Abstract
The two-component BvrS/BvrR system is essential for Brucella abortus virulence. It was shown previously that its dysfunction abrogates expression of some major outer membrane proteins and increases bactericidal peptide sensitivity. Here, we report that BvrS/BvrR mutants have increased surface hydrophobicity and susceptibility to killing by nonimmune serum. The bvrS and bvrR mutant lipopolysaccharides (LPSs) bound more polymyxin B, chimeras constructed with bvrS mutant cells and parental LPS showed augmented polymyxin B resistance, and, conversely, parental cells and bvrS mutant LPS chimeras were more sensitive and displayed polymyxin B-characteristic outer membrane lesions, implicating LPS as being responsible for the phenotype of the BvrS/BvrR mutants. No qualitative or quantitative changes were detected in other envelope and outer membrane components examined: periplasmic β(1-2) glucans, native hapten polysaccharide, and phospholipids. The LPS of the mutants was similar to parental LPS in O-polysaccharide polymerization and fine structure but showed both increased underacylated lipid A species and higher acyl-chain fluidity that correlated with polymyxin B binding. These lipid A changes did not alter LPS cytokine induction, showing that in contrast to other gram-negative pathogens, recognition by innate immune receptors is not decreased by these changes in LPS structure. Transcription of Brucella genes required for incorporating long acyl chains into lipid A (acpXL and lpxXL) or implicated in lipid A acylation control (bacA) was not affected. We propose that in Brucella the outer membrane homeostasis depends on the functioning of BvrS/BvrR. Accordingly, disruption of BvrS/BvrR damages the outer membrane, thus contributing to the severe attenuation manifested by bvrS and bvrR mutants.
Bacteria are able to survive in different environments by modulating the expression of their genes. This attribute is often accomplished by two-component transduction systems that assemble both sensors and regulators (46). Brucella organisms are intracellular α-Proteobacteria found in mammalian body fluids and within mammalian cells (52). Although genome sequencing has revealed 21 putative two-component regulatory systems in the Brucella genus (13, 40, 56), one of the best-characterized two-component systems involved in virulence is the BvrS/BvrR system. Indeed, the bvrS and bvrR mutants are avirulent in mice (63), show reduced invasiveness to epithelial cells and macrophages, and are incapable of inhibiting lysosome fusion and replicating intracellularly (42, 63). Dysfunction of BvrS and BvrR also diminishes the characteristic resistance of Brucella to bactericidal cationic peptides and increases its permeability to surfactants (63). Since the virulence of Brucella depends in part on its outer membrane (OM) properties (20, 44, 45, 55), we proposed that the BvrS/BvrR system plays a role in the homeostasis of the bacterial surface as well as in setting up the structures required for parasitism (42, 51). The B. abortus BvrS/BvrR system regulates transcription of at least two major outer membrane proteins (Omps) (30): a previously undescribed Omp (Omp22 or Omp3b) and Omp25 (also named Omp3a), which has been implicated in virulence (15, 16, 17). All other known Omps expressed in virulent Brucella abortus are detected to a similar level in the bvrS and bvrR mutants and the wild-type (wt) bacteria (30). However, although they are slightly attenuated, B. abortus omp25 and omp22 mutants do not show the high level of attenuation and sensitivity to bactericidal peptides displayed by the bvrS and bvrR mutants (15; López-Goñi et al., unpublished results). Therefore, it seems that other factors linked to virulence are regulated by the BvrS/BvrR system. In this study, we have investigated non-protein envelope molecules in the bvrS and bvrR mutants and discovered modifications in their lipopolysaccharide (LPS) lipid A moieties. We also found that the overall surface hydrophobicity of the envelope was altered and that recognition by complement in the absence of antibodies was enhanced. These results give clear new insights to explain the defective virulent phenotype of bvrS and bvrR mutants.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
B. abortus 2308 (parental wild-type virulent strain), B. abortus 2.13 (bvrS::Tn5 mutant, avirulent), B. abortus 65.21 (bvrR::Tn5 mutant, avirulent) and B. abortus 65.21p (bvrR::Tn5 mutant reconstituted, strain 65.21 with plasmid pBBR1MCS-4 bvrR+) (30, 63) were routinely grown in standard tryptic soy broth or agar, either plain or with the appropriate antibiotics. For extraction of cell envelope components, bacteria were propagated in a 15-liter Biostat fermentor (B. Braun Melsungen AG, Leinfelden, Germany) as described elsewhere (1). B. abortus per, wa**, and manBcore mutants are Tn5 mutants carrying rough LPSs with a complete core and a defective inner core and outer core, respectively (49).
Extraction and purification of Brucella cell envelope components.
Free lipids, LPSs, and polysaccharides were purified from dry bacteria or from OM fragments (23) following standard methods. LPSs were obtained from the phenol phase from water-phenol extracts (38) and extensively purified (1, 53), and free lipids were removed by extraction with chloroform-methanol (68), to yield preparations composed of smooth (80%)- and rough (20%)-type LPSs (21). Lipid A's were obtained by LPS hydrolysis in 1% sodium-dodecyl-sulfate (SDS), 10 mM sodium acetate (pH 4.5) at 100°C for 1 h; repeatedly washed first with ethanol-20 mM HCl and then with water; and freeze-dried (28). O-polysaccharides and core oligosaccharides were extracted by mild acid hydrolysis (1), and, after removal of the insoluble lipid A, they were separated on a Bio-Gel P2 (Bio-Rad) column (21). Native hapten (NH) polysaccharide, cyclic β(1-2) glucans, and total free lipids (mainly phospholipids) were extracted as described before (1, 4, 21).
Characterization of Brucella cell envelope components.
LPSs were analyzed by unidimensional polyacrylamide gel electrophoresis with SDS (36), deoxycholate (35), or Tricine-SDS (39, 61) or by two-dimensional gel electrophoresis (30) and stained by the periodate-alkaline silver method (66). The level of 3-deoxy-d-manno-2-octulosonic acid (Kdo) was measured by the thiobarbituric acid method (1). Western blots (49) were developed with antibodies from Brucella-infected bovines or rabbits immunized with B. abortus per, wa**, or manBcore mutants (49). Monoclonal antibodies against O-polysaccharide epitopes (anti-C, A76/07F09/B10; and anti-C/Y, A53/18H08/A02 and 04F9), core oligosaccharide (anti-R, A68/24G12/A08; anti-outer core, Baro-1; and anti-inner core, Baro-2) and lipid A (anti-lipid A, Bala-1, Bala-5, and Bala-13) (5, 21) were also used. O-polysaccharides, NH-polysaccharides, cyclic β(1-2) glucans, core oligosaccharides, lipid A's, and total free lipids were analyzed by high-performance thin-layer chromatography (HPTLC) on precoated silica gel plates (E. Merck, Darmstadt, Germany) (1, 21, 65). To determine the degree of lipid A acylation, samples were dissolved in chloroform-methanol-ammonium-water (25:14:1:2) and chromatographed on HPTLC plates. Plates were soaked in methanol-sulfuric acid and developed as described before (49). Lipid A preparations of Escherichia coli W3110 MLK3 (W3110 htrB1::Tn10, hexaacylated), W3110 MLK1067 (W3110 msbB::Ωcam, pentaacylated), and W3110 MLK986 (MLK53 msbB::Ωcam, tetraacylated) were used as standards (10). The structure of polysaccharide molecules was determined by 13C-nuclear magnetic resonance (NMR) and 1H-NMR in an Avance Bruker 400 Ultrashield spectrometer (Bruker Analytische Messtechnik) at 400 MHz. The amounts of O- and NH-polysaccharides were measured in a gel immunodiffusion test (1).
Action of polycationic bactericidal peptides on LPS chimeras and binding to LPS.
To establish the role of the various LPSs in the susceptibility to these agents, chimeras were constructed by inserting heterologous LPS (previously homogenized by sonication and sterilized in the autoclave) in the Brucella OMs (20). Fresh B. abortus cells were mixed with the same volume of heterologous LPS, and the mixtures were incubated for 18 h at 40°C. Cells were washed repeatedly to remove unbound LPS, resuspended in buffer, and tested immediately. Controls treated with plain broth were run in parallel. For the sensitivity test, chimeras and controls were incubated for 20 min at 37°C with polymyxin B or lactoferricin and CFU were counted on tryptic soy agar after 3 days at 37°C. Assays were done in triplicate, and the percentage of CFU was calculated with respect to that of controls incubated with no peptide. Microscopic observations were performed using a Hitachi 1100 transmission electron microscope (Hitachi Scientific Instruments, Mountain View, CA) operating at 100 kV (20).
To assess bactericidal peptide binding, LPSs were dispersed by sonication in 2.5 mM HEPES (pH 7.2) at 4 nmol Kdo/ml and incubated with different concentrations of dansyl-polymyxin B (62). The fluorescence was measured in continuous 400- to 600-nm scans in an LS-50 fluorimeter (Perkin-Elmer Ltd., Beaconsfield, England) (excitation, 340 nm; 2.5-nm slit width for both windows) at room temperature. The results were expressed as relative fluorescence units (RFU) of the maximum of the emission peak (from 470 to 490 nm, depending on the blue shift associated with the intensity of binding).
Determination of the acyl-chain fluidity of LPS.
The transition of the acyl chains of LPS from a well-ordered state (gel phase) to a fluid state (liquid crystalline phase) at a lipid-specific temperature was determined by Fourier transform infrared spectroscopy. A specific vibrational band, the symmetric stretching vibration of the methylene groups νs(CH2) around 2,850 cm−1, was analyzed since its peak position is a measure of the state of order (fluidity) of the acyl chains (6). Measurements were performed in a Bruker IFS 55 apparatus (Bruker, Karlsruhe, Germany) as described previously (6). To ensure homogeneity, LPS suspensions were prepared in 2.5 mM HEPES (pH 7.2) at room temperature, incubated at 56°C for 15 min, stirred, and cooled to 4°C. This heating/cooling step was repeated three times, and the suspensions were stored at 4°C for several hours before analysis. LPS suspensions (water content, >90%) were analyzed in CaF2 cuvettes with 12.5-μm Teflon spacers, and for each measurement, 50 interferograms were accumulated, Fourier transformed, and converted to absorbance spectra. The measurements were obtained in continuous heating scans (2°C/min) between 10°C and 60°C. To test the effect of polymyxin B, the experiments were performed in the presence of this lipopeptide at various LPS/polymyxin B ratios.
Aggregate structure of LPS.
The aggregate structure of lipid A's in the absence and presence of polymyxin B was determined by synchrotron radiation X-ray diffraction at the European Molecular Biology Laboratory (EMBL) outstation at the Hamburg synchrotron radiation facility HASYLAB using the double-focusing monochromator-mirror camera X33 (34). X-ray diffraction patterns, obtained with exposure times of 2 min using a linear gas proportional detector with delay line readout (22), were evaluated according to previously described procedures (7). These allow assigning the spacing ratios to defined three-dimensional aggregate structures, from which the conformations of the individual molecules are deduced. In the diffraction patterns presented here, the logarithm of the scattering intensity, log I(s), is plotted versus the scattering vector s = 2 sin θ/λ (2θ = scattering angle, λ = wavelength = 0.15 nm), and the spacings, d, are calculated according to d = 1/s.
Cell-surface hydrophobicity.
Exponentially growing bacteria were washed twice with 0.8 mM MgSO4, 53 mM urea in phosphate buffer (pH 7.0) and resuspended in this solution at an optical density at 470 nm of 1.0. A volume of this suspension was extracted with either xylene or chloroform, and the decrease in optical density of the aqueous phase was determined (59).
RT-PCR.
Total RNA was isolated from B. abortus (RNAeasy kit; QIAGEN S.A., Courtaboeuf, France), contaminating DNA was removed (DNA-free kit, Ambion Ltd., United Kingdom), and 1 μg of RNA was reverse transcribed into cDNA by using SuperScript III reverse transcriptase (RT; Invitrogen Life Technologies) using the random hexamers of the kit. Then, 2 μl of each RT reaction mixture was subjected to PCR using Immolase DNA polymerase (Bioline). Sense and antisense oligonucleotide primers for RT-PCR-amplified genes were designed using the Brucella melitensis genomic DNA sequence information obtained from the B. melitensis Genome Database (12) and with the Primer3 program (60). Primers, synthesized by Sigma-Genosys Ltd., were as follows: 5′-TAGAGCATGTTTCCGAAAA-3′ and 5′-AGCGCAAGACCGGCTTTCTT-3′ for open reading frame (ORF) BMEI1115 (lpxXL gene), 5′-TCGCCGAAACCAGTGAAATC-3′ and 5′-CGGAACCTTGCCTTCGTTTA-3′ for ORF BMEI1111 (acpXL gene), 5′-CGCGTCAAGAAAATCGTTGT-3′ and 5′-ACGCCGAATTCTTCTTCAAA-3′ for ORF BMEI1475 (acpXL gene), and 5′-GGCCGATTTTGCAGGCATTG-3′ and 5′-TCGGCAGCGTGCTGACCTTT-3′ for ORF BMEI1553 (bacA gene). PCR products were analyzed on a 1.0 to 1.2% agarose gels. Positive controls were performed with genomic DNA, and negative controls were performed with RNA that had not been subjected to reverse transcription.
LPS cytokine induction.
To determine the level of biological activity of the LPSs, murine C57BL/6 bone marrow-derived macrophages were tested for cytokine production (69). Briefly, macrophages were allowed to adhere overnight in 96-well plates under a 7% CO2 atmosphere at 37°C. Shigella flexneri serotype 5a (from P. Sansonetti, Institute Pasteur, France) and B. abortus LPSs were added to the macrophages in 200 μl of medium. Supernatants were collected after 48 h and frozen until tested by enzyme-linked immunosorbent assay for tumor necrosis factor alpha (TNF-α) (R&D Systems, Minneapolis, MN), interleukin-12p40 (IL-12p40) (R&D Systems), and IL-10 (BD Biosciences, San Diego, CA), following the manufacturer's instructions.
Sensitivity to the bactericidal action of nonimmune serum and LPS anticomplementary activity.
Exponentially growing bacteria were adjusted to 104 CFU/ml in saline and dispensed in duplicate in microtiter plates (45 μl per well) containing fresh normal calf or human serum (90 μl/well). After 30 or 90 min of incubation at 37°C, 200 μl/well of brain heart infusion broth was dispensed and mixed with the bacterial suspension and 100 μl was plated on tryptic soy agar. Results were expressed as the percentage CFU with respect to controls performed with serum decomplemented at 56°C for 30 min. To assess complement consumption, LPSs were homogenized at 250 μg/ml in 0.5% triethylamine in Veronal-Ca-Mg buffer (pH 7.5) as described for the acyl-chain fluidity analysis. Aliquots (250 μl) of these stocks were mixed with an equal volume of guinea pig complement (BioMerieux, Marcy l'Etoile, France), diluted at 1:50 in the same buffer, and incubated for 45 min at 37°C with gentle stirring. To measure the amount of complement consumed, 500 μl of 2% sheep erythrocytes and 0.33% anti-sheep erythrocyte immunoglobulin G (BioMerieux) in Veronal-Ca-Mg buffer was added, incubation was carried out for 45 min, and the hemolysis was determined at 540 nm after removal of cells and debris by centrifugation.
RESULTS
Disruption of the bvrS and bvrR genes has no effect on phospholipid profile or on cyclic β(1-2) glucan and native hapten polysaccharide contents.
Relatively large quantities of phosphatidylcholine, in addition to phosphatidylethanolamine and other phospholipids, are characteristic of α-Proteobacteria, and it has been hypothesized that the unusual stability of the Brucella OM with regard to the action of surfactants and polycationic peptides may be linked to the properties of phosphatidylcholine (52). Therefore, it was of interest to determine the presence of phosphatidylcholine and other phospholipids in the cell envelopes and OM fragments of the bvrS and bvrR mutants. No differences were observed between the phospholipid profiles of the parental wild-type (wt) and mutant brucellae (data not shown). Likewise, cells of α-Proteobacteria contain large amounts of periplasmic cyclic β(1-2) glucans, which have been implicated in Brucella virulence (8). In addition, smooth Brucella species contain NH polysaccharide, an OM component structurally related to the O-type polysaccharide, and antibodies precipitating NH are a marker of infection in animal brucellosis (14). Since it has been demonstrated that the Sinorhizobium meliloti ExoS/ChvI system, homologous to BvrS/BvrR (43), regulates production of extracellular polysaccharides necessary for symbiosis (9), we also investigated cyclic β(1-2) glucans and NH production in bvrS and bvrR mutants. Comparative quantifications of these molecules did not reveal significant differences compared to the wt B. abortus strain (data not shown).
The LPSs of bvrS and bvrR mutants show enhanced binding of bactericidal cationic peptides.
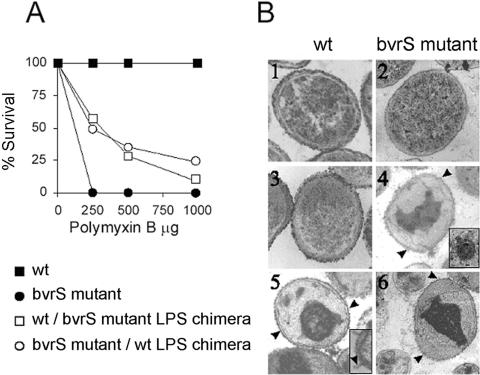
It is well known that LPS contains some of the main targets for the initial binding of polycationic bactericidal peptides to the OM (reviewed in reference 54). Accordingly, the LPSs from wt and mutant strains were probed with one cationic peptide, dansyl-polymyxin B. LPS from bvrS or bvrR mutant strains bound more dansyl-polymyxin B than LPS from the parental wt strain (Fig. 1A). The relevance of such differences was assessed using chimeras carrying heterologous LPS in the OM. It was shown previously using Brucella and Salmonella that such chimeras exhibit the permeability and bactericidal cationic peptide sensitivity of the donor bacteria (20). As illustrated in Fig. 2, chimeras of parental wt and bvrS mutant strains containing the heterologous LPS displayed different sensitivities to polymyxin B. Insertion of parental wt B. abortus 2308 LPS into the OM of the bvrS mutant resulted in chimeras more resistant to polymyxin B than the bvrS mutant. In contrast, the bvrS mutant LPS inserted into the OM of the parental wt B. abortus strain made the resulting chimeras more sensitive to this antibiotic than the wt strain (Fig. 2A). Similar results were obtained with the microbicidal cationic peptide lactoferricin B (data not shown). Transmission electron microscopy of LPS chimeras treated with polymyxin B corroborated the sensitivity assays. As reported before, polymyxin B did not affect the ultrastructure of the wt B. abortus strain (20, 45, 68), whereas the B. abortus wt 2308 chimeras containing LPS of the bvrS mutant showed morphological alterations similar to those demonstrated by the bvrS mutant (Fig. 2B). The alterations, characterized by vacuole formation, development of OM blebs, detachment of the inner membrane and DNA condensation, were those typical of the action of polymyxin B on gram-negative bacteria (20). Thus, although the presence of heterologous LPS did not completely change the sensitivity of the chimeric strains due to the presence of endogenous LPS, these experiments directly demonstrated that LPS is responsible for the increased binding of polymyxin B by bvrS and bvrR mutant strains.
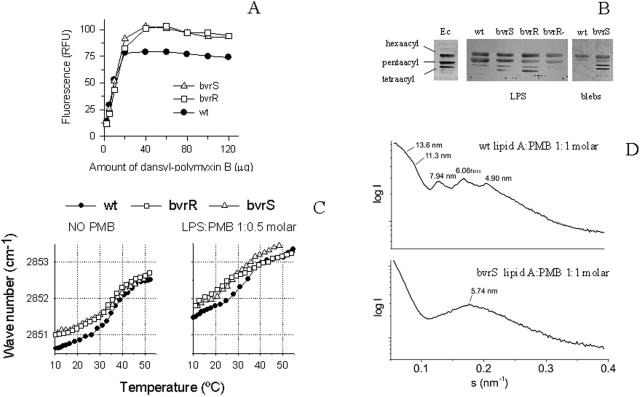
FIG. 1.
The LPS of B. abortus bvrS and bvrR mutants shows higher affinity for bactericidal cationic peptides and contains an altered lipid A. (A) Binding of dansyl-polymyxin B by LPS from the wt and bvrS and bvrR mutant strains. LPS suspensions were incubated with increasing amounts of dansyl-polymyxin B, and the fluorescence (RFU) at 480 nm was determined. (B) HPTLC analysis of lipid A acylation in the wt strain, bvrS and bvrR mutant strains, and reconstituted bvrR+ strain. Lipid A was extracted from purified LPS preparations or from OM fragments (blebs). Lane Ec contained lipid A of E. coli MLK53 controls: hexa-acylated, penta-acylated, and tetra-acylated. (C) Maximum of the peak position of the symmetric stretching vibration νs(CH2) versus temperature for the LPSs of the wt and bvrS and bvrR mutant strains in the absence or in the presence of polymyxin B. (D) Synchrotron radiation small-angle X-ray diffraction patterns of lipid A wt and bvrS mutant strain incubated with polymyxin B obtained at 40°C and high water content (90%). The logarithm of the scattering intensity (log I) is plotted versus the scattering vector s (= 1/d, where d = spacing).
FIG. 2.
Effect of polymyxin B on the viability of B. abortus chimeras. (A) wt and chimeric bacteria were exposed to increasing amounts of polymyxin B for 60 min at 37°C, and viability was assessed by plating on tryptic soy agar plates. Each experiment was run in triplicate, and the standard error at each point was less than 5% of the value. (B) Electron microscopy of polymyxin B-treated bacteria. Panels 1 and 2, untreated controls; 3 to 6, bacteria treated with 100 units of polymyxin B. The B. abortus wt strain (panels 1 and 3), bvrS mutant (panels 2 and 4), wt strain/bvrS mutant LPS chimera (panel 5), and bvrS mutant/wt strain LPS chimera (panel 6) are shown. The inserts in panels 4 and 5 show (×150,000) rolling of the inner membrane and blebbing. Arrowheads in panels 4, 5, and 6 show OM blebbing.
The lipid A of the LPSs of bvrS and bvrR mutants, but not the polysaccharide moiety, is modified and exhibits altered acylation, acyl-chain fluidity, and aggregate structure.
The peptide-binding experiments suggested that BvrS/BvrR dysfunction caused LPS structural changes. However, wt and mutant strains contained similar amounts of LPS and the LPS profiles obtained after SDS-, Tricine-SDS-, deoxycholate-, or two-dimensional polyacrylamide gel electrophoresis were those typical of smooth brucellae and similar for both mutants and the wt strain (not shown). No differences were observed either when these three LPSs were tested by Western blotting with immune sera from infected bovines or rabbits immunized with rough mutants with progressive LPS core deficiencies or with monoclonal antibodies against C, C/Y, LPS core, outer core, or inner core epitopes (data not shown). Absence of differences in the fine structure of the LPS sugar moiety was verified further. First, 13C-NMR and 1H-NMR spectroscopy yielded identical spectra for the O-polysaccharides obtained from the LPSs of the parental wt and mutant strains (data not shown). Second, the oligosaccharides obtained from the rough-type LPSs (normally present in the LPS extracts of smooth Brucellae) of the bvrS and bvrR mutants and the parental wt strain showed similar migration patterns after HPTLC analysis (data not shown), a method that reveals Brucella LPS core heterogeneity (21).
Western blots with the monoclonal antibodies recognizing the three epitopes in the sugar backbone of B. abortus lipid A (58) did not reveal differences among the LPSs (data not shown). Conspicuous differences in the heterogeneity of lipid A molecules were, however, noted between the bvrS bvrR mutants and the wt Brucella (Fig. 1). Whereas the lipid A's from the wt and the reconstituted bvrR strains contained two major lipid A forms, the lipid A's from the bvrS and bvrR mutants showed two additional forms with lower Rfs (Fig. 1B). These differences were more pronounced when the lipid molecules from OM fragments were analyzed (Fig. 1B). By comparison with E. coli lipid A molecules of known structure, a correlation was established between the underacylated lipid A's of E. coli and the Brucella lipid A bands with lower Rfs.
LPSs are amphipathic molecules forming aggregates in aqueous solutions that mimic the external leaflet of the OM. Thus, we examined whether the lipid A changes described above altered the acyl-chain fluidity in LPS aggregates, a property relevant to the overall structure and function of the OM. To this end, we determined the temperature-dependent shifts in the maximum peak position of the symmetric stretching vibration νs(CH2) of the acyl chains, a parameter that directly relates to the fluidity of these chains. As illustrated in Fig. 1C, the phase transitions were broader for the mutant LPSs, with transition temperatures (Tc) markedly different (37°C for the wt versus 32°C for the mutants). Moreover, for the mutant LPSs, the corresponding peak positions—expressed as wave number (cm−1)—were markedly higher at temperatures below Tc. These results demonstrate a higher acyl-chain fluidity in the LPSs of the mutants. The relevance of this difference in acyl-chain fluidity was proven when the assays were repeated in the presence of polymyxin B. The decrease of Tc to lower values and the higher peak positions observed with the LPSs of the bvrS and bvrR mutants showed that polymyxin B had a stronger effect on the LPS of the mutants (Fig. 1C). This result agrees with the data in Fig. 1A. Measurements performed with lipid A's corroborated that the changes in fluidity were attributable to this LPS moiety (data not shown).
In addition to the acyl-chain fluidity, the type of aggregate structure formed is an important parameter for understanding the bioactivity of LPSs (6, 7). Therefore, isolated lipid A preparations from wt and mutant bvrS were analyzed by synchrotron radiation X-ray diffraction. Lipid A's rather than LPSs were used since aggregate structures of the former are usually more readily assignable. We found that the diffraction patterns of both lipid A samples exhibited unspecific, weak, and broad reflections, which were difficult to interpret (data not shown). In the presence of polymyxin B, however, the patterns were interpretable (Fig. 1D). The lipid A aggregate structure from the wt strain at 40°C indicated a complex diffraction pattern with reflections ranging down to very low scattering vectors (corresponding to low scattering angles). The existence of particular relationships between the spacings of the reflections (for example, 7.94 nm = 11.3 nm/√2) was indicative of a cubic aggregate structure (Fig. 1D). In contrast, the lipid A from the bvrS mutant (Fig. 1D) showed one broad peak with only a slightly pronounced maximum around 5.74 nm, characteristic of a bilayer structure. It has been shown previously that the predominant aggregate structure of biologically active native lipid A is cubic (7). Therefore, these data allow us to conclude that the aggregate structure of the lipid A from the mutant strain is more significantly altered by polymyxin B than that of the wt, in agreement with the data in Fig. 1A and C.
The BvrS/BvrR system does not regulate transcription of genes involved in the biosynthesis of lipid A forms with very-long-chain acyl groups.
Analysis of the B. melitensis and B. suis genome sequences (13, 56) reveals two ORFs (BMEI1111 and BMEI1475) whose products display 95% and 66% similarity to the rhizobial AcpXL, the specialized acyl carrier protein involved in the synthesis of the very-long-chain fatty acids (VLCFAs) (C2827OH and C3029OH) of rhizobial lipid A. Moreover, Brucella also contains an ORF (BMEI1115) coding for a protein with 68% similarity to LpxXL, the AcpXL cognate acyltransferase that links VLCFAs to the beta-hydroxyl groups of tetra- or penta-acyl lipid A to generate the fully acylated forms (for a recent review, see reference 33). Ferguson et al. (19) recently found that the BacA protein, required for virulence (41), affects the VLCFA content of B. abortus lipid A. To determine whether the lipid A alterations reflect a deregulated transcription of those genes in the bvrR and bvrS mutants, RT-PCR experiments were performed with oligonucleotide primers specific for BMEI1111 and BMEI1475 (acpXL), BMEI1115 (lpxXL), and bacA. The same profiles and band intensities were obtained with the cDNA of the parental wt strain and the bvrS and bvrR mutants (not shown), demonstrating that transcription of these ORFs was not regulated by BvrS/BvrR.
The changes in the LPS of bvrS and bvrR mutants do not alter cytokine induction.
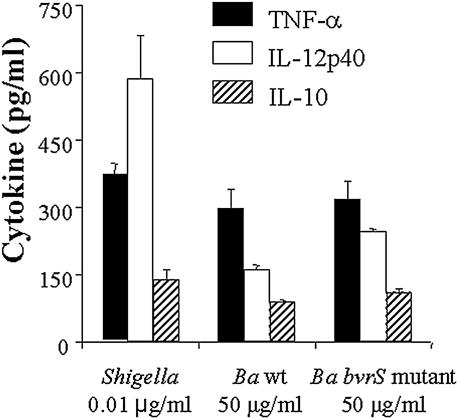
It is known that the biological activity of Brucella LPS is lower than that of its enterobacterial counterparts (50, 54). Also, some gram-negative bacteria are able to shift lipid A acylation, thus reducing the biological potency of the LPS and the responses of the innate and adaptive immune systems. Therefore, we tested if the LPSs of the bvrS and bvrR mutants had the same potency in triggering the generation of macrophage cytokines as wt Brucella LPS. TNF-α plays a major role in coordinating the inflammatory response and activating the cytokine cascade. Studies in vitro and in vivo demonstrate that TNF-α is a potent inducer of other cytokines, including IL-10, which has the function of downregulating and controlling proinflammatory responses. Similarly, IL-12p40 plays an important role in the normal host defense against infection by a variety of intracellular microorganisms, including Brucella. These three cytokines are secreted by macrophages in response to canonical LPSs (e.g., Salmonella), and their kinetics and secretion have been well established and used to determine the level of macrophage activation (54, 69). Consistent with previous reports, considerable quantities of Brucella LPS were required to induce the same level of activity as a standard enterobacterial LPS (Fig. 3). However, no differences in the induction of TNF-α, IL-12p40, or IL-10 between the mutant and wt LPSs were detected, supporting the concept that underacylated Brucella lipid A does not have any influence on the biological activity.
FIG. 3.
Cytokine induction by LPSs in murine bone marrow-derived macrophages. Macrophages were incubated with the indicated concentrations of LPS for 48 h, supernatants were collected, and the concentration of cytokines was determined by enzyme-linked immunosorbent assay. LPSs were obtained from Shigella flexneri serotype 5a, the B. abortus (Ba) wt, and the B. abortus bvrS mutant.
Surface hydrophobicity and killing by nonimmune serum are increased by BvrS/BvrR dysfunction.
Surface hydrophobicity is a property that depends on the topology of the OM surface. As mentioned above, the bvrS and bvrR mutants do not express some Omps (30) that are both exposed on the surface (11) and tightly bound to LPS (24, 58). Thus, we tested surface hydrophobicity changes that could reveal a deeply altered OM surface topology. In solvent partition experiments, it was observed that the optical density of a suspension of the wt strain decreased 19.0% ± 3.6% upon xylene extraction, whereas the decreases were 45.2% ± 2.5% and 39.3% ± 13.9% for the bvrS and bvrR mutant suspensions, respectively. This twofold-higher partition into the organic solvent demonstrates a higher surface hydrophobicity.
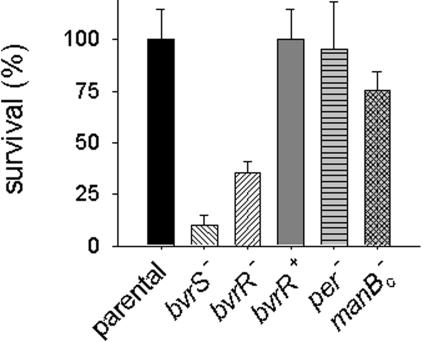
Smooth wt Brucella organisms are resistant to killing by nonimmune serum, an activity that denotes the ability of complement or associated collectins to recognize bacterial surfaces. The OM alterations of bvrS and bvrR mutants led us to test whether resistance to serum was also diminished. Figure 4 shows that both mutants were markedly more sensitive to nonimmune bovine serum than the parental wt strain, and similar results were obtained with human serum (data not shown). It is noteworthy that their sensitivity was even higher than that of sibling mutants with mutations in the genes per and manBcore carrying rough LPSs with no O-polysaccharide and complete or deeply truncated core oligosaccharides, respectively (49). To investigate if this feature was related to the lipid A acyl-chain shift, the complement-dependent hemolytic activity remaining after incubation of the LPSs of the mutant and wt strains in serum aliquots with fixed amounts of complement was assessed. The experiments showed that complement activation by the LPS of the mutants was not significantly altered (hemolytic activities 21%, 23%, and 25% of those of controls incubated without LPS for the bvrS and bvrR mutants and wt strain LPSs, respectively).
FIG. 4.
Mutants in the two-component system BvrS/BvrR are markedly sensitive to nonimmune serum. The survival of the B. abortus wt, bvrS mutant, bvrR mutant, reconstituted bvrR+ strain, and rough LPS mutant per and deep-rough LPS mutant manBcore strains in nonimmune bovine serum at 37°C is shown.
DISCUSSION
The B. abortus BvrS/BvrR system regulates the expression of at least two group 3 Omps, while other major Omps are not affected (30). Here, we have extended our observations and demonstrated that the lipid A moiety of the LPSs of the bvrS and bvrR mutants displays an altered acylation pattern compared to the parental strain, with the presence of underacylated forms. However, whereas Omp25 and Omp22 are transcriptionally regulated by BvrS/BvrR (30), transcription of BMEI1115 (lpxXL homologue), BMEI1111, and BMEI1475 (acpXL homologues) was not altered by dysfunction of the system. Since these are the only Brucella ORFs with homology to acyltransferase genes involved in lipid A biosynthesis or to acyl-carrier protein genes (33), a transcriptional regulation of lipid A acylation by BvrS/BvrR seems unlikely. Similarly, transcription of bacA remained unaffected. Thus, there is no obvious direct link between BvrS/BvrR and BacA. Disruption of bacA leads to the dominance of underacylated lipid A forms lacking mostly VLCFAs (19), and BacA shows sequence similarity to a family of peroxisomal membrane proteins affecting transport of activated VLCFAs out of the cytoplasm. On these bases, Ferguson et al. (19) proposed that BacA could be involved in exporting VLCFAs (and possibly other fatty acids) out of the cytoplasm where a hypothetical protein similar to Salmonella OM PagP (see below) could link them to lipid A. An alternative proposed by the same authors is that BacA could be necessary in the inner membrane for flipping the LPS precursors carrying VLCFAs. Accordingly, in the absence of BacA, translocation of underacylated forms lacking VLCFAs would be favored owing to their comparatively lower hydrophobicity. Although our results rule out transcriptional control over bacA, these hypotheses may also apply to BvrS/BvrR. It is conceivable that this system modulates the activity of some inner membrane or OM protein necessary to incorporate VLCFAs on the periplasmic side or that an altered OM topology hampers translocation of the more hydrophobic LPS forms. Concerning the first hypothesis, Brucella does not contain homologues of PagP (33), but this is not necessarily meaningful because the C16 acyl-chain substrate of PagP is markedly different from the VLCFAs. The second hypothesis is consistent with the observation that underacylated forms were enriched in the OM fragments because these fragments probably represent the detachment of zones where new materials are being rapidly incorporated into the OM (23). LPS and Omp25 form very hydrophobic, detergent-resistant complexes in OM fragments (24). Therefore, it is plausible that the absence of Omp25 in bvrS and bvrR mutants would somehow hamper insertion of the fully acylated, more hydrophobic LPS forms into the OM. However, B. abortus mutants carrying Omp25 deletions do not show enrichment of underacylated LPS (I. López-Goñi, unpublished results), and this shows that this simplification of the second hypothesis cannot account for our observations. Lipid A acylation patterns similar to those described here have been observed in rough LPS mutants of B. abortus (49) and other gram-negative bacteria (37), and it has been proposed that this underacylation is a mechanism to counterbalance the lack of the hydrophilic O-polysaccharide (49). This pleiotropic effect illustrates that surface alterations can also cause lipid A changes by compensatory mechanisms and not necessarily by direct regulation. In this respect, it may be relevant that the surface hydrophobicity of the bvrS and bvrR mutants was increased, perhaps due to the absence of key Omps. Since optimal OM stability requires a hydrophilic-to-hydrophobic equilibrium, lipid A underacylation may be an indirect counterbalancing adjustment.
It is well known that modifications of the lipid A moiety are used by some gram-negative bacteria for pathogenesis (2, 18, 25, 26, 28, 29, 32, 57). The best-studied case is that of Salmonella enterica serovar Typhimurium, where the lipid A is modified in response to host signals and sensed and modulated by a two-component regulatory network controlling over 40 genes (64). The essential components of this network are the PhoP/PhoQ and PmrA/PmrB systems, both involved in resistance to bactericidal cationic peptides and virulence (47, 48). Among the lipid A modifications observed in Salmonella are the addition of arabinosamine or phosphoethanolamine to the diaminoglucose backbone, the replacement of myristate with 2-OH myristate, and the addition of palmitate catalyzed by PagP (26-29, 31, 67, 70). Given their connection with virulence, polycationic peptide sensitivity, and lipid A modification, it may seem that the Brucella BvrS/BvrR and the S. enterica serovar Typhimurium PhoP/PhoQ or PmrA/PmrB systems represent functional counterparts. However, several lines of evidence demonstrate that the Brucella and S. enterica serovar Typhimurium systems differ widely. First, as described previously (63), these genes bear little sequence similarity, and we show here that BvrS/BvrR has no direct control on the genes putatively involved in the generation of the fully acylated lipid A forms of B. abortus or in obvious lipid A backbone or core modifications. Second, none of the three Brucella genomes sequenced so far contains genes homologous to the pmrJ, pmrM, and pmrD genes involved in lipid A backbone arabinosamine substitutions, and the genes homologous to other genes of the Salmonella pmrHFIJKLM operon (regulated by PhoP-PhoQ and PmrA-PmrB through pmrD) or to Salmonella pmrE are not organized in an operon but scattered in both chromosomes (33). Furthermore, in contrast to the 30 to 40% relative increase in E-selectin and TNF-α expression manifested by the LPSs of a PhoP− mutant (28), the biological activity of the LPS from the bvrS mutant was not significantly altered. As discussed elsewhere, an altered pathogen-associated molecular pattern not easily recognized by the innate immune system is a constitutive trait of B. abortus LPS (54) and not an environmentally modulated adaptive property. Thus, it is not surprising that the mode of action of BvrS/BvrR departs from the systems described in Enterobacteriaceae or Pseudomonadaceae (18).
Although the mechanism behind the appearance of underacylated LPSs remains to be elucidated, this structural shift and the altered Omp profile explain several properties of the bvrS and bvrR mutants, some clearly relevant to attenuation. First, underacylation correlated with both higher polymyxin B binding and augmented acyl-chain fluidity in LPS/lipid A aggregates (Fig. 1A, C, and D), observations that are directly connected. It is postulated that many bactericidal polycationic peptides act on membranes by a self-promoted mechanism in which an initial ionic interaction on the surface is followed by the insertion of the hydrophobic peptide section(s). Accordingly, the reduced lipid A hydrophobicity and the concomitant increase in acyl-chain fluidity should ease insertion and penetration of these peptides with a further increase in fluidity, as observed. Thus, although a lack of shielding of the LPS by Omp25 (not expressed in the mutants) could also contribute, the lipid A structural shift explains at least in part the increased sensitivity of mutant bacteria (63), as illustrated in the chimera experiments (Fig. 2). Second, the higher sensitivity of the bvrS and bvrR mutants to surfactants (63) is another phenotype possibly explained by our observations since a correlation between OM permeability and LPS acyl-chain fluidity has been observed previously (2, 3). Third, sensitivity to the bactericidal action of normal sera is a serious handicap for a pathogen. Since the bvrS and bvrR mutants have reduced ability to penetrate into host cells (63) and are thus exposed to humoral host defenses, their marked serum sensitivity undoubtedly contributes to their exceedingly rapid clearance from experimentally infected animals (63). It is remarkable that the sensitivity of bvrS and bvrR mutants to normal serum was higher than that of rough LPS mutants with complete or deeply truncated core and underacylated lipid A's (49). This suggests that the lipid A changes are not directly connected to the sensitivity to serum complement, and this interpretation is supported by the demonstration that LPS purified from the mutants did not display higher complement-activating activity than the wt LPS.
In summary, we have shown that BvrS/BvrR dysfunction weakens the overall stability of the B. abortus OM which becomes sensitive to humoral effectors (bactericidal peptides and complement) of the innate immune system. In Brucella, as well as in the intracellular pathogen Legionella, the lipid A is highly hydrophobic, mainly because of the presence of the VLCFAs that, in addition, are likely to span the OM, lending further stability to this structure. This ancestral property of some eukaryote-associated α- and γ-Proteobacteria may represent an advantage in the adaptation to intracellular parasitism (54). The phenotypic effects of the lipid A shift of BacA and BvrS/BvrR mutants lend further support to this hypothesis. Indeed, overall OM homeostasis is not the sole aspect of Brucella modulated by BvrS/BvrR, as this system is likely to be connected with other aspects of the biology of the microbe, including the regulation of some metabolic pathways (40, 43). Experiments are under way to identify all of the Brucella genes regulated by the two-component system BvrS/BvrR and their relationship to the intracellular life of these pathogenic bacteria.
Acknowledgments
Research at our laboratories is supported by the European Commission (QLK2-CT-2002-00918), Ministerio de Educación y Ciencia of Spain (AGL20004-01162/GAN), program Redes Temáticas de Investigación Cooperativa del FIS-Promoción de las Regiones (objetivo 2, 2000-2006) (Red Temática de Investigación en Brucellosis, G03/204), and the Network for Research and Training in Tropical Diseases in Central America (NeTropica). Fellowship support to L.M. from the Ministerio de Educación y Ciencia (Spain) is also gratefully acknowledged.
REFERENCES
- 1.Aragón, V., R. Díaz, E. Moreno, and I. Moriyón. 1996. Characterization of Brucella abortus and Brucella melitensis native haptens as outer membrane O-type polysaccharides independent from the smooth lipopolysaccharide. J. Bacteriol. 178:1070-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bengoechea, J. A., K. Brandenburg, M. D. Arraiza, U. Seydel, M. Skurnik, and I. Moriyón. 2003. Pathogenic Yersinia enterocolitica strains increase the outer membrane permeability in response to environmental stimuli by modulating lipopolysaccharide fluidity and lipid A structure. Infect. Immun. 71:2014-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bengoechea, J. A., K. Brandenburg, U. Seydel, R. Díaz, and I. Moriyón. 1998. Yersinia pseudotuberculosis and Yersinia pestis show increased outer membrane permeability to hydrophobic agents which correlates with lipopolysaccharide acyl-chain fluidity. Microbiology 144:1517-1526. [DOI] [PubMed] [Google Scholar]
- 4.Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911-917. [DOI] [PubMed] [Google Scholar]
- 5.Bowden, R. A., A. Cloeckaert, M. S. Zygmunt, S. Bernard, and G. Dubray. 1995. Surface exposure of outer membrane protein and lipopolysaccharide epitopes in Brucella species studied by enzyme-linked immunosorbent assay and flow cytometry. Infect. Immun. 63:3945-3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandenburg, K., S. Kusumoto, and U. Seydel. 1997. Conformational studies of synthetic lipid A analogues and partial structures by infrared spectroscopy. Biochim. Biophys. Acta 1329:183-201. [DOI] [PubMed] [Google Scholar]
- 7.Brandenburg, K., W. Richter, M. H. Koch, H. W. Meyer, and U. Seydel. 1998. Characterization of the nonlamellar cubic and HII structures of lipid A from Salmonella enterica serovar Minnesota by X-ray diffraction and freeze-fracture electron microscopy. Chem. Phys. Lipids 91:53-69. [DOI] [PubMed] [Google Scholar]
- 8.Briones, G., N. Iñón de Iannino, M. Roset, A. Vigliocco, P. S. Paulo, and R. A. Ugalde. 2001. Brucella abortus cyclic β-1,2-glucan mutants have reduced virulence in mice and are defective in intracellular replication in HeLa cells. Infect. Immun. 69:4528-4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng, H.-P., and G. C. Walker. 1998. Succinoglycan production by Rhizobium meliloti is regulated through the ExoS-ChvI two-component regulatory system. J. Bacteriol. 180:20-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clementz, T., Z. Zhou, and C. R. Raetz. 1997. Function of the Escherichia coli msbB gene, a multicopy suppressor of htrB knockouts, in the acylation of lipid A. Acylation by MsbB follows laurate incorporation by HtrB. J. Biol. Chem. 272:10353-10360. [DOI] [PubMed] [Google Scholar]
- 11.Cloeckaert, A., P. de Wergifosse, G. Dubray, and J. N. Limet. 1990. Identification of seven surface-exposed Brucella outer membrane proteins by use of monoclonal antibodies: immunogold labeling for electron microscopy and enzyme-linked immunosorbent assay. Infect. Immun. 58:3980-3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Bolle, X., C. Lambert, E. Depiereux, and J. J. Letesson. 2004. A Brucella melitensis genomic database, p. 69-84. In I. López-Goñi and I. Moriyón (ed.), Brucella: molecular and cellular biology. Horizon Bioscience, Norfolk, England.
- 13.DelVecchio, V. G., V. Kapatral, R. J. Redkar, G. Patra, C. Mujer, T. Los, N. Ivanova, I. Anderson, A. Bhattacharyya, A. Lykidis, G. Reznik, L. Jablonski, N. Larsen, M. D'Souza, A. Bernal, M. Mazur, E. Goltsman, E. Selkov, P. H. Elzer, S. Hagius, D. O'Callaghan, J. J. Letesson, R. Haselkorn, N. Kyrpides, and R. Overbeek. 2002. The genome sequence of the facultative intracellular pathogen Brucella melitensis. Proc. Natl. Acad. Sci. USA 99:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Díaz, R., P. Garatea, L. M. Jones, and I. Moriyón. 1979. Radial immunodiffusion test with a Brucella polysaccharide antigen for differentiating infected from vaccinated cattle. J. Clin. Microbiol. 10:37-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edmonds, M. D., A. Cloeckaert, N. J. Booth, W. T. Fulton, S. D. Hagius, J. V. Walker, and P. H. Elzer. 2001. Attenuation of a Brucella abortus mutant lacking a major 25 kDa outer membrane protein in cattle. Am. J. Vet. Res. 62:1461-1466. [DOI] [PubMed] [Google Scholar]
- 16.Edmonds, M. D., A. Cloeckaert, and P. H. Elzer. 2002. Brucella species lacking the major outer membrane protein Omp25 are attenuated in mice and protect against Brucella melitensis and Brucella ovis. Vet. Microbiol. 88:205-221. [DOI] [PubMed] [Google Scholar]
- 17.Edmonds, M. D., A. Cloeckaert, S. D. Hagius, L. E. Samartino, W. T. Fulton, J. V. Walker, F. M. Enright, N. J. Booth, and P. H. Elzer. 2002. Pathogenicity and protective activity in pregnant goats of a Brucella melitensis δ-omp25 deletion mutant. Res. Vet. Sci. 72:235-239. [DOI] [PubMed] [Google Scholar]
- 18.Ernst, R. K., E. C. Yi, L. Guo, K. B. Lim, J. L. Burns, M. Hackett, and S. I. Miller. 1999. Specific lipopolysaccharide found in cystic fibrosis airway Pseudomonas aeruginosa. Science 286:1561-1565. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson, G. P., A. Datta, J. Baumgartner, R. M. Roop, R. W. Carlson, and G. C. Walker. 2004. Similarity to peroxisomal-membrane protein family reveals that Sinorhizobium and Brucella BacA affect lipid-A fatty acids. Proc. Natl. Acad. Sci. USA 101:5012-5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freer, E., E. Moreno, I. Moriyón, J. Pizarro-Cerdá, A. Weintraub, and J.-P. Gorvel. 1996. Brucella-Salmonella lipopolysaccharide chimeras are less permeable to hydrophobic probes and more sensitive to cationic peptides and EDTA than are their native Brucella sp. counterparts. J. Bacteriol. 178:5867-5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freer, E., N. Rojas, A. Weintraub, A. A. Lindberg, and E. Moreno. 1995. Heterogeneity of Brucella abortus lipopolysaccharides. Res. Microbiol. 146:569-578. [DOI] [PubMed] [Google Scholar]
- 22.Gabriel, A. 1977. Position sensitive X-ray detector. Rev. Sci. Instrum. 48:1303-1305. [Google Scholar]
- 23.Gamazo, C., and I. Moriyón. 1987. Release of outer membrane fragments by exponentially growing Brucella melitensis cells. Infect. Immun. 55:609-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gamazo, C., A. I. Vitas, I. Moriyón, I. López-Goñi, and R. Díaz. 1993. Brucella group 3 outer membrane proteins contain a heat-modifiable protein. FEMS Microbiol. Lett. 112:141-146. [DOI] [PubMed] [Google Scholar]
- 25.Gunn, J. S. 2001. Bacterial modification of LPS and resistance to antimicrobial peptides. J. Endotoxin Res. 1:57-62. [PubMed] [Google Scholar]
- 26.Gunn, J. S., K. B. Lim, J. Krueger, K. Kim, L. Guo, M. Hackett, and S. I. Miller. 1998. PmrA-PmrB-regulated genes necessary for 4-aminoarabinose lipid A modification and polymyxin resistance. Mol. Microbiol. 27:1171-1182. [DOI] [PubMed] [Google Scholar]
- 27.Gunn, J. S., and S. I. Miller. 1996. PhoP-PhoQ activates transcription of pmrAB, encoding a two-component regulatory system involved in Salmonella typhimurium antimicrobial peptide resistance. J. Bacteriol. 178:6857-6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo, L., K. B. Lim, J. S. Gunn, B. Bainbridge, R. P. Darveau, M. Hackett, and S. I. Miller. 1997. Regulation of lipid A modifications by Salmonella typhimurium virulence genes.phoP-phoQ. Science 276:250-253. [DOI] [PubMed] [Google Scholar]
- 29.Guo, L., K. B. Lim, C. M. Poduje, M. Daniel, J. S. Gunn, M. Hackett, and S. I. Miller. 1998. Lipid A acylation and bacterial resistance against vertebrate antimicrobial peptides. Cell 95:189-198. [DOI] [PubMed] [Google Scholar]
- 30.Guzmán-Verri, C., L. Manterola, A. Sola-Landa, A. Parra, A. Cloeckaert, J. Garín, J. P. Gorvel, I. Moriyón, E. Moreno, and I. López-Goñi. 2002. The two-component system BvrR/BvrS essential for Brucella abortus virulence regulates the expression of outer membrane proteins with counterparts in members of the Rhizobiaceae. Proc. Natl. Acad. Sci. USA 99:12375-12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helander, I. M., I. Kilpelainen, and M. Vaara. 1994. Increased substitution of phosphate groups in lipopolysaccharides and lipid A of the polymyxin-resistant pmrA mutants of Salmonella typhimurium: a 31P-NMR study. Mol. Microbiol. 11:481-487. [DOI] [PubMed] [Google Scholar]
- 32.Hitchen, P. G., J. L. Prior, P. C. Oyston, M. Panico, B. W. Wren, R. W. Titball, H. R. Morris, and A. Dell. 2002. Structural characterization of lipo-oligosaccharide (LOS) from Yersinia pestis: regulation of LOS structure by the PhoPQ system. Mol. Microbiol. 44:1637-1650. [DOI] [PubMed] [Google Scholar]
- 33.Iriarte, M., D. González, R. M. Delrue, D. Monreal, R. Conde, I. López-Goñi, J. J. Letesson, and I. Moriyón. 2004. Brucella lipopolysaccharide: structure, biosynthesis and genetics, p. 159-192. In I. López-Goñi and I. Moriyón (ed.), Brucella: molecular and cellular biology. Horizon Bioscience, Norfolk, England.
- 34.Koch, M. H. J., and J. Bordas. 1983. X-ray diffraction and scattering on disordered systems using synchrotron radiation. Nucl. Instrum. Methods 208:461-469. [Google Scholar]
- 35.Krauss, J. H., J. Weckesser, and H. Mayer. 1988. Electrophoretic analysis of lipopolysaccharides of purple nonsulfur bacteria. Int. J. Syst. Bacteriol. 38:157-163. [Google Scholar]
- 36.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 37.Lebbar, S., D. Karibian, C. Deprun, and M. Caroff. 1994. Distribution of lipid A species between long and short chain lipopolysaccharides isolated from Salmonella, Yersinia, and Escherichia as seen by 252Cf plasma desorption mass spectrometry. J. Biol. Chem. 269:31881-31884. [PubMed] [Google Scholar]
- 38.Leong, D., R. Díaz, K. Milner, J. Rudbach, and J. B. Wilson. 1970. Some structural and biological properties of Brucella endotoxin. Infect. Immun. 1:174-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lesse, A. J., A. A. Campagnari, W. E. Bittner, and M. A. Apicella. 1990. Increased resolution of lipopolysaccharides and lipooligosaccharides utilizing tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J. Immunol. Methods 126:109-117. [DOI] [PubMed] [Google Scholar]
- 40.Letesson, J. J., and X. De Bolle. 2004. Brucella virulence: a matter of control, p. 117-158. In I. López-Goñi and I. Moriyón (ed.), Brucella: molecular and cellular biology. Horizon Bioscience, Norfolk, England.
- 41.LeVier, K., R. W. Phillips, V. K. Grippe, R. M. Roop, and G. C. Walker. 2000. Similar requirements of a plant symbiont and a mammalian pathogen for prolonged intracellular survival. Science 287:2492-2493. [DOI] [PubMed] [Google Scholar]
- 42.López-Goñi, I., C. Guzmán-Verri, L. Manterola, A. Sola-Landa, I. Moriyón, and E. Moreno. 2002. Regulation of Brucella virulence by the two-component system BvrR/BvrS. Vet. Microbiol. 90:329-339. [DOI] [PubMed] [Google Scholar]
- 43.López-Goñi, I., L. Manterola, and S. Q. Pan. 2004. The Brucella BvrS/BvrR and related two-component regulatory systems of the α-Proteobacteria: common regulatory strategies of animal and plant pathogens and endosymbionts, p. 213-230. In I. López-Goñi and I. Moriyón (ed.), Brucella: molecular and cellular biology. Horizon Bioscience, Norfolk, England.
- 44.Martínez de Tejada, G., and I. Moriyón. 1993. The outer membranes of Brucella spp. are not barriers to hydrophobic permeants. J. Bacteriol. 175:5273-5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martínez de Tejada, G., J. Pizarro-Cerdá, E. Moreno, and I. Moriyón. 1995. The outer membranes of Brucella spp. are resistant to bactericidal cationic peptides. Infect. Immun. 63:3054-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mekalanos, J. J. 1992. Environmental signals controlling expression of virulence determinants in bacteria. J. Bacteriol. 174:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller, S. I., A. M. Kukral, and J. J. Mekalanos. 1989. A two-component regulatory system (phoP/phoQ) controls Salmonella typhimurium virulence. Proc. Natl. Acad. Sci. USA 86:5054-5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller, S. I., W. S. Pulkkinen, M. E. Selsted, and J. J. Mekalanos. 1990. Characterization of defensin resistance phenotypes associated with mutations in the phoP virulence regulon of Salmonella typhimurium. Infect. Immun. 58:3706-3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Monreal, D., M. J. Grilló, D. González, C. M. Marín, M. J. De Miguel, I. López-Goñi, J. M. Blasco, A. Cloeckaert, and I. Moriyón. 2003. Characterization of Brucella abortus O-polysaccharide and core lipopolysaccharide mutants and demonstration that a complete core is required for rough vaccines to be efficient against Brucella abortus and Brucella ovis in the mouse model. Infect. Immun. 71:3261-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moreno, E., D. T. Berman, and L. M. Boettcher. 1981. Biological activities of Brucella abortus lipopolysaccharides. Infect. Immun. 31:362-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moreno, E., and I. Moriyón. 2002. Brucella melitensis: a nasty bug with hidden credentials for virulence. Proc. Natl. Acad. Sci. USA 99:1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moreno, E., and I. Moriyón. 2November2001, posting date. The genus Brucella. In M. Dworkin et al. (ed.), The prokaryotes: an evolving electronic resource for the microbiological community, 3rd ed., release 3.7. Springer-Verlag, New York, N.Y. [Online.] http://141.150.157.117:8080/prokPUB/index.htm.
- 53.Moreno, E., E. Stackebrandt, M. Dorsch, J. Wolters, M. Busch, and H. Mayer. 1990. Brucella abortus 16S rRNA and lipid A reveal a phylogenetic relationship with members of the alpha-2 subdivision of the class Proteobacteria. J. Bacteriol. 172:3569-3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moriyón, I. 2004. Against gram-negative bacteria: the lipopolysaccharide case, p. 204-230. In J. P. Gorvel (ed.), Intracellular pathogens in membrane interactions and vacuole biogenesis. Kluwer Academic/Plenum Publishers, New York, N.Y.
- 55.Moriyón, I., and D. T. Berman. 1982. Effects of nonionic, ionic, and dipolar detergents and EDTA on Brucella cell envelope. J. Bacteriol. 152:822-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paulsen, I. T., R. Seshadri, K. E. Nelson, J. A. Eisen, J. F. Heidelberg, T. D. Read, R. J. Dodson, L. Umayam, L. M. Brinkac, M. J. Beanan, S. C. Daugherty, R. T. Deboy, A. S. Durkin, J. F. Kolonay, R. Madupu, W. C. Nelson, B. Ayodeji, M. Kraul, J. Shetty, J. Malek, S. E. Van Aken, S. Riedmuller, H. Tettelin, S. R. Gill, O. White, S. L. Salzberg, D. L. Hoover, L. E. Lindler, S. M. Halling, S. M. Boyle, and C. M. Fraser. 2002. The Brucella suis genome reveals fundamental similarities between animal and plant pathogens and symbionts. Proc. Natl. Acad. Sci. USA 99:13148-13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Preston, A., E. Maxim, E. Toland, E. J. Pishko, E. T. Harvill, M. Caroff, and D. J. Maskell. 2003. Bordetella bronchiseptica PagP is a Bvg-regulated lipid A palmitoyl transferase that is required for persistent colonization of the mouse respiratory tract. Mol. Microbiol. 48:725-736. [DOI] [PubMed] [Google Scholar]
- 58.Rojas, N., E. Freer, A. Weintraub, M. Ramírez, S. Lind, and E. Moreno. 1994. Immunochemical identification of Brucella abortus lipopolysaccharide epitopes. Clin. Diagn. Lab. Immunol. 1:206-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosenberg, M., D. L. Gutnick, and E. Rosenberg. 1980. Adherence of bacteria to hydrocarbons: a simple method for measuring cell surface hydrophobicity. FEMS Microbiol. Lett. 9:29-33. [Google Scholar]
- 60.Rozen, S., and H. J. Skaletsky. 2000. Primer3 on the WWW for general users and for biologist programmers, p. 365-386. In S. Krawetz and S. Misener (ed.), Bioinformatics methods and protocols: methods in molecular biology. Humana Press, Totowa, N.J. [DOI] [PubMed]
- 61.Schagger, H., and G. von Jagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 62.Schindler, P. R. G., and M. Teuber. 1975. Action of polymyxin B on bacterial membranes: morphological changes in the cytoplasm and in the outer membrane of Salmonella typhimurium and Escherichia coli B. Antimicrob. Agents Chemother. 8:95-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sola-Landa, A., J. Pizarró-Cerdá, M. J. Grilló, E. Moreno, I. Moriyón, J. M. Blasco, J. P. Gorvel, and I. López-Goñi. 1998. A two-component regulatory system playing a critical role in plant pathogens and endosymbionts is present in Brucella abortus and controls cell invasion and virulence. Mol. Microbiol. 29:125-138. [DOI] [PubMed] [Google Scholar]
- 64.Soncini, F. C., and E. A. Groisman. 1996. Two-component regulatory systems can interact to process multiple environmental signals. J. Bacteriol. 178:6796-6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Touchstone, J. C., and M. F. Dobbins. 1983. Practice of thin layer chromatography, 2nd ed. John Wiley and Sons Inc., New York, N.Y.
- 66.Tsai, C., and C. E. Frasch. 1982. A sensitive stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]
- 67.Vaara, M., T. Vaara, and M. Sarvas. 1979. Decreased binding of polymyxin by polymyxin-resistant mutants of Salmonella typhimurium. J. Bacteriol. 139:664-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Velasco, J., J. A. Bengoechea, K. Brandenburg, B. Lindner, U. Seydel, D. González, U. Zahringer, E. Moreno, and I. Moriyón. 2000. Brucella abortus and its closest phylogenetic relative, Ochrobactrum spp., differ in outer membrane permeability and cationic peptide resistance. Infect. Immun. 68:3210-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weiss, D. S., B. Raupach, K. Takeda, S. Akira, and A. Zychlinsky. 2004. Toll-like receptors are temporally involved in host defense. J. Immunol. 172:4463-4469. [DOI] [PubMed] [Google Scholar]
- 70.Zhou, Z., A. A. Ribeiro, S. Lin, R. J. Cotter, S. I. Miller, and C. R. Raetz. 2001. Lipid A modifications in polymyxin-resistant Salmonella typhimurium: pmrA-dependent 4-amino-4-deoxy-L-arabinose, and phosphoethanolamine incorporation. J. Biol. Chem. 276:43111-43121. [DOI] [PubMed] [Google Scholar]