MMA, monomethylamine.
mcrBDCGA is essential in M. acetivorans.
The genes encoding MCR are predicted to be essential in M. acetivorans because MCR activity is required in each of the different routes of methanogenesis. To gather experimental support for this prediction, attempts were made to disrupt mcrA in wild-type M. acetivorans with the puromycin resistance cassette (linearized plasmid pPB41) under various growth conditions. In no case was a ΔmcrA::pac mutant obtained, which suggests that mcrA is essential in M. acetivorans. Next, a heterologous copy of the mcrBDCGA operon was inserted into the permissive hpt locus of M. acetivorans C2A by markerless exchange (Fig. 1). M. barkeri Fusaro was the source for the heterologous mcrBDCGA to avoid undesired recombination events (3). The resulting merodiploid strain, J1, showed a growth phenotype identical to the wild type when cultivated on methanol, trimethylamine, and acetate (data not shown), indicating that the presence of the heterologous mcr operon has no deleterious effect in M. acetivorans. With this second mcr operon on the chromosome, the endogenous mcrA could be disrupted. By double recombination events between the chromosome and the linearized plasmid pPB41 or pMR29 (see below), the endogenous gene was replaced with the pac cassette (Fig. 1 and data not shown). The resulting strains, J41 and J29, showed no difference in growth on methanol relative to their parental strain, J1 (Fig. 2 and data not shown), which demonstrates that mcrBDCGA from M. barkeri Fusaro is functionally expressed in M. acetivorans C2A. The fact that an mcrBDCGA-disrupted strain of M. acetivorans could be obtained only in the presence of a complementing copy provides compelling evidence that MCR is essential under the growth conditions employed.
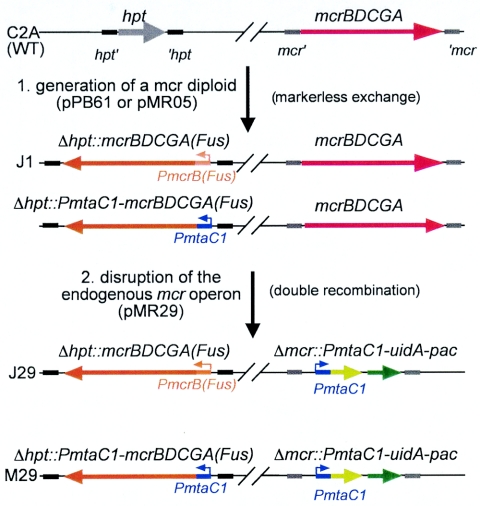
FIG. 1.
A scheme for creating a conditional mcrBDCGA deletion in M. acetivorans C2A. In the first step, the mcr operon from M. barkeri is inserted into the permissive hpt site by markerless exchange with the plasmid pPB61 or pMR05, generating the merodiploid strain J1 or MR05, respectively. In the second step, the endogenous mcr operon is disrupted with the PmtaC1-uidA-pac fusion cassette by a double recombination event with linearized pMR29, generating the strains J29 and M29. hpt, gene encoding hypoxanthine phosphoribosyl transferase; hpt′ and ′hpt, upstream and downstream regions of hpt on the M. acetivorans chromosome; mcr′ and ′mcr, upstream and downstream regions of the mcrBDCGA operon on the M. acetivorans chromosome; PmcrB(Fus), mcrB promoter from M. barkeri Fusaro; PmtaC1, mtaCB1 promoter region; and uidA, gene encoding β-glucuronidase. Note that uidA and pac do not comprise an operon in pMR29; see the text for details. The red arrows denote the M. acetivorans C2A mcrBDCGA operon, the orange arrows the M. barkeri Fusaro mcrBDCGA operon, the light green arrows the uidA gene, and the dark green arrows the pac gene.
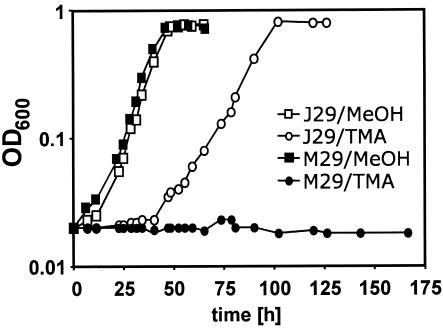
FIG. 2.
Growth phenotype of the M. acetivorans strains J29 and M29. Cells were pregrown in HS medium containing methanol before dilution (100-fold) into fresh medium containing the substrate indicated. □, J29 plus 125 mM methanol; ○, J29 plus 50 mM trimethylamine; ▪, M29 plus 125 mM methanol; •, M29 plus 50 mM trimethylamine.
Generation of a conditional M. acetivorans mcrBDCGA mutant.
To make mcrBDCGA expression, and thus growth of M. acetivorans, methanol dependent, the operon was placed under the control of the mtaC1 promoter. To this end, a “promoterless” mcrBDCGA operon was fused to the mtaC1 promoter region (identical to the PmtaC1-uidA fusion of WWM12), superimposing the translation start codons of mcrB and mtaC1. The resulting plasmid, pMR05, was inserted into the M. acetivorans C2A chromosome by markerless exchange, resulting in strain MR05, analogous to J1 (Fig. 1) but expressing the second copy of mcrBDCGA from the mtaC1 promoter rather than the native promoter. The endogenous mcrBDCGA operon of MR05 was then disrupted by the linearized pMR29, resulting in strain M29, a strain analogous to J29 (Fig. 1). In addition to the pac cassette, pMR29 carries a reporter gene fusion of uidA and PmtaC1 inserted into the mcr locus, which provides a means of monitoring transcription of PmtaC1-dependent gene expression in the two strains (see below).
It was predicted for strain M29 that the expression of the heterologous mcrBDCGA operon would be rendered methanol dependent by placing it under the control of PmtaC1. Turning off mtaC1-dependent gene expression should result in the elimination of mcrBDCGA expression and thus growth, because MCR is required for growth (see above). To test this prediction, growth experiments were conducted with J29 and M29 (Fig. 2). Both strains were pregrown on methanol and subsequently inoculated (10−2 dilution) into medium containing methanol into medium containing trimethylamine. The strains grew at comparable rates on methanol (Fig. 2). Upon shifting to trimethylamine, J29 lagged in growth for approximately 48 h before it grew with a rate somewhat slower than that on methanol (growth of the wild type is also somewhat slower on trimethylamine [TMA] [29]). M29, on the other hand, showed no growth for more than 240 h. This methanol-dependent growth phenotype of strain M29 indicates that expression of mcrBDCGA is turned off on trimethylamine due to regulation of the preceding mtaC1 promoter and provides further evidence that MCR is essential under these conditions.
The conditional mcr deletion phenotype is suppressed by mutation.
After prolonged incubation (for more than 250 h), M29 started to grow on trimethylamine and showed henceforth a growth phenotype identical to J29 (data not shown). The change in phenotype of M29 indicated that it acquired a mutation resulting in expression of the recombinant PmtaC1-mcrBDCGA fusion on trimethylamine. In order to determine the frequency of suppression of the conditional mcr deletion phenotype, single clones had to be isolated. Previous experiments showed that M. acetivorans is able to grow on agar plates even without an added growth substrate and that some energy source in the agar is metabolized via the methanol utilization pathway (M. A. Pritchett and W. W. Metcalf, unpublished results). Therefore, agar was unsuitable for the generation of methanol-free conditions. To be able to isolate methanol-independent mutants of M29 capable of growing on TMA as the sole energy source, a filter plating technique was developed. M. acetivorans strains M29 and J29 were grown on methanol-containing medium, serially diluted in substrate-free medium, “plated” on nylon membrane filters on top of filter pads soaked with medium containing the appropriate substrate, and incubated at 37°C. This method results in almost identical plating efficiencies as plating on agar plates. After 2 weeks of incubation, similar numbers of visible colonies were obtained for J29 on methanol and trimethylamine. On the other hand, M29 formed many fewer colonies on TMA-containing medium than on methanol-containing medium and only after extended incubation times (7 to 8 weeks). By comparing the ratios of colonies formed on methanol and TMA with respect to the original titer of the cultures of J29 and M29, respectively, the apparent mutation frequency of M29 on TMA is 8 × 10−5 ± 2 × 10−5.
Characterization of TMA-induced M29 mutants.
Both cis- and trans-acting mutations could be responsible for releasing the methanol-dependent mcrBDCGA expression in M29. To distinguish between the two possibilities, β-glucuronidase activity was determined in 10 independent mutant M29 clones (M29mut) isolated on TMA and subsequently grown on TMA or methanol (Table 3). A cis-active mutation would affect only the PmtaC1-mcrBDCGA fusion, whereas a trans-active mutation would also affect the PmtaC1-uidA fusion, which was inserted in the mcr locus (Fig. 1). β-Glucuronidase activity was comparably high in all M29mut clones both on methanol and on TMA, whereas in J29, uidA expression was, as expected, strictly methanol dependent (Table 3). The finding that not only mcrBDCGA but also uidA expression was rendered methanol independent in the M29mut strains strongly suggests that a trans-acting mutation, presumably affecting a regulatory protein of mtaCB1 transcription, caused the release of methanol regulation on PmtaC1.
TABLE 3.
PmtaC1-dependent reporter gene expression in spontaneous M29 mutants
| Strain | Specific β-glucuronidase activitya in:
|
|
|---|---|---|
| Methanol | TMA | |
| J29 | 383 ± 47 | 3.0 ± 0.3 |
| M29 | 570 ± 78 | n.d. |
| M29mut 1 | 315 ± 40 | 388 ± 34 |
| M29mut 2 | 555 ± 52 | 644 ± 59 |
| M29mut 3 | 580 ± 61 | 475 ± 23 |
| M29mut 4 | 561 ± 60 | 637 ± 60 |
| M29mut 5 | 536 ± 49 | 455 ± 41 |
| M29mut 6 | 375 ± 40 | 371 ± 47 |
| M29mut 7 | 485 ± 51 | 520 ± 56 |
| M29mut 8 | 492 ± 50 | 459 ± 47 |
| M29mut 9 | 504 ± 38 | 621 ± 60 |
| M29mut 10 | 391 ± 46 | 482 ± 51 |
Specific β-glucuronidase activities are given in mU (nmol × min−1 × mg−1) and are averages of at least three independent experiments. Strains were pregrown for approximately four generations on the respective substrate. n.d., not determined (the strain cannot grow on TMA without a suppressor mutation).
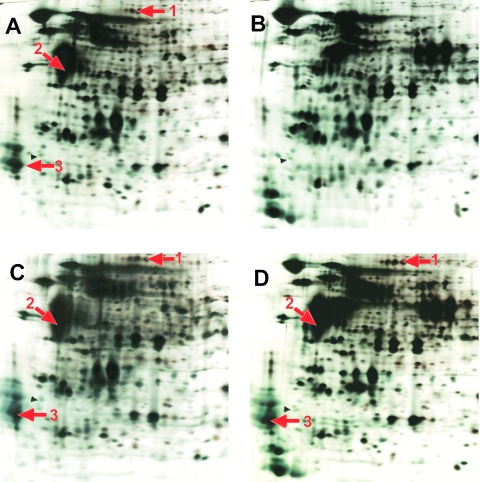
To corroborate this conclusion, crude cell extracts of J29, M29, and one M29mut clone (clone 3 [Table 3]) were subjected to 2D PAGE analysis. J29 was grown on methanol or TMA, M29 was grown on methanol, and M29mut was grown on TMA. The proteome patterns of J29 are significantly different, depending on the growth substrate (Fig. 3A and B). The proteome pattern of M29 grown on methanol corresponds to that of J29 grown on the same substrate (Fig. 3C). On the other hand, M29mut synthesized substantial amounts of three proteins when grown on TMA which are apparently methanol specific in J29 and M29 (Fig. 3, arrows). The proteins were identified as β-glucuronidase (spot 1), MtaB1 (spot 2), and MtaC1 (spot 3) (data not shown). This demonstrates that in M29mut, the products of genes under control of PmtaC1 are now synthesized in a methanol-independent fashion. Note that the products of the mcrBDCGA operon, although under the control of PmtaC1 in M29 and thus affected by the mutation leading to M29mut, were not identified because constitutive synthesis of MCR in J29 and deregulated synthesis of MCR in M29mut were not distinguishable.
FIG. 3.
Deregulation of methanol-dependent protein synthesis in M. acetivorans. Cells were grown in HS medium containing methanol (A and C) or trimethylamine (B and D). Crude cell extracts were separated by 2D PAGE, and the proteins were stained with silver. (A and B) Sections of gels with separated proteins from M. acetivorans J29. (C) Section of a gel with separated proteins from M. acetivorans M29. (D) Section of a gel with separated proteins from M. acetivorans M29mut. Arrows indicate the proteins that were subjected to peptide mass matching (fingerprinting).
DISCUSSION
In this study, we designed a system to test the essentiality of genes in the genetically tractable methanogenic archaeon M. acetivorans. Methyl-CoM reductase was chosen as a model because it is thought to be required in all methanogenic routes and is encoded in a single copy on the chromosome of this organism. A fusion of its promoter and uidA was highly expressed on all methanogenic substrates tested, providing further evidence that this operon is needed under all growth conditions examined. Interestingly, expression of the reporter gene fusion was threefold higher in cells grown on acetate than on methanol or methylamines. A possible explanation could be the maximization of substrate turnover during the low-energy-yielding aceticlastic methanogenesis pathway. This observation is in concurrence with the situation in M. barkeri Fusaro (40). In M. thermophila, however, different growth substrates do not result in a detectable differential synthesis of MCR subunits (7).
Because MCR could not be subjected directly to mutational analysis, we gathered statistical evidence to demonstrate its essentiality. Providing a heterologous complementing copy of the genes in trans allowed for the disruption of mcr in the partial diploid strain, whereas the respective mutation could not be obtained in the wild type. This finding strongly suggests that mcr is essential. An analogous approach was used to demonstrate that N5,N10-methenyl tetrahydromethanopterin cyclohydrolase is essential in M. acetivorans (13).
To corroborate our conclusion and to demonstrate the essentiality of the mcr genes in a nonstatistical manner, the complementing copy was placed under the control of the highly regulated mtaC1 promoter. This conditional gene inactivation approach has proven fruitful for exploring essential genes both in bacteria (5) and in eukaryotes (27). The finding that strain M29 grows only under conditions in which the mcr operon is expressed provides very strong support for the conclusion that MCR is essential in M. acetivorans. However, extended incubation under nonpermissive (nonexpressing) conditions of the strain carrying PmtaC1-mcrBDCGA resulted in the selection of mutants with deregulated PmtaC1-dependent gene expression, as evidenced by reporter gene fusion analysis and proteome analysis.
The surprisingly high frequency of mutation that led to the deregulation of PmtaC1 is probably due to properties both of the promoter and of mcrBDCGA itself. The expression of genes under control of PmtaC1 is 100-fold lower in the presence of TMA than in the presence of methanol but still at a detectable level (Table 2). Furthermore, mcrBDCGA transcripts were shown to have a half-life of about 15 min in Methanococcus vannielii (18). Thus, the basal expression and the mRNA stability of mcrBDCGA, taken together, probably allowed the strain to survive or even to grow very slowly in the absence of methanol until a mutation releasing the regulation of PmtaC1 occurred. Moreover, if the MCR protein is particularly stable, this effect could be magnified even further. Thus, the apparent mutation frequency reported here might be significantly higher than the true frequency. The tendency of “leaky” mutants to give rise to suppressed mutants by adaptive mutation at a high frequency, which reflects the selective pressure on the genetic system, has been studied in bacteria (1, 35) and eukaryotes (17).
The fact that only strains with trans-active mutations were isolated indicates that those occurred much more frequently than cis-active mutations. A possible explanation is that multiple mutations may be required in PmtaC1 to result in a deregulated but still active promoter. On the other hand, a single point mutation can render a regulatory protein unable to bind its target site (16). It should be noted that proteins other than a transcriptional regulator, regulatory RNA, or metabolites, which might interact with DNA sequences and/or components of the transcription machinery, could be affected by the mutation observed.
Despite the fact that constitutive mutations arise at high apparent frequencies, the system described here will be very useful for testing other potentially essential genes. However, a control strain that differs from the test strain only in the promoter preceding the complementing gene copy is required to reveal the expected lag phase upon switching from methanol to TMA, which should be substantially shorter for the control strain than for the strain with the regulated complementing copy. Further, the presence of a reporter gene fusion to the PmtaC1 promoter allows facile distinction between suppressed mutants (i.e., mutants with deregulated PmtaC1-dependent gene expression) and mutants which may be growing slowly under nonpermissive conditions due to leakiness of the regulated promoter.
Fusing the regulated mtaC1 promoter to an essential gene generates a high selective pressure towards mutating components of the respective regulatory system under nonpermissive conditions. In our case, trans-active components were affected, leading to the deregulation of the essential mcrBDCGA operon and all other genes under the control of PmtaC1. Consequently, mtaC1 and mtaB1, which comprise a transcriptional unit (34), and uidA were expressed on TMA in the mutated strain. Notably, the expression of the other mtaCB isogenes (mtaCB2 and mtaCB3) (7, 10) was apparently not affected by this deregulation, indicating distinct regulatory mechanisms for each of the methanol-dependent methyltransferase isoforms. The genetic system presented here can therefore be applied to a purpose other than testing essential genes: the dissection of regulons through isolation of regulatory mutants. By making a regulatory mutation directly selectable via a growth phenotype, all products of genes under the control of a particular regulator can be identified within the proteome of the resulting mutants, provided that their level of synthesis is sufficient for 2D PAGE analysis. Including the fusion of uidA and the promoter in question provides a means to screen for trans-active mutations. The method is analogous to the commonly used method for isolation of deregulated lacZ fusions by selection for growth on lactose medium in E. coli and other bacteria. In yeast, a similar approach led to the identification of proteins involved in protein modification (20). However, indirect effects of the suppressing mutations, which may alter regulation of genes, cannot be distinguished with this approach.
In summary, we show that fusing a regulated promoter to a gene in M. acetivorans can be used to demonstrate the essentiality of that gene as well as to identify other genes under the same transcriptional regulation. The method presented here may therefore help to elucidate the complex processes that enable adaptive responses to environmental changes in Methanosarcina species, which is an important prerequisite for the unsurpassed metabolic versatility among the methanogenic archaea.
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (RO 2445/1-1) to M.R. and grants from the Department of Energy (DE-FG-02ER15296) and the National Science Foundation (MCB987459 and MCB12466) to W.W.M.
We thank Adam M. Guss for construction of pAMG46.
REFERENCES
- 1.Andersson, D. I., E. S. Slechta, and J. R. Roth. 1998. Evidence that gene amplification underlies adaptive mutability of the bacterial lac operon. Science 282:1133-1135. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidmann, J. A. Smith, and K. Struhl. 1997. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 3.Boccazzi, P., J. K. Zhang, and W. W. Metcalf. 2000. Generation of dominant selectable markers for resistance to pseudomonic acid by cloning and mutagenesis of the ileS gene from the archaeon Methanosarcina barkeri Fusaro. J. Bacteriol. 182:2611-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Chow, W. Y., and D. E. Berg. 1988. Tn5tac1, a derivative of transposon Tn5 that generates conditional mutations. Proc. Natl. Acad. Sci. USA 85:6468-6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clauser, K. R., P. Baker, and A. L. Burlingame. 1999. Role of accurate mass measurement (+/− 10 ppm) in protein identification strategies employing MS or MS/MS and database searching. Anal. Chem. 71:2871-2882. [DOI] [PubMed] [Google Scholar]
- 7.Ding, Y. H., S. P. Zhang, J. F. Tomb, and J. G. Ferry. 2002. Genomic and proteomic analyses reveal multiple homologs of genes encoding enzymes of the methanol:coenzyme M methyltransferase system that are differentially expressed in methanol- and acetate-grown Methanosarcina thermophila. FEMS Microbiol. Lett. 215:127-132. [DOI] [PubMed] [Google Scholar]
- 8.Ellermann, J., R. Hedderich, R. Bocher, and R. K. Thauer. 1988. The final step in methane formation. Investigations with highly purified methyl-CoM reductase (component C) from Methanobacterium thermoautotrophicum (strain Marburg). Eur. J. Biochem. 172:669-677. [DOI] [PubMed] [Google Scholar]
- 9.Fiedler, S., and R. Wirth. 1988. Transformation of bacteria with plasmid DNA by electroporation. Anal. Biochem. 170:38-44. [DOI] [PubMed] [Google Scholar]
- 10.Galagan, J. E., C. Nusbaum, A. Roy, M. G. Endrizzi, P. Macdonald, W. FitzHugh, S. Calvo, R. Engels, S. Smirnov, D. Atnoor, A. Brown, N. Allen, J. Naylor, N. Stange-Thomann, K. DeArellano, R. Johnson, L. Linton, P. McEwan, K. McKernan, J. Talamas, A. Tirrell, W. Ye, A. Zimmer, R. D. Barber, I. Cann, D. E. Graham, D. A. Grahame, A. M. Guss, R. Hedderich, C. Ingram-Smith, H. C. Kuettner, J. A. Krzycki, J. A. Leigh, W. Li, J. Liu, B. Mukhopadhyay, J. N. Reeve, K. Smith, T. A. Springer, L. A. Umayam, O. White, R. H. White, E. Conway de Macario, J. G. Ferry, K. F. Jarrell, H. Jing, A. J. Macario, I. Paulsen, M. Pritchett, K. R. Sowers, R. V. Swanson, S. H. Zinder, E. Lander, W. W. Metcalf, and B. Birren. 2002. The genome of M. acetivorans reveals extensive metabolic and physiological diversity. Genome Res. 12:532-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gharahdaghi, F., C. R. Weinberg, D. A. Meagher, B. S. Imai, and S. M. Mische. 1999. Mass spectrometric identification of proteins from silver-stained polyacrylamide gel: a method for the removal of silver ions to enhance sensitivity. Electrophoresis 20:601-605. [DOI] [PubMed] [Google Scholar]
- 12.Graham, D. E., N. Kyrpides, I. J. Anderson, R. Overbeek, and W. B. Whitman. 2001. Genome of Methanocaldococcus (Methanococcus) jannaschii. Methods Enzymol. 330:40-123. [DOI] [PubMed] [Google Scholar]
- 13.Guss, A. M., B. Mukhopadhyay, J. K. Zhang, and W. W. Metcalf. 2005. Genetic analysis of mch mutants in two Methanosarcina species demonstrates multiple roles for the methanopterin-dependent C-1 oxidation/reduction pathway and differences in H2 metabolism between closely related species. Mol. Microbiol. 55:1671-1680. [DOI] [PubMed] [Google Scholar]
- 14.Hanahan, D. 1985. Techniques for transformation of E. coli, p. 109-135. In D. M. Glover (ed.), DNA cloning. IRL Press, Oxford, United Kingdom.
- 15.Harms, U., and R. K. Thauer. 1996. Methylcobalamin: coenzyme M methyltransferase isoenzymes MtaA and MtbA from Methanosarcina barkeri. Cloning, sequencing and differential transcription of the encoding genes, and functional overexpression of the mtaA gene in Escherichia coli. Eur. J. Biochem. 235:653-659. [DOI] [PubMed] [Google Scholar]
- 16.Hecht, B., G. Müller, and W. Hillen. 1993. Noninducible Tet repressor mutations map from the operator binding motif to the C terminus. J. Bacteriol. 175:1206-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heidenreich, E., and U. Wintersberger. 1998. Replication-dependent and selection-induced mutations in respiration-competent and respiration-deficient strains of Saccharomyces cerevisiae. Mol. Gen. Genet. 260:395-400. [DOI] [PubMed] [Google Scholar]
- 18.Hennigan, A. N., and J. N. Reeve. 1994. mRNAs in the methanogenic archaeon Methanococcus vannielii: numbers, half-lives and processing. Mol. Microbiol. 11:655-670. [DOI] [PubMed] [Google Scholar]
- 19.Jablonski, P. E., A. A. DiMarco, T. A. Bobik, M. C. Cabell, and J. G. Ferry. 1990. Protein content and enzyme activities in methanol- and acetate-grown Methanosarcina thermophila. J. Bacteriol. 172:1271-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson, D. R., S. J. Cok, H. Feldmann, and J. I. Gordon. 1994. Suppressors of nmtl-181, a conditional lethal allele of the Saccharomyces cerevisiae myristoyl-CoA:protein N-myristoyltransferase gene, reveal proteins involved in regulating protein N-myristoylation. Proc. Natl. Acad. Sci. USA 91:10158-10162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Metcalf, W. W. 1999. Genetic analysis in the domain archaea, p. 277-326. In M. Smith and L. Sockett (ed.), Methods in microbiology: genetic methods for diverse prokaryotes, vol. 29. Academic Press, London, United Kingdom. [Google Scholar]
- 22.Metcalf, W. W., J. K. Zhang, E. Apolinario, K. R. Sowers, and R. S. Wolfe. 1997. A genetic system for Archaea of the genus Methanosarcina: liposome-mediated transformation and construction of shuttle vectors. Proc. Natl. Acad. Sci. USA 94:2626-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Metcalf, W. W., J. K. Zhang, X. Shi, and R. S. Wolfe. 1996. Molecular, genetic, and biochemical characterization of the serC gene of Methanosarcina barkeri Fusaro. J. Bacteriol. 178:5797-5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nölling, J., and J. N. Reeve. 1997. Growth- and substrate-dependent transcription of the formate dehydrogenase (fdhCAB) operon in Methanobacterium thermoformicicum Z-245. J. Bacteriol. 179:899-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Connell, K. L., and J. T. Stults. 1997. Identification of mouse liver proteins on two-dimensional electrophoresis gels by matrix-assisted laser desorption/ionization mass spectrometry of in situ enzymatic digests. Electrophoresis 18:349-359. [DOI] [PubMed] [Google Scholar]
- 26.O'Farrell, P. H. 1975. High resolution two-dimensional electrophoresis of proteins. J. Biol. Chem. 250:4007-4021. [PMC free article] [PubMed] [Google Scholar]
- 27.Patel, D., and J. S. Butler. 1992. Conditional defect in mRNA 3′ end processing caused by a mutation in the gene for poly(A) polymerase. Mol. Cell. Biol. 12:3297-3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paul, L., and J. A. Krzycki. 1996. Sequence and transcript analysis of a novel Methanosarcina barkeri methyltransferase II homolog and its associated corrinoid protein homologous to methionine synthase. J. Bacteriol. 178:6599-6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pritchett, M. A., and W. W. Metcalf. 2005. Genetic, physiological and biochemical characterization of multiple methanol methyltransferase isozymes in Methanosarcina acetivorans C2A. Mol. Microbiol. 56:1183-1194. [DOI] [PubMed] [Google Scholar]
- 30.Pritchett, M. A., J. K. Zhang, and W. W. Metcalf. 2004. Development of a markerless genetic exchange method for Methanosarcina acetivorans C2A and its use in construction of new genetic tools for methanogenic archaea. Appl. Environ. Microbiol. 70:1425-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rospert, S., D. Linder, J. Ellermann, and R. K. Thauer. 1990. Two genetically distinct methyl coenzyme M reductases in Methanobacterium thermoautotrophicum strain Marburg and DELTA-H. Eur. J. Biochem. 194:871-878. [DOI] [PubMed] [Google Scholar]
- 32.Rother, M., and W. W. Metcalf. 2004. Anaerobic growth of Methanosarcina acetivorans C2A on carbon monoxide: an unusual way of life for a methanogenic archaeon. Proc. Natl. Acad. Sci. USA 101:16929-16934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rouviere, P. E., T. A. Bobik, and R. S. Wolfe. 1988. Reductive activation of the methyl coenzyme M methylreductase system of Methanobacterium thermoautotrophicum delta H. J. Bacteriol. 170:3946-3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sauer, K., U. Harms, and R. K. Thauer. 1997. Methanol:coenzyme M methyltransferase from Methanosarcina barkeri. Purification, properties and encoding genes of the corrinoid protein MT1. Eur. J. Biochem. 243:670-677. [DOI] [PubMed] [Google Scholar]
- 35.Slechta, E. S., J. Harold, D. I. Andersson, and J. R. Roth. 2002. The effect of genomic position on reversion of a lac frameshift mutation (lacIZ33) during non-lethal selection (adaptive mutation). Mol. Microbiol. 44:1017-1032. [DOI] [PubMed] [Google Scholar]
- 36.Sowers, K. R., J. E. Boone, and R. P. Gunsalus. 1993. Disaggregation of Methanosarcina spp. and growth as single cells at elevated osmolarity. Appl. Environ. Microbiol. 59:3832-3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sowers, K. R., S. F. Baron, and J. G. Ferry. 1984. Methanosarcina acetivorans sp. nov., an acetotrophic methane-producing bacterium isolated from marine sediments. Appl. Environ. Microbiol. 47:971-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stathopoulos, C., W. Kim, T. Li, I. Anderson, B. Deutsch, S. Palioura, W. Whitman, and D. Söll. 2001. Cysteinyl-tRNA synthetase is not essential for viability of the archaeon Methanococcus maripaludis. Proc. Natl. Acad. Sci. USA 98:14292-14297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thauer, R. K. 1998. Biochemistry of methanogenesis: a tribute to Marjory Stephenson. Microbiology 144:2377-2406. [DOI] [PubMed] [Google Scholar]
- 40.Vaupel, M., and R. K. Thauer. 1998. Two F420-reducing hydrogenases in Methanosarcina barkeri. Arch. Microbiol. 169:201-205. [DOI] [PubMed] [Google Scholar]
- 41.Wanner, B. L. 1986. Novel regulatory mutants of the phosphate regulon in Escherichia coli K-12. J. Mol. Biol. 191:39-58. [DOI] [PubMed] [Google Scholar]
- 42.Weil, C. F., D. S. Cram, B. A. Sherf, and J. N. Reeve. 1988. Structure and comparative analysis of the genes encoding component C of methyl coenzyme M reductase in the extremely thermophilic archaebacterium Methanothermus fervidus. J. Bacteriol. 170:4718-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang, J. K., A. K. White, H. C. Kuettner, P. Boccazzi, and W. W. Metcalf. 2002. Directed mutagenesis and plasmid-based complementation in the methanogenic archaeon Methanosarcina acetivorans C2A demonstrated by genetic analysis of proline biosynthesis. J. Bacteriol. 184:1449-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]