Abstract
The tick-borne bacterium Borrelia burgdorferi has over 20 different circular and linear plasmids. Some B. burgdorferi plasmids are readily lost during in vitro culture or genetic manipulation. Linear plasmid 25, which is often lost in laboratory strains, is required for the infection of mice. Strains missing linear plasmid 25 (lp25−) are able to infect mice if the BBE22 gene on lp25 is provided on a shuttle vector. In this study, we examined the role of lp25 and BBE22 in tick infections. We tested the hypothesis that complementation with BBE22 in spirochetes lacking lp25 would restore the ability of spirochetes to infect ticks. A natural tick infection cycle was performed by feeding larvae on mice injected with the parental, lp25−, or lp25− BBE22-complemented spirochete strains. In addition, larvae and nymphs were artificially infected with different strains to study tick infections independent of mouse infections. B. burgdorferi missing lp25 was significantly impaired in its ability to infect larval and nymphal ticks. When an lp25− strain was complemented with BBE22, the ability to infect ticks was partially restored. Complementation with BBE22 allowed spirochetes lacking lp25 to establish short-term infections in ticks, but in most cases the infection prevalence was lower than that of the wild-type strain. In addition, the number of infected ticks decreased over time, suggesting that another gene(s) on lp25 is required for long-term persistence in ticks and completion of a natural infection cycle.
Borrelia burgdorferi, the etiological agent of Lyme disease, has a genome consisting of a linear chromosome in addition to 12 linear plasmids and 9 circular plasmids (2, 6). The plasmids are important components of the genome and are predicted to contain 535 genes (2). The study of the function of the plasmid genes has been advanced by recent developments in genetic-manipulation techniques (5, 7, 11, 17, 23, 24). Several studies have characterized the ability of spirochetes without a full plasmid complement to infect mice and ticks (8-10, 12, 13, 15, 17, 19, 20). Some of the plasmids contain genes with necessary functions in the tick vector and/or mammalian host. For instance, linear plasmid 25 (lp25), lp28-1, and lp54 are plasmids required for the persistent infection of mice, while lp54 and circular plasmid 26 (cp26) may be required for the establishment of tick infection or migration from the tick gut to the salivary glands, respectively (3, 9, 12, 13, 15, 17, 19, 20, 28).
A significant obstacle to creating viable mutants of B. burgdorferi is that genetic transformation of infectious spirochete strains with autonomously replicating vectors may result in mutants that do not have lp25 (lp25−) (14). A DNA restriction-modification system encoded by the BBE02 gene on lp25 imparts resistance to the introduction of foreign DNA; thus, transformation with introduced vectors that are stably maintained is generally achieved only in spirochetes that do not have lp25 (14). Unfortunately, B. burgdorferi lacking lp25 is unable to infect mice when injected intraperitoneally (12, 13, 20). Linear plasmid 25 also has a role in infecting nymphal ticks; spirochetes lacking lp25 are associated with reduced gut infectivity (26).
Purser et al. (19) demonstrated that the BBE22 gene on lp25 provides a necessary nicotinamidase function in mice and that transformation of an lp25− strain with a vector containing the BBE22 gene restores the ability of the spirochetes to infect mice. It is not known if BBE22 would also restore the ability of lp25− strains to effectively infect and persist in tick guts. We conducted a series of experiments to determine if BBE22 is able to restore the ability of an lp25− mutant to infect larval and nymphal ticks by both natural and artificial tick infection routes.
MATERIALS AND METHODS
Borrelia burgdorferi strains and creation of mutants.
A clone of Borrelia burgdorferi strain B31 (CDC, Fort Collins, CO) was used in experiments as a positive control (B31-C1). B. burgdorferi strain B31-A3, kindly provided by Patricia Rosa (Rocky Mountain Laboratories, NIAID, NIH, Hamilton, MT), is a clone of B31 MI, a low-passage nonclonal infectious strain of B. burgdorferi B31. Strain B31-A3 was derived by subsurface plating of B31 MI in solid Barbour-Stoenner-Kelly (BSK) medium (21) as described by Elias et al. (4). The shuttle vector pBSV2, also graciously provided by Patricia Rosa, is a derivative of B. burgdorferi B31 cp9 and contains three open reading frames from cp9, Escherichia coli ColE1, and a kanamycin resistance cassette (25). The shuttle vector pBSV2 was modified in the present study by insertion of the BBE22 gene to create the vector pBSV2+22. The BBE22 gene of lp25 was amplified from B. burgdorferi B31 by using the methods and primers described by Purser et al. (19) from DNA prepared using QIAGEN’s DNeasy tissue kit (QIAGEN, Chatsworth, CA). Each primer contained a KpnI site and amplified a 2.074-kb region of DNA containing BBE22 from lp25, including the BBE23 gene located directly upstream of BBE22. The vector pBSV2 and the primer amplicon were digested with KpnI, and then the BBE22 amplicon was ligated into the shuttle vector to create pBSV2+22.
Two mutants of strain B31-A3 were created in this study by transformation with the vectors pBSV2 and pBSV2+22. Even though the parental strain contained lp25, we created our shuttle vectors in anticipation of selecting transformants that had lost lp25 and its restriction-modification system. Strain A3-pB was created by transformation with pBSV2 by using methods modified from those of Samuels (22). In brief, a 100-μl aliquot containing 2 × 109 spirochetes and 20 μg of DNA was given a single electrical pulse of 2.5 kV, 25 μF, and 200 Ω, transferred to 3 ml of BSK-H complete medium (Sigma, St. Louis, MO), and incubated for 36 h at 35°C. The transformation mixture was then transferred to 50 ml of BSK-H containing kanamycin (Sigma, St. Louis, MO) and incubated at 37°C. Live spirochetes were visible in the 50-ml cultures starting at 5 days and were plated onto semisolid plating BSK medium (22) when densities reached early log phase. Individual clones were selected after 1 to 3 weeks (wk). Strain A3-pB22 was created by transformation of B31-A3 with the vector pBSV2+22 by following the methods of Yang et al. (28). This method was adopted during the course of this study due to its ability to isolate clones more easily. A 100-μl aliquot containing 2 × 109 spirochetes and 20 μg of DNA was given a single electrical pulse of 2.5 kV, 25 μF, and 200 Ω and transferred to 100 ml of BSK-H complete medium. After overnight recovery at 35°C, kanamycin was added to the transformation mixture that was then transferred to 96-well plates having 200 μl per well. Plates were incubated at 35°C and 2% CO2 for 2 to 4 wk, and transformants were identified from individual wells by dark-field microscopy. A3-pB and A3-pB22 transformants were confirmed as carrying the shuttle vector by using M13 primers located within the shuttle vector and spanning the insert region. The parental B31-A3 and its two derived mutants were screened for plasmid content by using a set of 29 primers (4) (Table 1).
TABLE 1.
B. burgdorferi strains used in this study
| Strain | Introduced DNA | Plasmid(s) missing | Source or reference |
|---|---|---|---|
| B31-C1 | None | None | CDC |
| B31-A3 | None | cp9 | 4 |
| A3-pB | pBSV2 | cp9, lp25 | This study |
| A3-pB22 | pBSV2 + BBE22 | cp9, lp25 | This study |
Larval-tick feeding on infected mice.
The B. burgdorferi strains B31-A3, A3-pB, and A3-pB22 were grown to late log stage in 6 ml BSK-H complete medium. Naïve female C3H/HeNcr mice (4 to 6 wk old; NIH) were injected subcutaneously above the shoulder with 100 μl of culture containing 1 × 104 to 1 × 106 spirochetes of each of the strains (methods approved by the UNC Institutional Animal Care and Use Committee). Two weeks after the mice were injected, approximately 100 uninfected larval Ixodes scapularis ticks (kindly provided by Jerry Bowman, Oklahoma State University, Stillwater, OK) were placed on each mouse on the neck and shoulder region. Mice were housed individually in cages containing one-half inch of water and a wire grid that supported the mice above the water. Larvae were allowed to feed to repletion and fall off the mice. Larvae were collected from the water, and subsets were analyzed for spirochete infection by using a direct fluorescent antibody (DFA) stain 7 days after ticks were placed on the mice. Whole live ticks were homogenized on glass slides in 3 μl of phosphate-buffered saline (PBS) by using the blunt ends of disposable pestles (Kontes Glass Company, Vineland, NJ), and the slides were acetone fixed and then blocked for 30 min in 5% fetal bovine serum (FBS)-PBS. Slides were next stained with a 1:200 dilution of fluorescein isothiocyanate-conjugated goat anti-Borrelia species polyclonal antibody (KPL, Gaithersburg, MD) in 5% FBS-PBS for 1 h. After three washes in PBS, coverslips were mounted and infection status was determined by fluorescence microscopy (Nikon Eclipse E600 with epifluorescence attachment; Melville, NY). An additional subset of larvae was homogenized and checked for spirochete infection 2 wk after ticks were placed on the mice. Mice were sacrificed 3 wk after needle inoculation. Blood was collected, and ear, heart, urinary bladder, and left hind tibiotarsal joints were harvested and cultured in 6 ml BSK-H medium. Blood samples were separated using a Microtainer serum separator tube (Becton Dickinson, Franklin Lakes, NJ), and the serum was run against Western blots of the parental B31-A3 strain. Briefly, 109 spirochetes were boiled, loaded into a 12% gel, and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The gel was transferred to a nitrocellulose membrane and blocked for 30 min in 2% skim milk in Tris-buffered saline (TBS). The membranes were then cut into strips and reacted against a 1:100 dilution of mouse serum in blocking solution for 1 h. Strips were washed three times in TBS-Tween 20 and then reacted against a 1:500 dilution of alkaline phosphatase-conjugated goat anti-mouse (KPL, Gaithersburg, MD) for 45 min. Strips were washed an additional three times in TBS-Tween 20 and once in TBS and then developed with BCIP (5-bromo-4-chloro-3-indolylphosphate)-nitroblue tetrazolium phosphatase substrate (KPL, Gaithersburg, MD).
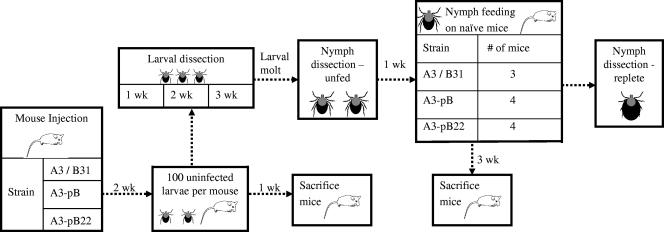
The remaining larvae that had fed to repletion on infected mice were kept in humidity-controlled incubators at room temperature until molt. At 1 wk after molt, a subset of nymphs (unfed condition) from each group were homogenized on glass slides and assessed for spirochete infection. At 2 wk after molt, the remaining nymphs were placed on naïve C3H/HeNcr mice, allowed to feed to repletion, and then homogenized on slides and assessed for infection after all nymphs had fallen off the mice (fed condition). Three weeks after nymphs had fed on the mice, the mice were sacrificed, blood was collected, and tissues were cultured in BSK-H complete medium. The complete mouse/tick/mouse experimental protocol is outlined in Fig. 1.
FIG. 1.
Experimental mouse/tick/mouse infection cycle protocol. Mice were needle inoculated with three strains of spirochetes, and after 2 wk, larvae were fed on the mice. After feeding to repletion, larvae were held through molt and then fed as nymphs on naïve mice. Larvae and nymphs were tested for infection at different time points after feeding on mice, and all mice were sacrificed and tested for infection either 3 wk after needle inoculation or 3 wk after being fed upon by nymphs.
The above experiment was repeated as outlined above with the exceptions that strain B31-C1 was used instead of B31-A3 as a positive control and that larvae were tested at 2 and 3 wk after placement on mice. In a third repeat of the above experiment, the same protocol was used with B31-A3 as the positive control, except that replete larvae were tested for infection at 1, 2, and 3 wk after placement on mice, and larvae were not tested after molt.
Nymphal-tick feeding on infected mice.
Three naïve female C3H/HeNcr mice were needle inoculated subcutaneously above the shoulder with 100 μl of culture containing 1 × 104 spirochetes of B. burgdorferi strain B31-A3, and an additional three mice were inoculated with the same number of spirochetes of strain A3-pB22. After 3 wk, 30 uninfected I. scapularis nymphs were placed on each of the mice. Nymphs were allowed to feed to repletion and fall off. Replete nymphs were homogenized on glass slides at 1, 2, and 3 wk after placement on mice and stained by DFA as outlined previously. The experiment was repeated by feeding nymphs on two additional mice, each injected with either B31-C1 or A3-pB22, and nymphs were tested at 2 and 3 wk after placement on mice. All mice were confirmed positive 3 wk after tick feeding by serology as outlined previously, and all organs tested were positive.
Larval-tick infection by immersion.
Larvae were infected with spirochetes by larval immersion following the procedure of Policastro and Schwan (18). Immersion was used due to the small size of larvae, which rules out other artificial-infection methods. Briefly, spirochete strains B31-A3, A3-pB, and A3-pB22 were grown to late log stage in BSK-H complete medium and centrifuged at 8,000 × g for 10 min, and then the supernatant was removed and the pellet was resuspended in fresh BSK-H medium at a concentration of 1 × 108 spirochetes per ml. Larval I. scapularis ticks were cooled to 4°C and transferred to each of three 1.5-ml centrifuge tubes. Five hundred microliters of each of the spirochete cultures was added to the tubes that were then incubated at 32°C for 2 h, with occasional vortexing. Tubes were next centrifuged for 30 s at 200 × g, and the supernatant was removed, followed by two additional washes in PBS. Whatman filter papers were added to absorb excess moisture, and then larvae were placed on each of three naïve female C3H/HeNcr mice. Larvae were allowed to feed to repletion and fall off. At 1 and 3 wk after placement on mice, replete larvae were homogenized on glass slides and stained for infection by DFA. Three wk after larvae had fallen off the mice, the mice were sacrificed and tested for infection by serology and organ culture.
Nymphal-tick infection by capillary feeding.
Nymphal ticks were capillary fed B31-A3, A3-pB, and A3-pB22 according to the methods of Broadwater et al. (1). Spirochetes were grown to late log stage, pelleted, and resuspended at a concentration of 1 × 108 spirochetes per ml in equal volumes of BSK-H complete medium and Shen's saline (16). Glass micropipettes (5 μl) were filled with these solutions and placed over the hypostomes and palps of unfed nymphal ticks immobilized on glass slides. Ticks were allowed to drink from the micropipettes for 1 h and then held for 24 h in a humidified incubator at room temperature. After the 24-h recovery period, nymphs were placed on the necks and shoulders of naïve female C3H/HeNcr mice and fed to repletion. Replete ticks were homogenized on glass slides and tested by DFA for infection at 1 wk and 3 wk after placement on mice. Mice were sacrificed 3 wk after being fed upon by ticks, and infection status was determined by serology and organ culture. In a repeat of this experiment, the same protocol was followed except that capillary-fed nymphs were given a recovery period of 3 days in a humidified incubator. On the third day, nymphs were placed on mice and allowed to feed to repletion, and 1 wk after placement, the nymphs were homogenized on glass slides and tested for spirochete infection by DFA.
RESULTS
B. burgdorferi strains and creation of mutants.
Previous work has shown that complementation of lp25− spirochetes with BBE22 restores the ability of spirochetes to infect mice (19). In order to test whether BBE22-complemented lp25− spirochetes are able to establish infection in ticks, two mutants of strain B31-A3 were derived by transformation with the shuttle vectors pBSV2 and pBSV2+22 (Table 1). Strain A3-pB was transformed with the vector pBSV2 only, while strain A3-pB22 was transformed with pBSV2 containing the BBE22 gene. A plasmid profile of the parental B31-A3 strain revealed a complete plasmid set except for cp9, while plasmid profiles of the two mutants revealed that they were additionally missing lp25 as expected. Loss of cp9 in spirochetes is not associated with ability to infect either mice or ticks (20, 27). B31-C1 contained a complete set of all plasmids. During the course of this study, the eight mice injected with the parental B31-A3 or B31-C1 strain and used for feeding uninfected ticks were positive for infection by serology, and all organs tested (ear punch, heart, bladder, and joint) were positive. The seven mice injected with A3-pB22 were also positive by serology, and all organs tested were positive except for two mice, which had three out of four organ cultures positive. The lp25− A3-pB strain did not infect any of the four mice tested, demonstrating that the BBE22-complemented mutants that we created effectively expressed BBE22 and were restored in their ability to infect mice, confirming previous studies by Purser et al. (19).
Larval-tick feeding on infected mice.
In order to test the ability of the three spirochete strains to complete a natural infectious cycle, mice were injected subcutaneously with each strain and uninfected larvae were placed on the mice 2 weeks later (Fig. 1). Replete larvae were tested for infection by DFA at 1, 2, and 3 wk after placement on mice. Nearly all of the larvae that fed on mice injected with strain B31-A3 or B31-C1 were positive at all time points (Table 2). In contrast, the infection rate of larvae that fed on mice injected with A3-pB22 was significantly lower than that of larvae fed on mice injected with the parental strain at each time point, with infection rates ranging from 89.6% down to 60% over the course of 3 wk (Fisher's exact test, P < 0.05). In addition, the infection rate of larvae infected with A3-pB22 dropped significantly at each subsequent time point tested, and the numbers of spirochetes within individual tick homogenates at 2 and 3 wk were smaller in A3-pB22-infected larvae than in larvae infected with B31-A3 or B31-C1 (data not shown). As expected, none of the larvae that fed on A3-pB-injected mice contained visible spirochetes at this or any other time point. Mice were sacrificed 3 wk after injection, blood was collected, and organs were cultured. All of the mice injected with B31-A3, B31-C1, and A3-pB22 were positive by organ culture and serology, while none of the mice given A3-pB were positive by either method. Spirochetes were cultured from all of the organs from the B31-A3-injected and B31-C1-injected mice, while three out of four organ cultures from each of the A3-pB22-injected mice were positive.
TABLE 2.
Experimental mouse/tick/mouse infection cyclea
| Strain | No. positive for infection/total no. (%)
|
|||||
|---|---|---|---|---|---|---|
| Larvaec
|
Molted nymphsd
|
Micee | ||||
| 1 wk | 2 wk | 3 wk | Unfed | Fed | ||
| B31-A3 or B31-C1b | 104/108 (96.3) | 76/82 (92.7) | 70/71 (98.6) | 27/27 (100) | 28/29 (96.6) | 3/3 (100) |
| A3-pB | 0/93 (0)* | 0/41 (0)* | ND | 0/54 (0)* | 0/59 (0)* | 0/4 (0) |
| A3-pB22 | 121/135 (89.6)* | 72/93 (77.4)* | 21/35 (60)* | 3/51 (5.9)* | 6/53 (11.3)* | 0/4 (0) |
Data are pooled from three separate experiments. ND, not determined. *, tick infection mean was significantly different than the infection mean of ticks that fed on B31-A3-injected or B31-C1-injected mice for each column (P < 0.05, Fisher's exact test).
B31-A3 was used in the first and third repeats of the experiment, while B31-C1 was used in the second repeat of the experiment.
Larvae were tested for infection at 1, 2, and 3 wk after placement on needle-inoculated mice.
Nymphs were tested for infection 1 wk after molt as unfed nymphs, as well as 2 wk after molt after feeding to repletion on naïve mice.
Mice were sacrificed and tested for infection 3 wk after being fed on by molted nymphs.
The remaining larvae that had fed to repletion on injected mice were allowed to molt to nymphs. Half of the nymphs were kept in the unfed condition, while the other half were allowed to feed on naïve mice (Table 2). Unfed nymphs and replete nymphs were tested for infection. Almost all of the unfed and replete nymphs that had fed as larvae on B31-C1-injected or B31-A3-injected mice were positive, whereas nymphs that fed on A3-pB22-injected mice as larvae were infected at significantly lower rates (Table 2). The number of spirochetes in the homogenates of individual nymphs infected with A3-pB22 were also noticeably fewer than the numbers in nymphs infected with B31-C1 or B31-A3. Only nymphs infected with B31-A3 or B31-C1 were able to transmit infection to mice, with all organs testing positive (Table 2).
Nymphal-tick feeding on infected mice.
In the above experiment, the abilities of the four spirochete strains to survive in nymphs that had molted from infected larvae were tested. To further characterize the abilities of the spirochete strains to infect nymphs, uninfected nymphs were fed directly on needle-inoculated mice. Mice were needle inoculated with B31-A3, B31-C1, or A3-pB22, and nymphs were placed on the mice 3 wk later. Nymphs were allowed to feed to repletion, and then infection status was determined by DFA at 1, 2, and 3 wk after placement on the mice. At 1 wk after placement of nymphs on the mice, the infection rate of nymphs that had fed on A3-pB22-infected mice was 52.2%, a significantly lower rate than that of nymphs fed on mice infected with the parental strain (P < 0.05) (Table 3). The infection rate of nymphs that fed on A3-pB22-infected mice steadily dropped during the experiment, and by the third week, only 5.7% of these nymphs were infected, and the numbers of spirochetes per gut were visibly fewer than those in nymphs fed on mice infected with the parental strain.
TABLE 3.
Nymphal feeding on mice needle inoculated with two strains of spirochetesa
| Strain | No. of nymphs positive for infection/total tested (%) atb:
|
||
|---|---|---|---|
| 1 wk | 2 wk | 3 wk | |
| B31-A3 or B31-C1c | 21/24 (87.5) | 30/34 (88.2) | 25/28 (89.3) |
| A3-pB22 | 12/23 (52.2)* | 7/38 (18.4)* | 2/35 (5.7)* |
Data are pooled from two separate experiments.
*, tick infection mean was significantly different than the infection mean of ticks that fed on B31-A3-injected or B31-C1-injected mice for each column (P < 0.05, Fisher's exact test).
B31-A3 was used in the first repeat of the experiment, while B31-C1 was used in the second repeat of the experiment.
Larval-tick infection by immersion.
Mice needle inoculated with the A3-pB strain do not become infected because of the lack of lp25, and therefore the ability of ticks to acquire A3-pB must be studied using artificial-infection methods. An artificial-immersion method recently described by Policastro and Schwan (18) was used to infect larvae. Uninfected larvae were immersed in medium containing the B31-A3, A3-pB, or A3-pB22 spirochete strain and then allowed to feed to repletion on naïve mice. At 3 wk after feeding, larvae immersed in A3-pB (10%) and A3-pB22 (30.8%) had fewer infected larvae than larvae immersed in the parental B31-A3 (60%) strain; however, only differences between larvae immersed in B31-A3 and A3-pB were statistically significant (P < 0.05) (Table 4). The average numbers of spirochetes in the homogenates of larvae immersed in A3-pB and A3-pB22 at both time periods were noticeably fewer than the numbers in B31-A3-immersed larvae (data not shown). Mice were sacrificed and tested for infection by serology and organ culture. The mice fed on by larvae immersed in B31-A3 and A3-pB22 were positive by serology, and all organs tested were positive, whereas mice fed on by A3-pB-immersed larvae were not infected (Table 4). Although only one mouse was tested per strain, which precludes drawing strong conclusions about the ability of infected larvae to transmit infection, the results are nonetheless interesting and can be studied in more detail in future studies.
TABLE 4.
Artificial feeding of larvae and nymphs with three strains of spirochetesa
| Strain | No. positive for infection/total no. (%)
|
||||
|---|---|---|---|---|---|
| Immersed larvaeb
|
Capillary-fed nymphsc
|
||||
| 1-day hold
|
3-day hold | ||||
| 1 wk | 3 wk | 1 wk | 3 wk | 1 wk | |
| B31-A3 | 12/15 (80) | 9/15 (60) | 23/23 (100) | 21/22 (95.5) | 23/28 (82.1) |
| A3-pB | 3/11 (27.3)* | 1/10 (10)* | 3/26 (11.5)* | 2/25 (8)* | 0/21 (0)* |
| A3-pB22 | 10/14 (71.4) | 4/13 (30.8) | 13/24 (54.2)* | 14/21 (67.7)* | 2/34 (5.9)* |
*, infection rate was significantly different than the infection rate of ticks that fed on B31-A3-injected mice for each column (P < 0.05, Fisher's exact test).
Immersed larvae were tested for infection at 1 and 3 wk after placement on mice. Of the mice fed on by B31-A3-infected, A3-pB-infected, or A3-pB22-infected larvae, respectively, one of one, zero of one, and one of one tested positive for infection.
Nymphs were held for 1 or 3 days after capillary feeding before placement on mice and then tested for infection 1 and 3 wk after placement on mice. Of the mice fed on by B31-A3-infected, A3-pB-infected, or A3-pB22-infected nymphs held for 1 day, two of two, zero of two, and one of two mice, respectively, tested positive for infection.
Nymphal-tick infection by capillary feeding.
The abilities of the B31-A3, A3-pB, and A3-pB22 spirochete strains to infect nymphs were directly tested by artificial-infection capillary feeding (1). Uninfected unfed nymphs were capillary fed the three spirochete strains, held for either 24 or 72 h, placed on naïve mice, and allowed to feed to repletion. After all nymphs had fallen off, they were tested for infection at 1 and 3 wk after placement on the mice. All of the nymphs that were capillary fed B31-A3 and held for 24 h prior to placement on mice were positive at the 1-wk time point, and nearly all were positive at the 3-wk time point (Table 4). Nymphs capillary fed A3-pB and A3-pB22 had significantly lower infection prevalences than nymphs capillary fed B31-A3 at both time periods (P < 0.05), and A3-pB-fed nymphs additionally had a significantly lower infection prevalence than A3-pB22-fed nymphs. The numbers of spirochetes seen in the homogenates of positive nymphs fed either A3-pB or A3-pB22 were also noticeably smaller than the numbers in nymphs fed parental B31-A3 at both time points (data not shown). Mice were sacrificed 3 wk after being fed on by the nymphs. Both mice fed on by B31-A3-infected nymphs were positive by serology, and all organ cultures were positive, while only one mouse fed on by A3-pB22-infected nymphs was positive for infection, with all organs testing positive, and no mice fed on by nymphs capillary fed A3-pB were infected. In the second part of this experiment, 82.1% of the nymphs capillary fed the B31-A3 strain and held for 72 h prior to feeding on mice were positive (Table 4). None of the nymphs held for 72 h that were capillary fed A3-pB were positive, and 5.9% that were fed A3-pB22 were positive, a significantly lower rate than that of nymphs fed the parental strain.
DISCUSSION
The results of this study have demonstrated that the complementation of an lp25− strain with BBE22 restored the ability of spirochetes to infect the guts of larvae that fed on needle-inoculated mice but at a frequency lower than the parental strain. In addition, the BBE22-complemented spirochetes exhibited a lack of persistence in the larvae during the ensuing weeks, and the infection rate plummeted during the molt from larvae to nymphs. Importantly, the small numbers of spirochetes seen in molted nymphs were unable to infect naïve mice. A natural infection cycle from infected mice to larval ticks and back to mice via molted nymphs did not occur in spirochetes missing lp25 that contained BBE22 on a shuttle vector.
We also tested the ability of nymphs to directly acquire the strains B31-A3 and A3-pB22 by feeding on infected mice. Within 1 week of placing the nymphs, spirochetes could be found in over half of the nymphs that fed on A3-pB22-infected mice, but the number of A3-pB22-infected nymphs had dropped significantly by the third week. Thus, lp25− spirochetes complemented with BBE22 were able to establish a nominal infection in nymphs, but as with larvae that had fed on A3-pB22-infected mice, the infection dropped markedly during the course of 3 weeks. The stability of the pB22 vector in spirochetes during tick infection was not addressed in this study; however, the vector was stably maintained in the mice during 3 wk of infection, as seen by organ culture under kanamycin selection. In addition, Stewart et al. (25) tested the stability of the pBSV2 shuttle vector in transformed spirochetes and found that the vector was stable for at least 25 generations without antibiotic selection in vitro.
The experiments in which larvae and nymphs were fed on infected mice might be confounded by the abilities of our strains to thrive in mice or to move from the mouse into the tick. We used artificial-infection methods (immersion for larvae and capillary feeding for nymphs) to directly introduce our strains (B31-A3, A3-pB, and A3-pB22) into larval and nymphal ticks. Spirochetes missing lp25 (A3-pB) infected significantly fewer larvae and nymphs than the parental strain or the lp25− strain that was complemented with BBE22. This result clearly indicates that BBE22 enhances the ability of lp25− strains to infect ticks. However, there are other genes on lp25 required for efficient infection and persistence in ticks, as the BBE22-complemented strain infected fewer nymphs than the parental strain. One other gene on lp25 whose requirement for infecting mice has been studied is BBE02 (11). A spirochete strain with this gene disrupted was able to infect mice at rates comparable to those of the parental strain, and therefore this gene is not essential for mouse infection. In our lab, we have studied the ability of a BBE02-disrupted mutant to infect larvae and nymphs and did not see an impairment of this strain to infect ticks (unpublished data), and so the other genes on lp25 can be studied in order to determine their requirements for tick infection. These results indicate that BBE22 on lp25 incompletely restores infection of ticks and that an additional gene(s) on lp25 may be required for efficient infection and persistence in ticks. Alternatively, the levels of nicotinamidase produced by the BBE22-complemented strain may be adequate for mouse infection but not sufficient for completely restoring tick infection. The strain complemented with BBE22 did do well in ticks over the short term and was even transmitted from ticks to mice, indicating that additional genes on lp25 were not required for transient tick infections and transmission to the host.
In summary, complementation of B. burgdorferi lacking lp25 with BBE22 restored the short-term ability of spirochetes to infect I. scapularis larvae and nymphs and reestablished the ability of spirochetes to be transmitted to naïve mice by both life stages. Complementation with BBE22, however, did not allow long-term survival of spirochetes within larvae or nymphs, nor did it allow the completion of a mouse/tick/mouse infection cycle, suggesting that other genes on lp25 are required for tick infection. This idea can be tested in future studies by adding lp25 back into the lp25− mutants complemented with BBE22 by using the techniques of Grimm et al. (7) in order to see if the addition of lp25 restores the ability of the spirochetes to persistently infect ticks. Our study has demonstrated that in mice, the addition of BBE22 in shuttle vectors and the production of nicotinamidase can be used as selectable markers in lp25− spirochetes; however, our results negate the ability to use BBE22 in ticks as a selectable marker, due to its inability to restore infection levels to wild-type levels. Other studies have demonstrated that individual plasmids have determinants important in infecting both ticks and mice. Spirochetes without lp28-4 are associated with an attenuation of the spirochetes' ability to infect tick guts and mice (12, 13, 20, 26). The gene(s) on lp25 besides BBE22 that is responsible for long-term persistence in ticks is not known. Linear plasmid 25 has 39 putative genes with varied hypothetical functions, including roles associated with the cell envelope and cellular processes such as cell division and DNA metabolism, replication, and repair (http://www.tigr.org), and future studies can address the roles of these genes in tick infection and persistence. The development of B. burgdorferi strains resistant to the loss of lp25 should also be considered when manipulating spirochetes by the introduction of autonomously replicating vectors. Kawabata et al. (11) developed strains with the restriction-modification gene (BBE02) on lp25 disrupted, thereby allowing the introduction of foreign DNA into spirochetes that retain lp25.
ADDENDUM IN PROOF
During review of the manuscript, a relevant paper was published (A. T. Revel, J. S. Blevins, C. Almazan, L. Neil, K. M. Kocan, J. de la Fuente, K. E. Hagman, and M. V. Norgard, Proc. Natl. Acad. Sci. USA 102:6972-6977, 2005). Revel et al. describe the essential nature of BBE16 on lp25 for persistence of spirochetes in ticks. Thus, BBE22 and BBE16 are required for persistence of spirochetes in ticks. The identification of these two genes does not rule out the possibility that another gene(s) on lp25 may also be required.
Acknowledgments
We thank Abdallah Elias, Dorothee Grimm, and Patricia Rosa (Rocky Mountain Laboratories, NIAID, NIH, Hamilton, MT) for providing Borrelia strains and the shuttle vector. We also thank Jerry Bowman for supplying ticks used in this study.
This work was supported by grants from the Arthritis Foundation and NIH.
REFERENCES
- 1.Broadwater, A. H., D. E. Sonenshine, W. L. Hynes, S. Ceraul, and S. A. De. 2002. Glass capillary tube feeding: a method for infecting nymphal Ixodes scapularis (Acari: Ixodidae) with the Lyme disease spirochete Borrelia burgdorferi. J. Med. Entomol. 39:285-292. [DOI] [PubMed] [Google Scholar]
- 2.Casjens, S., N. Palmer, R. van Vugt, W. M. Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. M. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490-516. [DOI] [PubMed] [Google Scholar]
- 3.Dever, L. L., J. H. Jorgensen, and A. G. Barbour. 1993. In vitro activity of vancomycin against the spirochete Borrelia burgdorferi. Antimicrob. Agents Chemother. 37:1115-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elias, A. F., P. E. Stewart, D. Grimm, M. J. Caimano, C. H. Eggers, K. Tilly, J. L. Bono, D. R. Akins, J. D. Radolf, T. G. Schwan, and P. Rosa. 2002. Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect. Immun. 70:2139-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher, M. A., D. Grimm, A. K. Henion, A. F. Elias, P. E. Stewart, P. A. Rosa, and F. C. Gherardini. 2005. Borrelia burgdorferi σ54 is required for mammalian infection and vector transmission but not for tick colonization. Proc. Natl. Acad. Sci. USA 102:5162-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. C. Venter, et al. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 7.Grimm, D., C. H. Eggers, M. J. Caimano, K. Tilly, P. E. Stewart, A. F. Elias, J. D. Radolf, and P. A. Rosa. 2004. Experimental assessment of the roles of linear plasmids lp25 and lp28-1 of Borrelia burgdorferi throughout the infectious cycle. Infect. Immun. 72:5938-5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grimm, D., A. F. Elias, K. Tilly, and P. A. Rosa. 2003. Plasmid stability during in vitro propagation of Borrelia burgdorferi assessed at a clonal level. Infect. Immun. 71:3138-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimm, D., K. Tilly, R. Byram, P. E. Stewart, J. G. Krum, D. M. Bueschel, T. G. Schwan, P. F. Policastro, A. F. Elias, and P. A. Rosa. 2004. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc. Natl. Acad. Sci. USA 101:3142-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Indest, K. J., J. K. Howell, M. B. Jacobs, D. Scholl-Meeker, S. J. Norris, and M. T. Philipp. 2001. Analysis of Borrelia burgdorferi vlsE gene expression and recombination in the tick vector. Infect. Immun. 69:7083-7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawabata, H., S. J. Norris, and H. Watanabe. 2004. BBE02 disruption mutants of Borrelia burgdorferi B31 have a highly transformable, infectious phenotype. Infect. Immun. 72:7147-7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Labandeira-Rey, M., J. Seshu, and J. T. Skare. 2003. The absence of linear plasmid 25 or 28-1 of Borrelia burgdorferi dramatically alters the kinetics of experimental infection via distinct mechanisms. Infect. Immun. 71:4608-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Labandeira-Rey, M., and J. T. Skare. 2001. Decreased infectivity in Borrelia burgdorferi strain B31 is associated with loss of linear plasmid 25 or 28-1. Infect. Immun. 69:446-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawrenz, M. B., H. Kawabata, J. E. Purser, and S. J. Norris. 2002. Decreased electroporation efficiency in Borrelia burgdorferi containing linear plasmids lp25 and lp56: impact on transformation of infectious B. burgdorferi. Infect. Immun. 70:4798-4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masuzawa, T., T. Kurita, H. Kawabata, and Y. Yanagihara. 1994. Relationship between infectivity and OspC expression in Lyme disease Borrelia. FEMS Microbiol. Lett. 123:319-324. [DOI] [PubMed] [Google Scholar]
- 16.Oliver, J. H., P. R. Wilkinson, and G. M. Kohls. 1972. Observations on hybridization of three species of North American Dermacentor ticks. J. Parasitol. 58:380-384. [PubMed] [Google Scholar]
- 17.Pal, U., X. Yang, M. Chen, L. K. Bockenstedt, J. F. Anderson, R. A. Flavell, M. V. Norgard, and E. Fikrig. 2004. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J. Clin. Investig. 113:220-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Policastro, P. F., and T. G. Schwan. 2003. Experimental infection of Ixodes scapularis larvae (Acari: Ixodidae) by immersion in low passage cultures of Borrelia burgdorferi. J. Med. Entomol. 40:364-370. [DOI] [PubMed] [Google Scholar]
- 19.Purser, J. E., M. B. Lawrenz, M. J. Caimano, J. K. Howell, J. D. Radolf, and S. J. Norris. 2003. A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Mol. Microbiol. 48:753-764. [DOI] [PubMed] [Google Scholar]
- 20.Purser, J. E., and S. J. Norris. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 97:13865-13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosa, P., D. S. Samuels, D. Hogan, B. Stevenson, S. Casjens, and K. Tilly. 1996. Directed insertion of a selectable marker into a circular plasmid of Borrelia burgdorferi. J. Bacteriol. 178:5946-5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samuels, D. S. 1995. Electrotransformation of the spirochete Borrelia burgdorferi, p. 253-259. In J. A. Nickoloff (ed.), Methods in molecular biology, vol. 47. Humana Press, Inc., Totowa, N.J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sartakova, M., E. Dobrikova, and F. C. Cabello. 2000. Development of an extrachromosomal cloning vector system for use in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 97:4850-4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stewart, P. E., R. Byram, D. Grimm, K. Tilly, and P. A. Rosa. 2005. The plasmids of Borrelia burgdorferi: essential genetic elements of a pathogen. Plasmid 53:1-13. [DOI] [PubMed] [Google Scholar]
- 25.Stewart, P. E., R. Thalken, J. L. Bono, and P. Rosa. 2001. Isolation of a circular plasmid region sufficient for autonomous replication and transformation of infectious Borrelia burgdorferi. Mol. Microbiol. 39:714-721. [DOI] [PubMed] [Google Scholar]
- 26.Strother, K., A. Broadwater, and A. M. de Silva. Plasmid requirements for infection of ticks by Borrelia burgdorferi. Vector Borne Zoonotic Dis., in press. [DOI] [PubMed]
- 27.Tilly, K., D. Grimm, D. M. Bueschel, J. G. Krum, and P. Rosa. 2004. Infectious cycle analysis of a Borrelia burgdorferi mutant defective in transport of chitobiose, a tick cuticle component. Vector Borne Zoonotic Dis. 4:159-168. [DOI] [PubMed] [Google Scholar]
- 28.Yang, X. F., U. Pal, S. M. Alani, E. Fikrig, and M. V. Norgard. 2004. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J. Exp. Med. 199:641-648. [DOI] [PMC free article] [PubMed] [Google Scholar]