Abstract
The survival of pathogenic mycobacteria in macrophages requires the eukaryotic enzyme-like serine/threonine protein kinase G. This kinase with unknown specificity is secreted into the cytosol of infected macrophages and inhibits phagosome-lysosome fusion. The pknG gene is the terminal gene in a putative operon containing glnH, encoding a protein potentially involved in glutamine uptake. Here, we report that the deletion of pknG did not affect either glutamine uptake or intracellular glutamine concentrations. In vitro growth of Mycobacterium bovis BCG lacking pknG was identical to that of the wild type. We conclude that in M. bovis BCG, glutamine metabolism is not regulated by protein kinase G.
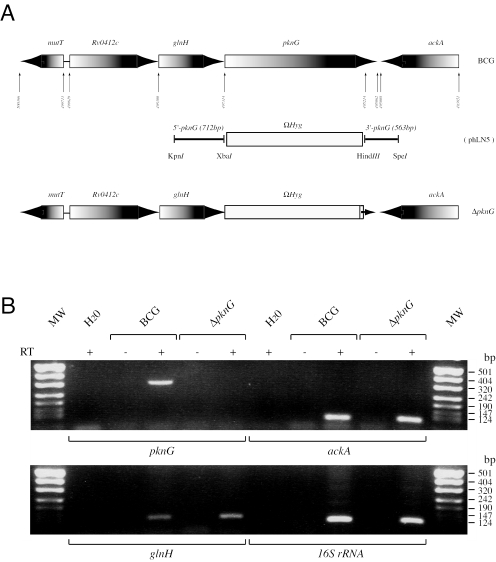
The resistance of pathogenic mycobacteria to host innate immune responses has been attributed to their ability to block phagosome-lysosome fusion. This results in their capability to persist inside macrophages, avoiding bactericidal activities of the host macrophages (9, 15, 16). The genome of Mycobacterium tuberculosis encodes 11 eukaryotic enzyme-like serine/threonine protein kinases (1, 7). Among these, protein kinase G (PknG) was recently found to promote mycobacterial survival in macrophages. Although a role for PknG in mycobacterial physiology remains to be established, PknG blocks phagosome-lysosome fusion in infected macrophages where it is secreted into the cytosol. Disruption of the pknG gene in Mycobacterium bovis BCG by homologous recombination, as well as chemical inhibition of PknG by a specific inhibitor, leads to accelerated phagosome maturation and a growth defect in macrophages (19). The pknG gene is the last gene in a putative operon that includes Rv0412c (encoding an unknown membrane protein) and glnH (Fig. 1A) in all mycobacterial genomes sequenced. The glnH gene is predicted to encode a glutamine-binding lipoprotein that might be involved in glutamine import through the membrane (1, 6). In Escherichia coli and Bacillus subtilis, glnH is located in the glutamine permease operon including glnP and glnQ, which together form an ABC transporter. This operon is essential for glutamine import activity (13). In the M. tuberculosis genome, the putative glutamine importing system consists of five proteins encoded by genes locating in three different regions (GlnH, Rv2563 and GlnQ, and Rv0072 and Rv0073). GlnQ and Rv0073 are homologous to nucleotide binding proteins, while Rv2563 and Rv0072 are membrane-spanning proteins. These two pairs of proteins are expected to form one or two separate glutamine transporters, while glnH is thought to encode the substrate binding protein (4).
FIG. 1.
(A) Genomic organization at the pknG locus. (B) RT-PCR analysis for expression of genes ackA, glnH, and pknG predicted to share the same operon. Expression of 16S rRNA genes was used as a control.
Glutamine biosynthesis is catalyzed by glutamine synthase, which ligates the ammonium group to l-glutamate. The deletion of glutamine synthase in M. tuberculosis resulted in l-glutamine auxotrophicity and attenuated growth in human THP-1 macrophages (18). In addition, the glutamine synthase-negative mutant is avirulent in the highly susceptible guinea pig model of pulmonary tuberculosis (18). Depletion of glutamine synthase activity by a specific inhibitor (11) or antisense technology (12) has been shown to effectively inhibit growth of M. tuberculosis.
Given the importance of glutamine metabolism for mycobacterial survival and the possible location of pknG and glnH within the same operon, we analyzed the roles of PknG in glutamine metabolism and growth of M. bovis BCG.
Transcription of glnH and ackA in the presence or absence of pknG.
To analyze possible coregulation of the genes located surrounding pknG, transcription of glnH (forward primer, 5′-TCGGGATCAACCTGGACAA-3′; reverse primer, 5′-GAGCACCGTCAGCCACTTG-3′), ackA encoding an acetate kinase (forward primer, 5′-GGGCGTCATCAGCTACTTGTG-3′; reverse primer, 5′-CCGCCAACCCCAACATC-3′), and pknG (forward primer, 5′-GCCACCGACATCTACACCGT-3′; reverse primer, 5′-GGTGTGCGCCACCAGCAG-3′) was measured by RT-PCR. As a control, transcription of the 16S rRNA gene (forward primer, 5′-ACGAACAACGCGACAAACC-3′; reverse primer, 5′-CCAGCAGCCGCGGTAA-3′) was also analyzed. RNA samples were isolated from wild-type M. bovis BCG and M. bovis BCG ΔpknG (BCG-ΔpknG) (19) by using the RNeasy midi kit (QIAGEN Inc., Valencia, CA) and treated with DNase for 3 h at 37°C. To analyze transcription, cDNA was synthesized from 1 μg of total RNA by using Superscript III (Invitrogen Life Technologies, Carlsbad, CA) and random primers (Promega Inc., Madison, WI), followed by amplification of the products by using gene-specific primers (see above).
No pknG transcript was detected in M. bovis BCG-ΔpknG, whereas transcription of glnH and ackA was not effected in the ΔpknG mutant (Fig. 1B), suggesting that PknG does not control the transcription of glnH and ackA. In addition, this result excludes the possibility that the deletion of pknG by homologous recombination in the ΔpknG mutant resulted in impaired expression of glnH and ackA.
Effect of PknG depletion on in vitro growth.
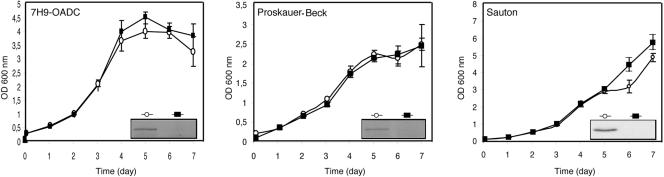
Changes in glutamine metabolism have been reported to alter the in vitro growth characteristics of mycobacteria (12, 18). To analyze a contribution of PknG to the in vitro growth of M. bovis BCG, wild-type M. bovis BCG, and M. bovis BCG-ΔpknG (strain Montreal) were grown in 7H9-OADC medium as well as in two different defined media, Sauton and Proskauer-Beck (PB) media, whose nitrogen sources were based on asparagines, supplemented with 0.05% Tween 80 (14). The in vitro growth of M. bovis BCG-ΔpknG and the growth of its parental strain were monitored by measuring the optical densities of liquid cultures at a wavelength of 600 nm (OD600). Saturated cultures (OD600 of 1.5) stored at −70°C were used to inoculate fresh media to the final OD600 of 0.1, and cultures were incubated at 37°C with orbital shaking. In none of the media tested was there any detectable alteration in the growth of M. bovis BCG-ΔpknG compared to that of its parental strain (Fig. 2).
FIG. 2.
In vitro growth of M. bovis BCG (○) and M. bovis BCG-ΔpknG (▪) in 7H9-OADC, Sauton, and PB media. The expression levels of PknG in the different strains were analyzed at day 6 by immunoblotting of lysates separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by immunoblotting (inset).
Effect of PknG depletion on glutamine uptake.
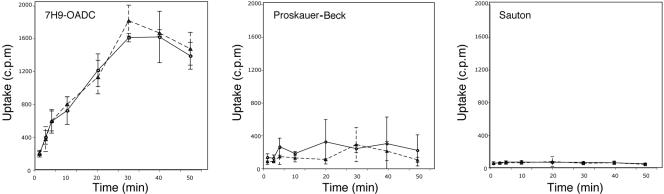
As glnH, the gene adjacent to pknG, has been suggested to be part of a glutamine transport system (4, 7, 8) and glnH and pknG may be part of the same operon, the contribution of PknG to glutamine transport was investigated. To that end, the uptake of [3H]glutamine (Amersham) by M. bovis BCG or M. bovis BCG-ΔpknG grown in 7H9-OADC, PB, and Sauton media supplemented with 0.05% Tween 80 was analyzed. Bacterial cultures growing at exponential phase (OD600 of 0.5) were washed and diluted to an OD600 of 0.2 with fresh medium. Diluted cultures were then distributed in 96-well plates (in triplicate) and incubated for 2 h at 37°C before the addition of 1 μCi [3H]glutamine to each well (final concentration of [3H]glutamine, 0.1 μM). After incubation times, cultures from plates were harvested to UniFilter plates (Packard) and washed to remove extracellular glutamine. Cell-incorporated radioactivity was counted by using the Topcount microplate scintillation counter (Packard). As shown in Fig. 3, no glutamine uptake activity was observed for the bacteria grown in PB and Sauton media. As expected, glutamine was readily internalized by strains grown in 7H9-OADC, reflecting the accelerated extracellular glutamine synthesis and transport when mycobacteria are grown in 7H9, which is supplemented with l-glutamate, the direct substrate of extracellular glutamine synthase (10). However, glutamine uptake was identical in M. bovis BCG lacking pknG (Fig. 3), indicating that PknG does not contribute to glutamine uptake.
FIG. 3.
Effects of PknG depletion on uptake of glutamine. Uptake of [3H]glutamine by M. bovis BCG (○) and M. bovis BCG-ΔpknG (▪) in 7H9-OADC, Sauton, and PB media.
Effect of PknG depletion on intracellular glutamine concentration.
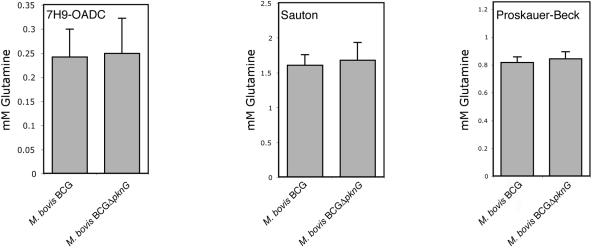
An impaired uptake of glutamine would affect the intracellular level of glutamine and other amino acids. To analyze intracellular glutamine levels, M. bovis BCG and M. bovis BCG-ΔpknG were grown in the different media indicated in Fig. 4 and homogenized, and aliquots equalized for similar protein amounts (bicinchoninic acid; Pierce) were analyzed spectrophotometrically for the presence of glutamine via enzymatic deamination by glutaminase (Sigma). M. bovis BCG and M. bovis BCG-ΔpknG grown in the different media indicated in Fig. 3 displayed equal levels of glutamine. To obtain an independent assessment of the levels of glutamine in wild-type M. bovis BCG and M. bovis BCG-ΔpknG, homogenates were either analyzed directly (Tables 1 and 2) or after hydro-lysis (Table 3), followed by concentration and phenylisothiocyanate derivatization of the amino acids as described previously (2, 3, 5). The levels of the amino acids measured were similar between wild-type and pknG mutant bacteria (expressed as percentages of total amino acids determined [Tables 1 and 2]). Importantly, no difference in the levels of glutamine and glutamate was observed, indicating that in M. bovis BCG, glutamine does not accumulate upon pknG deletion.
FIG. 4.
Glutamine levels in lysates of M. bovis BCG and M. bovis BCG-ΔpknG as measured via enzymatic deamination by glutaminase.
TABLE 1.
Free glutamine/glutamate levels in M. bovis BCG and M. bovis BCG-ΔpknG grown in Proskauer-Beck medium
| Amino acid | Amino acid level (pmol) (mean ± SD) of indicated M. bovis strain
|
|
|---|---|---|
| BCG | BCG-ΔpknG | |
| Glutamine | 5.77 ± 0.24 | 6.1 ± 0.21 |
| Glutamate | 36.57 ± 1.11 | 40.20 ± 1.17 |
TABLE 2.
Free glutamine/glutamate levels in M. bovis BCG and M. bovis BCG-ΔpknGgrown in Sauton medium
| Amino acid | Amino acid level (pmol) (mean ± SD) of indicated M. bovis strain
|
|
|---|---|---|
| BCG | BCG-ΔpknG | |
| Glutamine | 0.85 ± 0.09 | 0.64 ± 0.10 |
| Glutamate | 28.11 ± 2.81 | 29.12 ± 2.00 |
TABLE 3.
Total glutamine levels in M. bovis BCG and M. bovis BCG-ΔpknG grown in Sauton and Proskaur-Beck media
| Medium | Glutamine level (pmol) (mean ± SD) of indicated M. bovis strain
|
|
|---|---|---|
| BCG | BCG-ΔpknG | |
| Proskaur-Beck | 20.73 ± 0.19 | 20.00 ± 0.13 |
| Sauton | 11.28 ± 0.15 | 11.20 ± 0.41 |
Concluding remarks.
The availability of glutamine for mycobacteria has been suggested to be crucial for growth and survival both in vitro and within macrophages (18). The positioning of a gene encoding a putative glutamine binding protein, glnH, adjacent to pknG, has led to the suggestion that in M. tuberculosis, one function of PknG is associated with glutamine metabolism (1, 8). While PknG may perform an as yet unknown function in mycobacterial physiology, the results presented here show that the depletion of PknG in M. bovis BCG has no effect on in vitro growth. In addition, there was no detectable defect in glutamine uptake or altered intracellular concentration observed in our experiments, suggesting that modulation of glutamine metabolism does not contribute to the intracellular degradation of M. bovis BCG lacking PknG (19).
There have been examples suggesting variations in gene functions and pathogenesis among M. tuberculosis, M. bovis, and M. bovis BCG (17). The molecular basis for different roles described for PknG in these bacteria needs to be further characterized.
Acknowledgments
We thank U. Kampfer and J. Schaller for analysis of amino acid concentrations and M. Weber and B. Zanolari for expert technical assistance.
This work was supported by a FEBS fellowship (to A.W.) and grants from the World Health Organization and the Swiss National Science Foundation (to J.P.).
REFERENCES
- 1.Av-Gay, Y., and M. Everett. 2000. The eukaryotic-like Ser/Thr protein kinases of Mycobacterium tuberculosis. Trends Microbiol. 8:238-244. [DOI] [PubMed] [Google Scholar]
- 2.Bidlingmeyer, B. A., S. A. Cohen, and T. L. Tarvin. 1984. Rapid analysis of amino acids using pre-column derivatization. J. Chromatogr. 336:93-104. [DOI] [PubMed] [Google Scholar]
- 3.Bidlingmeyer, B. A., S. A. Cohen, T. L. Tarvin, and B. Frost. 1987. A new, rapid, high-sensitivity analysis of amino acids in food type samples. J Assoc. Off. Anal. Chem. 70:241-247. [PubMed] [Google Scholar]
- 4.Braibant, M., P. Gilot, and J. Content. 2000. The ATP binding cassette (ABC) transport systems of Mycobacterium tuberculosis. FEMS Microbiol. Rev. 24:449-467. [DOI] [PubMed] [Google Scholar]
- 5.Chang, J. Y., and R. Knecht. 1991. Direct analysis of the disulfide content of proteins: methods for monitoring the stability and refolding process of cystine-containing proteins. Anal. Biochem. 197:52-58. [DOI] [PubMed] [Google Scholar]
- 6.Cole, S. T. 1999. Learning from the genome sequence of Mycobacterium tuberculosis H37Rv. FEBS Lett. 452:7-10. [DOI] [PubMed] [Google Scholar]
- 7.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, B. G. Barrell, et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 8.Cowley, S., M. Ko, N. Pick, R. Chow, K. J. Downing, B. G. Gordhan, J. C. Betts, V. Mizrahi, D. A. Smith, R. W. Stokes, and Y. Av-Gay. 2004. The Mycobacterium tuberculosis protein serine/threonine kinase PknG is linked to cellular glutamate/glutamine levels and is important for growth in vivo. Mol. Microbiol. 52:1691-1702. [DOI] [PubMed] [Google Scholar]
- 9.Gatfield, J., and J. Pieters. 2003. Molecular mechanisms of host-pathogen interaction: entry and survival of mycobacteria in macrophages. Adv. Immunol. 81:45-96. [DOI] [PubMed] [Google Scholar]
- 10.Harth, G., D. L. Clemens, and M. A. Horwitz. 1994. Glutamine synthetase of Mycobacterium tuberculosis: extracellular release and characterization of its enzymatic activity. Proc. Natl. Acad. Sci. USA 91:9342-9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harth, G., and M. A. Horwitz. 1999. An inhibitor of exported Mycobacterium tuberculosis glutamine synthetase selectively blocks the growth of pathogenic mycobacteria in axenic culture and in human monocytes: extracellular proteins as potential novel drug targets. J. Exp. Med. 189:1425-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harth, G., P. C. Zamecnik, J. Y. Tang, D. Tabatadze, and M. A. Horwitz. 2000. Treatment of Mycobacterium tuberculosis with antisense oligonucleotides to glutamine synthetase mRNA inhibits glutamine synthetase activity, formation of the poly-l-glutamate/glutamine cell wall structure, and bacterial replication. Proc. Natl. Acad. Sci. USA 97:418-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nohno, T., T. Saito, and J. S. Hong. 1986. Cloning and complete nucleotide sequence of the Escherichia coli glutamine permease operon (glnHPQ). Mol. Gen. Genet. 205:260-269. [DOI] [PubMed] [Google Scholar]
- 14.Parish, T., and N. G. Stoker. 1998. Mycobacteria protocols. Humana Press, Totowa, N.J.
- 15.Pieters, J. 2001. Evasion of host cell defense mechanisms by pathogenic bacteria. Curr. Opin. Immunol. 13:37-44. [DOI] [PubMed] [Google Scholar]
- 16.Russell, D. G. 2001. Mycobacterium tuberculosis: here today, and here tomorrow. Nat. Rev. Mol. Cell Biol. 2:569-577. [DOI] [PubMed] [Google Scholar]
- 17.Steyn, A. J., D. M. Collins, M. K. Hondalus, W. R. Jacobs, Jr., R. P. Kawakami, and B. R. Bloom. 2002. Mycobacterium tuberculosis WhiB3 interacts with RpoV to affect host survival but is dispensable for in vivo growth. Proc. Natl. Acad. Sci. USA 99:3147-3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tullius, M. V., G. Harth, and M. A. Horwitz. 2003. Glutamine synthetase GlnA1 is essential for growth of Mycobacterium tuberculosis in human THP-1 macrophages and guinea pigs. Infect. Immun. 71:3927-3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walburger, A., A. Koul, G. Ferrari, L. Nguyen, C. Prescianotto-Baschong, K. Huygen, B. Klebl, C. Thompson, G. Bacher, and J. Pieters. 2004. Protein kinase G from pathogenic mycobacteria promotes survival within macrophages. Science 304:1800-1804. [DOI] [PubMed] [Google Scholar]