Abstract
The products of the hexacistronic spoVA operon of Bacillus subtilis may be involved in the transport of dipicolinic acid into the forespore during sporulation and its release during spore germination. The major hydrophilic coding region of B. subtilis spoVAD was cloned, the protein was expressed in Escherichia coli as a His tag fusion protein, and a rabbit antiserum was raised against the purified protein. Western blot analyses of fractions from B. subtilis spores showed that SpoVAD is an integral inner membrane protein present at levels >50-fold higher than those of the spore's nutrient germinant receptors that are also present in the inner membrane. SpoVAD also persisted in outgrowing spores.
Spore formation and spore germination are two crucial processes in the life cycle of spore-forming bacteria. Sporulation is induced by nutrient deprivation and generates a dormant spore that can survive long periods under unfavorable growth conditions. The process of spore germination and then outgrowth returns the spore to life in response to better conditions, in particular, the presence of nutrients. In addition, for spores of pathogenic species, spore germination can lead to rapid production of toxins or enzymes that cause disease or food spoilage.
The mechanisms of spore formation and germination in Bacillus species, in particular, Bacillus subtilis, have been extensively studied (10, 31, 35). A characteristic feature of the spores of Bacillus and Clostridium species is high levels of pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]), which usually comprises ∼10% of the spore's dry weight (9). DPA is synthesized in the mother cell compartment of a sporulating cell, enters the developing spore by moving across the two membranes that surround the spore core, and likely exists in the core as a 1:1 chelate with divalent cations, predominantly Ca2+. The high DPA level in the spore core is important for spore resistance to wet heat and spore stability, as DPA-less spores lyse rapidly during sporulation and stabilized DPA-less spores are much more susceptible to wet heat than are wild-type spores (15, 27). DPA is released, most likely as a 1:1 chelate with divalent cations, in the first minutes of spore germination triggered by nutrients. This DPA exit facilitates the rehydration of the spore core and also triggers subsequent steps in the germination process (30, 35). Clearly, the entry of DPA into, its presence in, and its exit from the spore core, respectively, are essential processes for these spore-forming bacteria.
Unfortunately, very little is known of the mechanisms of DPA entry into the developing forespore and its exit during spore germination, although it has been suggested that proteins encoded by the spoVA operon are involved in DPA entry (13). The B. subtilis spoVA operon encodes six proteins that are likely to be membrane proteins, and the operon is transcribed in the forespore by RNA polymerase containing σG at or about the time of DPA synthesis in the mother cell (12, 13, 14, 25, 33, 34, 36). Evidence for the involvement of SpoVA proteins in the entry of DPA into the developing spore has been obtained using strains with null mutations in spoVA (13, 38). In addition, the involvement of SpoVA proteins in both DPA entry during spore formation and its release during nutrient-triggered spore germination was suggested recently by analysis of a temperature sensitive B. subtilis spoVA mutant (40).
Several of the proteins of the germinant receptors which recognize the nutrients that trigger spore germination have been localized to the inner membrane of spores of B. subtilis (20, 29). Since nutrient binding to the spore's germinant receptors triggers the rapid release of DPA from the spore core, if SpoVA proteins are involved in this DPA release, the SpoVA proteins will also be in the spore's inner membrane. In this study, we have prepared an antiserum to one SpoVA protein, SpoVAD, and have used this antiserum to localize SpoVAD in B. subtilis spores.
MATERIALS AND METHODS
Strains used and spore preparation.
The B. subtilis strains used in this work are isogenic with strain PS832, a prototrophic derivative of strain 168. The sleB spoVA strain PS3406, which produces relatively stable, albeit DPA-less spores, has been described previously (38). To place spoVA under the control of the strong σG-dependent forespore-specific promoter of the sspB gene (PsspB) (5, 36), a 354-bp region stretching from bp 1 to 354 in the spoVAA coding sequence was amplified by PCR (all primer sequences are available on request). The primers also contained an NdeI site in the upstream primer and an XbaI site in the downstream primer. The PCR product was ligated into plasmid pCR2.1 (Invitrogen, Carlsbad, CA) and transformed into Escherichia coli TG1 to obtain plasmid pPS3386. Plasmid pFE133/140, which carries the ermC gene (16) and PsspB in plasmid pUC19, has been described previously (4, 29). The spoVAA fragment from pPS3386 was excised by digestion with NdeI and XbaI and the fragment inserted between the same sites in plasmid pFE133/140 to obtain plasmid pPS3393 in E. coli. This plasmid was used to transform B. subtilis strain PS832 as previously described (28) to resistance to erythromycin and lincomycin by a single-crossover event in spoVAA, giving strain PS3411, in which the spoVA operon is under the control of PsspB.
Spores of all strains were prepared on 2× SG medium plates at 37°C, cleaned by repeated washing with water, and stored as previously described (26). All spore preparations were free (>95%) of sporulating cells, germinated spores, and cell debris as determined by observation in a phase-contrast microscope.
Expression and purification of a SpoVAD fusion protein.
Since a Hopp-Woods hydropathy plot (19) revealed that SpoVAD has hydrophilic patches extending from amino acid 14 to 318, the region of spoVAD encoding this part of the protein was amplified from genomic DNA of B. subtilis strain PS832, with an NdeI site at the upstream end and a BamHI site at the downstream end introduced into the primers. The PCR product was digested with NdeI and BamHI and cloned into the pET16b expression vector (Novagen, Madison, WI), fusing a His10 tag to the protein's N terminus; the resultant plasmid, pPS3732, was isolated in E. coli and sequenced to ensure that the sequence and orientation of the spoVAD open reading frame were as expected.
Expression of His-tagged SpoVAD (SpoVAD fusion protein) from plasmid pPS3732 was in E. coli BL21/pLysS(DE3) (Stratagene, La Jolla, CA). The expression strain was grown for 16 h in Terrific broth (32) containing ampicillin (100 μg/ml) and chloramphenicol (20 μg/ml) at 37°C with 2 mM isopropyl-β-d-thiogalactopyranoside (IPTG), the pellet from 500 ml of culture suspended in 50 ml of buffer (50 mM NaPO4 [pH 8.0], 300 mM NaCl), frozen, thawed, and sonicated (five 10-s bursts with pauses of 30 s between bursts). The sonicated suspension was centrifuged at 13,000 × g for 15 min at 4°C, and the pellet was suspended in 5 ml of denaturing buffer (buffer plus 8 M urea), incubated for 5 min at 37°C, sonicated briefly to dissolve the pellet, and centrifuged to remove cell debris. The final supernatant fluid was termed the pellet fraction.
The pellet fraction was added to 3 ml of cobalt resin (BD Talon metal affinity resin; BD Biosciences, Palo Alto, CA) and shaken for 20 min at 23°C to allow binding of the SpoVAD fusion protein to the resin. The suspension was centrifuged in a microcentrifuge, and the supernatant fluid was discarded as unbound material. The resin-protein complexes were suspended in 25 ml of denaturing buffer at pH 7.0 and poured into a column, the column was washed with 5 volumes of denaturing buffer, and protein was eluted with 3 ml of 100 mM EDTA (pH 8). The eluate was diluted to 30 ml with water and concentrated twice to 3 ml using Microcon concentration spin columns (Millipore Corporation, Bedford, MA), reducing the concentrations of urea, cobalt, and EDTA ∼100-fold and giving ∼3 mg of protein. Fractions from the purification procedure were analyzed by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (SDS-PAGE) (22).
Production of antiserum to the SpoVAD fusion protein.
Purified SpoVAD fusion protein, 0.5 ml at 1.4 mg/ml, was supplied to Pocono Rabbit Farm and Laboratory, Canadensis, PA, for the production of antiserum in rabbits. Antibody against the SpoVAD fusion protein was detected in blood drawn 2 months after the initial injection, at which time the rabbits were exsanguinated.
Western blot analyses.
Purified SpoVAD fusion protein was run on SDS-PAGE and the protein transferred to a polyvinylidene difluoride membrane (Immobilon; Millipore) in accordance with the manufacturer's instructions. This Western blot was probed with mouse anti-His tag antibody (Novagen), followed by a 1:10,000 dilution of goat anti-mouse immunoglobulin G-alkaline phosphatase conjugate (17), and the alkaline phosphatase was detected using a chemiluminescence Western blotting kit (Roche Diagnostics, Indianapolis, IN) in accordance with the manufacturer's instructions.
The purified SpoVAD fusion protein and spore extracts were analyzed on Western blots using a 1:5,000 dilution of the anti-SpoVAD serum, followed by a 1:10,000 dilution of goat anti-rabbit immunoglobulin G-alkaline phosphatase conjugate (Southern Biotech Associates, Birmingham, AL) as previously described (17).
Preparation and fractionation of spore lysates.
Spores at 6 to 10 mg (dry weight)/ml were decoated by treatment for 30 min at 70°C in 0.1 M NaCl-0.1 M NaOH-1% SDS-0.1 M dithiothreitol, and the treated spores were washed 10 times with 1.5 ml water (29). This decoating procedure removes not only the spore coat but also the spore's outer membrane proteins (3).
Approximately 7 mg of dry decoated spores was resuspended in 0.5 ml of lysis buffer (per ml: 2 mg lysozyme, 2 μg pancreatic RNase, 2 μg bovine DNase I, and 40 μg MgCl2 in 50 mM Tris-HCl [pH 7.4]-5 mM EDTA-1 mM PMSF), incubated for 5 min at 37°C, followed by 20 min on ice. Disruption of the lysozyme-treated spores was in a Mini-Bead Beater (BioSpec Products, Bartlesville, OK) with ∼2 g of 0.1-mm glass beads for 18 1-min pulses with 1 min of cooling on ice between pulses. After >80% of the spores had been disrupted, the fluid was recovered and centrifuged at 13,000 × g for 5 min at 4°C. The supernatant fluid (membranes plus soluble protein) was centrifuged at 100,000 × g for 1 h at 4°C, giving a soluble fraction (S100) and a pellet (membrane) fraction (P100) (29). The pellet fractions were dissolved in 1× SDS-PAGE loading buffer (22), and the S100 fractions were concentrated ∼10-fold in a Microcon centrifugal filter (Millipore).
For isolation of membranes from outgrowing spores (29), heat-activated (70°C, 30 min) spores (2 ml, 7 mg [dry weight]/ml) were germinated in 5 ml of 10 mM Tris-HCl (pH 8.2)-10 mM l-alanine for 1 h at 37°C, diluted into 50 ml of 2 × YT medium (29), and incubated with shaking for 2 h at 28°C, by which time more than 70% of the growing spores had a rod-like morphology. The spores were centrifuged and suspended in 1 ml of lysis buffer, and the S100 and P100 fractions were isolated as previously described (29).
RESULTS
Expression and purification of the SpoVAD fusion protein and production of antiserum.
The amino acid sequence of SpoVAD in the region from the 14th amino acid to the 318th has several putative transmembrane domains, as well as hydrophilic patches on a Hopp-Woods (19) plot. This relatively hydrophilic coding region of spoVAD was cloned, and the encoded protein was expressed in E. coli as a fusion protein with a His10 tag at the SpoVAD N terminus, giving the SpoVAD fusion protein.
Affinity purification of the pellet fraction from the IPTG-induced E. coli expression strain gave a protein of approximately the expected size of the SpoVAD fusion protein that contained a His tag (Fig. 1A and B). This protein migrated at ∼32 kDa, although the calculated mass of the SpoVAD fusion protein is ∼35 kDa. Analysis of crude extracts of the induced E. coli strain indicated that the SpoVAD fusion protein comprised ∼10% of the total E. coli protein and was all in the pellet fraction (data not shown).
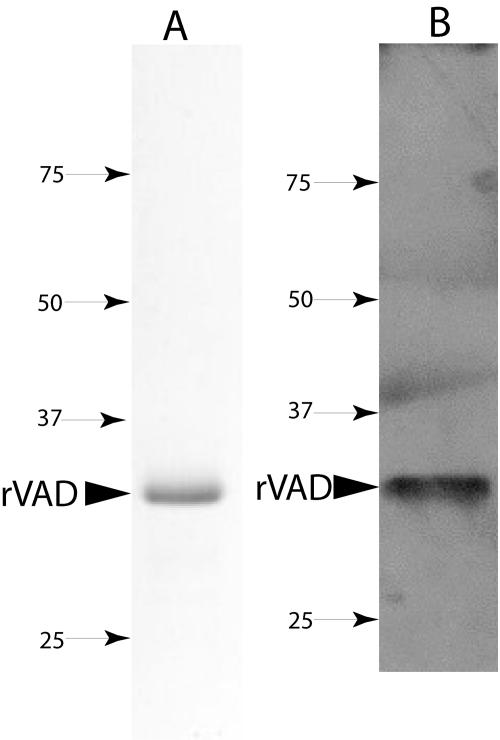
FIG. 1.
SDS-PAGE of the purified SpoVAD fusion protein (A) and detection of the SpoVAD fusion protein with anti-His tag antibody (B). (A) The SpoVAD fusion protein was purified from the pellet fraction of induced E. coli as described in Materials and Methods. An aliquot (∼10 μg) of the purified protein was run on SDS-PAGE and the protein visualized by Coomassie blue staining. (B) Approximately 0.5 μg of the SpoVAD fusion protein was run on SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and detected with anti-His tag antibody as described in Materials and Methods. In panels A and B, the arrowhead denotes the purified SpoVAD fusion protein (∼32 kDa) and numbered arrows show the migration positions of molecular mass markers (sizes are in kilodaltons). Note that the apparent bands in panel B above the 37- and 50-kDa markers do not actually fall in the lane where the sample was run and are only smudges on the X-ray film.
Purified SpoVAD fusion protein was used to immunize naive rabbits, and after confirming that preimmune serum did not react with the SpoVAD fusion protein (data not shown), the antiserum obtained from one rabbit was used at a 1:5,000 dilution without further purification. While the major bands of SpoVAD and SpoVAD fusion protein detected with the antiserum migrated at 36 and 32 kDa, respectively, at times bands of lower molecular masses were also detected. These are probably degradation products of SpoVAD, as repeated boiling and loading of the same sample increased the intensity of the smaller products on Western blot analysis (data not shown).
Detection of SpoVAD in spores.
To test the specificity of the antiserum toward SpoVAD in spores, extracts of spore coat-outer membrane protein or disrupted decoated spores from strains with (PS832) and without (PS3406) spoVA and from spores overexpressing spoVA under the control of PsspB (strain PS3411) were run on SDS-PAGE and analyzed by Western blotting (Fig. 2A and B). No immunoreactive proteins were detected in samples of either coat-outer membrane proteins or total proteins from decoated spores of the sleB spoVA mutant strain (Fig. 2A and B, lanes 1). Analysis of the coat-outer membrane fraction or total proteins from fourfold more sleB spoVA mutant strain spores than analyzed in Fig. 2 also failed to detect any immunoreactive protein (data not shown). These data indicate that the antiserum against the SpoVAD fusion protein is specific for SpoVAD.
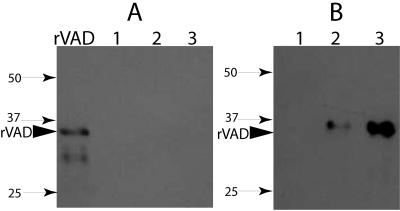
FIG. 2.
Detection of SpoVAD in the coat-outer membrane fraction (A) and disrupted decoated spores of B. subtilis strains (B). Samples of the coat-outer membrane extract (A) or the total extract from disrupted decoated spores of various strains (B) were run on SDS-PAGE and analyzed by Western blotting as described in Materials and Methods. The samples run in the various lanes were from 70 μg dry spores of strains PS3406 (sleB spoVA) (lane 1), PS832 (wild-type) (lane 2), and PS3411 (PsspB::spoVA) (lane 3). The arrowhead denotes the migration position of the SpoVAD fusion protein, and the numbered arrows denote the migration positions of molecular mass markers (sizes are in kilodaltons).
With spores of both the wild-type and spoVA-overexpressing strains, there again was no SpoVAD in the coat-outer membrane extracts (Fig. 2A, lanes 2 and 3, and Fig. 3, lane 1). However, a protein migrating at the size expected for SpoVAD (∼36 kDa) was detected in the extracts from decoated wild-type spores; this band was about fivefold more intense in extracts from decoated spores of the strain with spoVA under the control of PsspB (Fig. 2B, compare lanes 2 and 3, and see below). Previous work has shown that decoating of spores by a regimen similar to that used in the present work removes not only coat proteins but also proteins found in the spore's outer membrane, but not inner spore membrane proteins (3, 29).
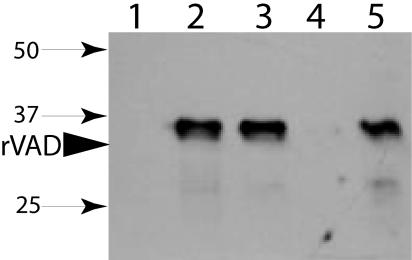
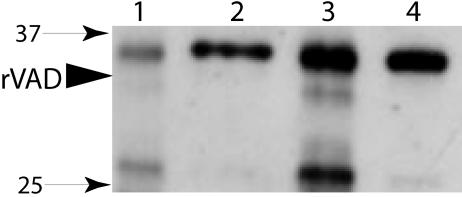
FIG. 3.
SpoVAD in different fractions of wild-type B. subtilis spores. Wild-type B. subtilis (PS832) spores were disrupted, extracted, and fractionated as described in Materials and Methods, and aliquots from 70 μg of dry spores (lanes 1, 2, and 3) or 325 μg of dry spores (lanes 4 and 5) were run on SDS-PAGE and SpoVAD detected by Western blot analysis. The samples in the various lanes were from the coat-outer membrane extract (lane 1), uncentrifuged disrupted decoated spores (lane 2), supernatant fluid from the decoated spore extract (lane 3), the S100 fraction from the decoated spore extract (lane 4), and the P100 fraction from the decoated spore extract (lane 5). The arrowhead labeled rVAD denotes the migration position of the SpoVAD fusion protein, and the numbered arrows denote the migration positions of molecular mass markers (sizes are in kilodaltons).
To locate SpoVAD more precisely within the spore, wild-type spores were decoated and lysed, the S100 and P100 fractions (see Materials and Methods) were isolated, and aliquots run on SDS-PAGE and analyzed by Western blotting (Fig. 3). As expected, there was no SpoVAD in the coat-outer membrane extract (Fig. 3, lane 1), while the protein was readily detected in the lysed decoated spores (Fig. 3, lane 2). SpoVAD was not pelleted by low-speed centrifugation (Fig. 3, compare lanes 2 and 3) but was pelleted by high-speed centrifugation, giving the P100 fraction containing the spore's inner membrane fragments (Fig. 3, lane 5). There was no immunoreactive protein in the S100 fraction containing the spore's soluble proteins (Fig. 3, lane 4). In other experiments, the coat-outer membrane extract and the S100 fraction from threefold more wild-type spores than shown in Fig. 3 also contained no SpoVAD (data not shown).
To further characterize the location of SpoVAD, the effects of detergent and salt treatments on the distribution of SpoVAD in the P100 fraction were determined (Fig. 4). Triton X-100 treatment of disrupted spore lysates markedly reduced (>80%) the amount of SpoVAD recovered in the P100 fraction, with a concomitant appearance of SpoVAD in the S100 fraction (Fig. 4, lanes 3 and 4). In contrast, treatment with a high salt concentration did not affect the location of SpoVAD (Fig. 4, lanes 5 and 6).
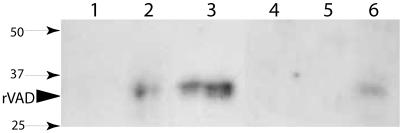
FIG. 4.
Effects of detergent and salt treatments on SpoVAD fractionation. The low-speed supernatant fluid from disrupted decoated wild-type (PS832) spores (1.25 mg initial dry weight) was incubated for 30 min on ice with no additions, with 1% Triton X-100, or with 0.5 M NaCl, followed by centrifugation at 100,000 × g to obtain S100 and P100 fractions as described in Materials and Methods. Aliquots equivalent to 70 μg of dry spores were then run on SDS-PAGE and SpoVAD detected by Western blot analysis. The samples run in the various lanes were the S100 fraction from the untreated extract (lane 1), the P100 fraction from the untreated extract (lane 2), the S100 fraction from the Triton X-100-treated extract (lane 3), the P100 fraction from the Triton X-100-treated extract (lane 4), the S100 fraction from the 0.5 M NaCl-treated extract (lane 5), and the P100 fraction from the 0.5 M NaCl-treated extract (lane 6). The arrowhead labeled rVAD denotes the migration position of the SpoVAD fusion protein, and the numbered arrows denote the migration positions of molecular mass markers (sizes are in kilodaltons). All lanes are from one gel, but lane 3 has been moved from its original position.
SpoVAD abundance in spores.
The ability to readily detect SpoVAD in spores even using a 1:5,000 dilution of crude anti-SpoVAD antiserum suggested that SpoVAD is present in spores at fairly high levels. To estimate the number of SpoVAD molecules per spore, the SpoVAD signals from different quantities of spores of the wild-type and spoVA-overexpressing strain were compared to those obtained with different amounts of purified SpoVAD fusion protein (data not shown). These comparisons indicated that there are ∼15,000 molecules of SpoVAD per wild-type spore and ∼75,000 molecules per spore overexpressing spoVA from PsspB.
SpoVAD presence and location in outgrown spores.
As spores outgrow, the dormant spore's inner membrane becomes the plasma membrane of the outgrowing spore and the outer membrane and the coat layers of the dormant spore are shed. Since at least one of the spore's nutrient germinant receptors persists in the outgrown spore's plasma membrane (29), it was of interest to determine the fate of SpoVAD during spore germination and growth. Wild-type spores and spores with overexpressed SpoVAD were germinated in l-alanine and outgrown in a rich medium, and P100 fractions were isolated and analyzed for SpoVAD (Fig. 5). This analysis showed that SpoVAD persists in the membrane of outgrown spores (Fig. 5, lanes 2 and 4), while the S100 fractions from the outgrown spores again gave no SpoVAD signal (data not shown). However, the amount of SpoVAD in outgrown wild-type spores was reduced two- to threefold over that in dormant spores (Fig. 5, compare lanes 1 and 2, noting the larger amount of spores analyzed in lane 2; data not shown), presumably due to some SpoVAD degradation. A similar two- to threefold decrease in SpoVAD was found when the P100 fractions from dormant and outgrown spores of the strain that overexpresses SpoVAD were analyzed (Fig. 5, compare lanes 3 and 4, noting the larger amount of spores analyzed in lane 4; data not shown).
FIG. 5.
SpoVAD in outgrowing spores. P100 fractions from dormant and outgrown spores of strains PS832 (wild type) and PS3411 (overexpressing spoVA) were prepared as described in Materials and Methods. Samples of the P100 fractions from dry dormant spores and outgrown spores were run on SDS-PAGE, and SpoVAD was detected by Western blot analysis. The P100 samples run in the various lanes were from 325 μg of dormant wild-type spores (lane 1), outgrown wild-type spores from 1.25 mg of initial dry dormant spores (lane 2), 325 μg of dormant spores overexpressing SpoVA (lane 3), and outgrown spores overexpressing SpoVA from 625 μg of initial dry dormant spores (lane 4). The arrowhead labeled rVAD denotes the migration position of the SpoVAD fusion protein, and the numbered arrows denote the migration positions of molecular mass markers (sizes are in kilodaltons).
DISCUSSION
The spoVA operon encodes six proteins, each of which has at least several putative membrane-spanning domains, and together they have been suggested to be involved in DPA transport into and out of the spore (13, 38, 40). In the present study, Western blot analysis was used to localize SpoVAD in spore fractions using an antiserum raised against a SpoVAD fusion protein. The results show that SpoVAD is located in the spore's inner membrane but not in the outer membrane. The presence of SpoVAD or any SpoVA protein in a membrane is not surprising given the likely presence of multiple transmembrane domains in these proteins. Since spoVA is transcribed only in the developing forespore and not in the mother cell (12, 13, 31), the most likely location of SpoVA proteins including SpoVAD would be the inner membrane that is derived from the forespore rather than the outer membrane derived from the mother cell. However, this was not a foregone conclusion, as the close apposition of both membranes at some period in sporulation might allow proteins made in the forespore to move to the outer membrane.
The solubilization of SpoVAD on treatment of spore lysates with Triton X-100 but not with 0.5 M NaCl indicates that SpoVAD is an integral membrane protein. This is consistent with a hydropathy analysis of SpoVAD that suggests that this protein has about seven membrane-spanning domains. With 15,000 molecules per spore, SpoVAD is a moderately abundant protein. We do not know if all SpoVA proteins are as abundant as SpoVAD, although this seems likely since the SpoVA proteins are encoded in one operon. That the spore's inner membrane may have a rather high protein content has been suggested previously (11). Perhaps the high protein content in this membrane is important in maintaining the lipids in this membrane in their relatively immobile state in the dormant spore (8). A high protein content in the dormant spore's inner membrane may also be important for the successful expansion of the inner membrane during spore germination that takes place without new membrane lipid synthesis (8).
The localization of SpoVAD, and by inference other SpoVA proteins, in the dormant spore's inner membrane is certainly consistent with the SpoVA proteins being involved in DPA movement across this membrane (13, 38, 40). However, the lack of SpoVAD in the outer membrane indicates that SpoVA proteins are not likely to be involved in DPA transport across this membrane. In germinating spores, this may not be a major problem, since the outer membrane may not be a significant permeability barrier in the dormant spore (9, 10). If this is indeed true, SpoVA proteins may allow rapid DPA exit from the spore core upon germination, the DPA concentration will rise in the cortex-outer membrane-coat region, and then the DPA will slowly diffuse into the surrounding medium. This slow loss of DPA from outer spore layers during germination may be important, since it may facilitate the activation of the cortex lytic enzyme CwlJ by Ca2+-DPA (35). Other factors that might modulate DPA movement out of the spore in germination may be the state of the inner and outer membrane lipids. Inner membrane lipids appear to be largely immobile in the dormant spore, and while the precise physical state of this membrane is not known, it has exceedingly low passive permeability to small molecules, even to molecules as small as uncharged methylamine and perhaps even water (6, 7, 8, 37, 42). In contrast, the outer membrane does not appear to have these permeability constraints, if this membrane is a permeability barrier at all.
While the presence of SpoVA proteins exclusively in the spore's inner membrane is consistent with these proteins playing a key role in DPA exit from the spore core during germination, this does not explain the uptake of DPA by the forespore in sporulation. DPA is synthesized in the mother cell compartment and must cross both the outer and the inner forespore membranes to reach the spore core (13), and the outer membrane is most likely a functional membrane in the developing forespore (3, 9, 31). How then does DPA cross the outer forespore membrane? We cannot answer this question, but possible explanations include the following: (i) some combination of SpoVA proteins that does not include SpoVAD is located at least in part in the spore's outer membrane, and this subcomplex of SpoVA proteins is involved in DPA transport across this membrane; (ii) diffusion of DPA, perhaps as Ca2+-DPA, across the outer membrane may be sufficiently fast for DPA uptake; or (iii) there is another system entirely for DPA transport across the outer membrane.
Another observation consistent with the involvement of SpoVA proteins in DPA movement across the inner membrane in spore germination is the relatively high level of SpoVAD, and thus presumably all SpoVA proteins. This high level of SpoVA proteins may be important in facilitating the movement of the enormous amount of DPA (∼20% of the core's dry weight) that must traverse the inner membrane during spore germination in only 1 to 2 min (18, 39). Since 1 mg of dry spores has ∼109 spores and 110 μg of DPA (38), if we assume that there is one SpoVAD molecule per DPA channel, then during spore germination one channel would have to move ∼25,000 molecules of DPA/min (18, 35, 39). This value is well within the capacity of channels and/or pumps to facilitate the movement of molecules across membranes (21).
It is also notable that the spore's level of SpoVAD, and by inference all SpoVA proteins, is much higher than that of the nutrient germinant receptors. While the inner membrane of a B. subtilis spore has ∼15,000 molecules of SpoVAD, there are only ∼25 molecules of the GerB nutrient germinant receptor (29), likely not much more than this of the GerA receptor, and perhaps even less of the GerK receptor (2, 28). This high ratio of SpoVA proteins to germinant receptors suggests that there could be a huge amplification of the nutrient germinant signal if the activation of the rare germinant receptors opens a gated DPA efflux channel composed of abundant SpoVA proteins. Preliminary data obtained by yeast two-hybrid analysis have suggested that GerA and SpoVAC physically interact (41), and if this is confirmed by more detailed studies, it would provide further support for a role for SpoVA proteins in DPA transport across the membrane.
The high level of the SpoVAD protein in the spore's inner membrane was increased about fivefold when the spoVA operon was expressed under the control of PsspB. This increase in the SpoVAD level, and presumably those of the other SpoVA proteins, did not alter DPA uptake or release in sporulation and nutrient-mediated spore germination, respectively (41), suggesting that the amounts of SpoVA proteins are normally not rate limiting for these processes.
Finally, given the relatively high level of SpoVAD in the spore's inner membrane, it may be possible to localize SpoVAD in this membrane by immunoelectron microscopy. While this analysis may show that SpoVAD is uniformly distributed in the membrane, it is also possible that SpoVA is located in one region of the inner membrane, much as is the case with chemoreceptors and other proteins in a number of bacteria (1, 23, 24).
Acknowledgments
This work was supported by grants from the National Institutes of Health (GM19698) and the Army Research Office.
We thank F. Tovar-Rojo for the construction of strains and B. Setlow for the protocol for inner membrane isolation.
REFERENCES
- 1.Angela, C. M., N. Usha, J. P. Armitage, and J. R. Maddock. 2003. Polar localization of CheA2 in Rhodobacter sphaeroides requires specific Che homologs. J. Bacteriol. 185:4667-4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black, E. P., K. Koziol-Dube, D. Guan, J. Wei, B. Setlow, D. E. Cortezzo, D. G. Hoover, and P. Setlow. Factors influencing the germination of Bacillus subtilis spores via the activation of nutrient receptors by high pressure. Appl. Environ. Microbiol., in press. [DOI] [PMC free article] [PubMed]
- 3.Buchanan, C. E., and S. L. Neyman. 1986. Correlation of penicillin-binding protein composition with different functions of two membranes in Bacillus subtilis forespores. J. Bacteriol. 165:498-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cabrera-Martinez, R.-M., F. Tovar-Rojo, V. R. Vepachedu, and P. Setlow. 2003. Effects of overexpression of nutrient receptors on germination of spores of Bacillus subtilis. J. Bacteriol. 185:2457-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connors, M. J., J. M. Mason, and P. Setlow. 1986. Cloning and nucleotide sequence of genes for three small, acid-soluble proteins of Bacillus subtilis spores. J. Bacteriol. 166:417-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cortezzo, D. E., K. Koziol-Dube, B. Setlow, and P. Setlow. 2004. Treatment with oxidizing agents damages the inner membrane of spores of Bacillus subtilis and sensitizes spores to subsequent stress. J. Appl. Microbiol. 97:838-852. [DOI] [PubMed] [Google Scholar]
- 7.Cortezzo, D. E., and P. Setlow. 2005. Analysis of factors that influence the sensitivity of spores of Bacillus subtilis to DNA damaging chemicals. J. Appl. Microbiol. 98:606-617. [DOI] [PubMed] [Google Scholar]
- 8.Cowan, A. E., E. M. Olivastro, D. E. Koppel, C. A. Loshon, B. Setlow, and P. Setlow. 2004. Lipids in the inner membrane of dormant spores of Bacillus species are largely immobile. Proc. Natl. Acad. Sci. USA 101:7733-7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Driks, A., and P. Setlow. 1999. Morphogenesis and properties of the bacterial spore, p. 191-218. In Y. V. Brun and L. J. Shimkets (ed.), Prokaryotic development. American Society for Microbiology, Washington, D.C.
- 10.Driks, A. 2002. Proteins of the spore core and coat, p. 527-536. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, D.C.
- 11.Ellar, D. J. 1978. Spore specific structures and their functions. Symp. Soc. Gen. Microbiol. 28:295-325. [Google Scholar]
- 12.Errington, J., and J. Mandelstam. 1986. Use of a lacZ gene fusion to determine the dependence pattern and the spore compartment expression of sporulation operon spoVA in spo mutants of Bacillus subtilis. J. Gen. Microbiol. 132:2977-2985. [DOI] [PubMed] [Google Scholar]
- 13.Errington, J. 1993. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol. Rev. 57:1-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fort, P., and J. Errington. 1985. Nucleotide sequence and complementation analysis of a polycistronic sporulation operon, spoVA, in Bacillus subtilis. J. Gen. Microbiol. 131:1091-1105. [DOI] [PubMed] [Google Scholar]
- 15.Gerhardt, P., and R. E. Marquis. 1989. Spore thermoresistance mechanisms, p. 43-63. In I. Smith, R. A. Slepecky, and P. Setlow (ed.), Regulation of prokaryotic development. American Society for Microbiology, Washington, D.C.
- 16.Guerot-Fleury, A.-M., K. Shazand, N. Frandsen and P. Stragier. 1985. Antibiotic resistance cassettes for Bacillus subtilis. Gene 167:335-336. [DOI] [PubMed] [Google Scholar]
- 17.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 18.Hashimoto, T., W. R. Frieben, and S. F. Conti. 1969. Microgermination of Bacillus cereus spores. J. Bacteriol. 100:1385-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hopp, T. P., and K. R. Woods. 1981. Prediction of protein antigenic determinants from amino acid sequences. Proc. Natl. Acad. Sci. USA 78:3824-3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hudson, K. D., B. M. Corfe, E. H. Kemp, I. M. Feavers, P. J. Coote, and A. Moir. 2001. Localization of GerAA and GerAC germination proteins in the Bacillus subtilis spore. J. Bacteriol. 183:4317-4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kung, C., and P. Blount. 2004. Channels in microbes: so many holes to fill. Mol. Microbiol. 53:373-380. [DOI] [PubMed] [Google Scholar]
- 22.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 23.Lai, E. M., N. Usha, N. D. Phadke, and J. R. Maddock. 2004. Proteomic screening and identification of differentially distributed membrane proteins in Escherichia coli. Mol. Microbiol. 52:1029-1044. [DOI] [PubMed] [Google Scholar]
- 24.Maddock, J. R., M. R. Alley, and L. Shapiro. 1993. Polarized cells and polar actions. J. Bacteriol. 175:7125-7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moldover, B., P. J. Piggot, and M. D. Yudkin. 1991. Identification of the promoter and the transcriptional start site of the spoVA operon of Bacillus subtilis and Bacillus licheniformis. J. Gen. Microbiol. 137:527-531. [DOI] [PubMed] [Google Scholar]
- 26.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination, and outgrowth, p. 391-450. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley & Sons, Ltd., Chichester, England.
- 27.Paidhungat, M., B. Setlow, A. Driks, and P. Setlow. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J. Bacteriol. 182:5505-5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paidhungat, M., and P. Setlow. 2000. Role of Ger proteins in nutrient and nonnutrient triggering of spore germination in Bacillus subtilis. J. Bacteriol. 182:2513-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paidhungat, M., and P. Setlow. 2001. Localization of a germinant receptor protein (GerBA) to the inner membrane of Bacillus subtilis spores. J. Bacteriol. 183:3982-3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paidhungat, M., and P. Setlow. 2002. Spore germination and outgrowth, p. 537-548. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, D.C.
- 31.Piggot, P. J., and R. Losick. 2002. Sporulation genes and intercompartmental regulation, p. 483-518. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, D.C.
- 32.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 33.Savva, D., and J. Mandelstam. 1984. Cloning of the Bacillus subtilis spoIIA and spoVA genes in phage 105DI:lt. J. Gen. Microbiol. 130:2137-2145. [DOI] [PubMed] [Google Scholar]
- 34.Savva, D., and J. Mandelstam. 1986. Synthesis of spoIIA and spoVA RNA in Bacillus subtilis. J. Gen. Microbiol. 132:3005-3011. [DOI] [PubMed] [Google Scholar]
- 35.Setlow, P. 2003. Spore germination. Curr. Opin. Microbiol. 6:550-556. [DOI] [PubMed] [Google Scholar]
- 36.Sun, D., P. Stragier, and P. Setlow. 1989. Identification of a new σ-factor involved in compartmentalized gene expression during sporulation in Bacillus subtilis. Genes Dev. 3:141-149. [DOI] [PubMed] [Google Scholar]
- 37.Swerdlow, B. M., B. Setlow, and P. Setlow. 1981. Levels of H+ and other monovalent cations in dormant and germinated spores of Bacillus megaterium. J. Bacteriol. 148:20-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tovar-Rojo, F., M. Chander, B. Setlow, and P. Setlow. 2002. The products of the spoVA operon are involved in dipicolinic acid uptake into developing spores of Bacillus subtilis. J. Bacteriol. 184:584-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vary, J. C., and H. O. Halvorson. 1965. Kinetics of germination of Bacillus spores. J. Bacteriol. 89:1340-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vepachedu, V. R., and P. Setlow. 2004. Analysis of germination of spores of Bacillus subtilis with temperature sensitive spo mutations in the spoVA operon. FEMS Microbiol. Lett. 239:71-77. [DOI] [PubMed] [Google Scholar]
- 41.Vepachedu, V. R., and P. Setlow. 2005. Unpublished results.
- 42.Westphal, A. J., P. B. Price, T. J. Leighton, and K. E. Wheeler. 2003. Kinetics of size changes of individual Bacillus thuringiensis spores in response to changes in relative humidity. Proc. Natl. Acad. Sci. USA 100:3461-3466. [DOI] [PMC free article] [PubMed] [Google Scholar]