Abstract
The tpr gene family of Treponema pallidum subsp. pallidum, the causative agent of syphilis, has recently become the focus of intensive investigation. TprF and TprI sequences are highly conserved among different isolates and are the targets of strong humoral and cellular immune responses of the host, and immunization with a recombinant peptide from the amino terminus of these antigens has been shown to alter significantly lesion development following homologous challenge. This indicates that these antigens are expressed during infection and strongly suggests a key functionality. tprF and tprI are located immediately downstream of the tprG and tprJ genes, respectively, separated by very short intergenic spacers (55 nucleotides for G-F and 56 nucleotides for J-I). Preliminary analysis using gene-specific primers failed to amplify tprJ in the Sea 81-4 isolate. In this study, sequence and transcriptional analysis of these loci showed a similar gene organization in the Nichols and Sea 81-4 strains, a complex pattern of transcription, and the presence of G homopolymeric repeats of variable lengths upstream of the tprF, tprI, tprG, and tprJ transcriptional start sites. However, distinctive features were also identified in the Sea 81-4 isolate, including a tprG-like open reading frame in the tprJ locus, a frameshift and a premature termination in the tprG coding sequence, a longer tprG-tprF intergenic spacer, and absence of cotranscription of the tprG-tprF genes.
Treponema pallidum subsp. pallidum, the etiologic agent of venereal syphilis, is highly pathogenic and able to cause a chronic, lifelong infection in the infected individual despite a vigorous humoral and cellular immune response (22). The Nichols strain genome sequence revealed 48 open reading frames (ORFs) coding for potential virulence factors (8). Among them, the tpr gene family consists of 12 paralogs with homology to the major sheath protein of Treponema denticola and accounts for approximately 2% of the small 1.138-Mb T. pallidum genome. According to their predicted amino acid homologies, the Tprs are divided into three subfamilies: subfamily I (tprC, -D, -F, and -I), subfamily II (tprE, -G, and -J), and subfamily III (tprA, -B, -H, -K, and -L) (1). Subfamily I and subfamily II Tprs, respectively, show common amino and carboxyl termini flanking central domains that differ in sequence and length. Only limited homology among its members characterizes subfamily III Tprs (1). TprF is unique in that it contains a deletion of the central nonconserved region and a portion of the C-terminal conserved region, resulting in a frameshift and premature termination. Analysis of the tprF and tprI ORFs in the genome sequence of the Nichols strain revealed that they are located immediately downstream of the tprG and tprJ genes separated by very short intergenic spacers (55 nucleotides for G-F and 56 nucleotides for J-I). Preliminary analysis using gene-specific primers showed that the tpr genes are conserved among syphilis isolates; however, tprJ-specific primers failed to demonstrate the presence of this gene in the Sea 81-4 strain, suggesting either the absence of tprJ in this strain or sequence differences in the primer binding sites.
In seven T. pallidum subsp. pallidum isolates examined to date (20), tprF and tprI are invariant, suggesting a key functionality of these genes. TprI and TprF are predicted to have NH2-terminal cleavable signal peptides and a putative outer membrane location. Immunization with a recombinant peptide comprising the conserved amino terminus of TprF/TprI altered significantly lesion development following intradermal homologous challenge, although it did not prevent infection (20). Additionally, antibody and T-cell responses to TprF/TprI during infection with different syphilis isolates have been recently studied in the rabbit model (12, 20). Overall, the TprF/TprI NH2-terminal conserved region elicits a marked antibody response during infection with the T. pallidum subsp. pallidum Chicago isolate and also strong T-cell responses during infection with different T. pallidum subsp. pallidum isolates (Nichols, Bal-3, Chicago, and Sea 81-4 strains) (20). Because the sequences of TprF and TprI are identical, differential reactivities cannot be explained by differences in inherent antigenicity or sequence heterogeneity in the antigens, suggesting modulation of tpr gene expression during infection. This is supported by recent microarray and real-time PCR studies (18). Our own studies using real-time PCR also support the hypothesis of differential transcription of the tpr genes among syphilis strains (unpublished data). Taken together, these data strongly suggest that these antigens are playing a significant role in the immune response to syphilis and likely in syphilis pathogenesis.
Gene regulation in T. pallidum has been little studied, and the mechanisms and regulatory elements of gene expression of the tpr genes remain to be defined. Because of the apparent importance of the TprF/TprI antigens in the immune response during syphilis infection and because strain-specific differences in neuroinvasion and clinical phenotype (21) between the Sea 81-4 and Nichols strains have been reported (strain Sea 81-4 induces higher pleocytosis in cerebrospinal fluid than Nichols, fewer indurated skin lesions after intravenous inoculation, and lower Venereal Disease Research Laboratory [VDRL] titers), we examined the sequence organization, promoter regions, and transcription patterns with respect to tprG and tprJ in the Nichols and Sea 81-4 isolates in a further attempt to understand the role of the tpr gene family during infection. We have identified in this study distinctive features in the Sea 81-4 isolate, such as a unique sequence composition of the tprJ locus (which contains a tprG-like ORF), a frameshift and premature termination of the ORF in the tprG locus, a longer tprG-tprF intergenic spacer, and a different pattern of transcription than the Nichols isolate.
MATERIALS AND METHODS
T. pallidum strain propagation and nucleic acid extraction.
T. pallidum subsp. pallidum Nichols and Sea 81-4 strains were propagated in New Zealand White rabbits as reported elsewhere (14). The Sea 81-4 strain was isolated in 1981 from the primary lesion of a patient attending the STD Clinic at Harborview Medical Center, Seattle, Wash. The Nichols strain, originally isolated from cerebrospinal fluid in 1912 (16), was provided by James N. Miller (University of California, Los Angeles) in 1979. Treponemes were extracted from infected rabbit testes as described elsewhere (3). Briefly, organisms were separated from host cellular debris by low-speed centrifugation and, then, supernatants were spun in a microcentrifuge for 30 min at 12,000 rpm at 4°C and the pellets were resuspended in 200 μl of 1× lysis buffer (10 mM Tris, pH 8.0; 0.1 M EDTA; 0.5% sodium dodecyl sulfate) for DNA extraction or in 400 μl of Ultraspec buffer (Biotecx Laboratories, Inc., Houston, TX) for RNA isolation. DNA extraction was performed as previously described (2) using the QIAamp DNA Mini kit (QIAGEN Inc., Chatsworth, CA). RNA extraction was performed according to the manufacturer's instructions followed by treatment with DNase I (Invitrogen, Carlsbad, CA). DNase-treated RNA was stored at −80°C until use. Every extraction was performed taking careful precautions to prevent cross-contamination.
PCR amplification of Sea 81-4 tpr genes, cloning, sequencing, and sequence analysis.
Primers were designed upstream of tprJ and tprG as well as downstream of tprI and tprF, based on the Nichols sequences. They were used to amplify the corresponding regions spanning the tprJ/tprI and the tprG/tprF ORFs from the Sea 81-4 strain (Table 1). The reactions were performed using the LA-PCR long PCR kit (Takara Bio Inc., Shiga, Japan) in a 100-μl final volume containing 200 μM deoxynucleoside triphosphates (dNTPs), 2.5 mM MgCl2, and 0.05 U/μl of LA-Taq. The cycling conditions were denaturation at 94°C for 1 min, followed by 35 cycles of 98°C for 20 s and 68°C for 5 min. Final extension was 72°C for 10 min. The products were separated in 1% agarose gels, purified using the QIAquick gel extraction kit (QIAGEN), and cloned into the TOPO-XL cloning vector (Invitrogen). Plasmid DNA from colonies containing inserts was extracted using the QIAGEN Plasmid Mini kit (QIAGEN) and sequenced in both directions with the Applied Biosystems dye terminator sequencing kit (Perkin-Elmer, Foster City, CA) using the primer walking approach. Sequences were aligned using the Multiple Alignment program (http://searchlauncher.bcm.tmc.edu/multi-align/multi-align.html), and shading of identical bases was done with the Boxshade 3.21 program (http://www.ch.embnet.org/software/BOX_form.html). Open reading frames were determined using the NCBI ORF finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html) and by sequence comparison with the Nichols tpr loci.
TABLE 1.
Primers used in this study
| Purpose and primer name | 5′ to 3′ sequence | Product size (bp)a, primer combination | Annealing temp (°C) |
|---|---|---|---|
| Cloning | |||
| TPRJ-L | CGAGTGAGGCTCATCAAGAA | 5,205, TPRJ-L with I-R | 68 |
| I-R | AGTAAGCCCTGCCCAAGAAC | ||
| PG 1 | CCCTGCGTTTCCCATCTG | 4,233, PG1 with TPRF-R | 68 |
| TPRF-R | GTACTACCTTCCCCCGGTCT | ||
| RT-PCR (controls) | |||
| JS | TCTTCACACCCCGCAGGGAA | 364, JS with JAs | 65 |
| JAs | CGTTATTTCCGTTCGCATCATC | ||
| ISb | GACCCTGCCGATGCAGGTAAT | 336, IS with IAs | 65 |
| IAs | TAAGCACGATGTCCGACTGACT | ||
| GS | GAAGGTGTTCATTACCGACCCT | 359, GS with GAs | 65 |
| GAs | TTGTAGCCTCAGCCGTAAGCTT | ||
| FSb | GACCCTGCCGATGCAGGTAAT | 266, FS with Fas | 65 |
| FAs | TCAGCAAGCACCCCCTGTTC | ||
| tp47S | CGTGTGGTATCAACTATGG | 310, tp47S with tp47As | 63 |
| tp47As | TCAACCGTGTACTCAGTGC | ||
| JI 1 | CCTTCCGGCGGTTCCTC | 558 (N.), 559 (S.), JI 1 with JIGF 1; 504 (N), 505 (S.), JI 1 with JIGF 2 | 60 |
| RT-PCR (intergenic spacers) | |||
| JI 2 | TCCTCAGGGCACATTGGCCT | 546 (N), 547 (S.), JI 2 with JIGF 1; 492 (N.), 493 (S.), JI 2 with JIGF 2 | 63 |
| GF 1 (Nichols specific) | GTGCGTGGCATTCAGGAAAA | 562, GF 1 with JIGF 1 | 60 |
| 508, GF 1 with JIGF 2 | 60 | ||
| sGF 1 (Sea 81-4 specific) | GACGCTATACAAAAATAATAACG | 604, sGF 1 with JIGF 1 | 62 |
| sGF 2 (Sea 81-4 specific) | CCGGCAGTTCCTCAGGAAAA | 507, sGF 2 with JIGF 2 | 62 |
| JIGF 1 | GCCAGTGCGTGGATTCTT | ||
| JIGF 2 | ACTGACCTGCGGAGTGAGTA | ||
| PJ 1 | AAGTTTGCTTTCAGATCCGC | 344 (N.), 343 (S.), PJ 1 with PJG | 60 |
| RT-PCR (5′-flanking regions) | |||
| PJ 2 | AAAGAAAAAGGATTTCCGCA | 284 (N.), 283 (S.), PJ 2 with PJG | |
| PJ 3 | GAACAATGCGCAGCACCG | 224 (N.), 223 (S.), PJ 3 with PJG | 60 |
| PG 1 | CCCTGCGTTTCCCATCTG | 365 (N.), 368 (S.), PG 1 with PJG | 60 |
| PG 2 | CGCGTACCCACTTCTCTCTC | 287, PG 2 with PJG | 60 |
| PG 3 | AAACATGCGCAAAATAAGGG | 242, PG 3 with PJG | 60 |
| PJG | GTGGCAGAGCCAGTTAGCTT | ||
| 5′-RACE | |||
| J/G RACE-1c | CATACAGTGCCGGGTGCT | cDNA synthesis only | |
| J/G-RACE-2 | GCATAACCAGGGGAAAGGAT | 395, J/G-RACE with RACE AAP primer | 63 |
| F/I-RACE-1c | GACGCAAGCTCTACTGCCA | cDNA synthesis only | |
| F/I-RACE-2 | TTCAGCTGCAGCTGTGCC | Expected size, 337 | 63 |
| F/I-RACE-3 | GCGGGTGTGGGTGTGCT | 293, F3-RACE with RACE AUA primer | 63 |
| IVT | |||
| PJ 1 | AAGTTTGCTTTCAGATCCGC | 351, PJ 1 with JG-IVT-rev | 60 |
| PG 1 | CCCTGCGTTTCCCATCTG | 372, PJ 1 with JG-IVT-rev | 60 |
| JG-IVT-rev | CTGCAGTGTGGCAGAGCCAG | ||
| E/I 1 | ACAGCTGCGTGCTGGTATTT | 520 (N.), 521 (S.), E 1 with E-IVT-rev | 60 |
| E/I-IVT-rev | CTGCAGAGACGCAAGCTCTACTGC |
(N.) indicates product size in the Nichols strain; (S.) indicates product size in the Sea 81-4 strain.
IS and FS primers are identical.
J/I-RACE-1 is specific for both tprJ and tprI, and G/F-RACE-1 is specific for both tprG and tprF.
RT-PCR analysis.
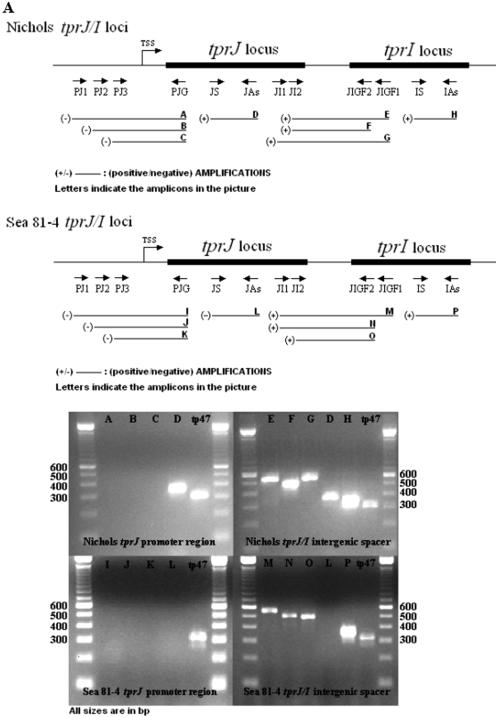
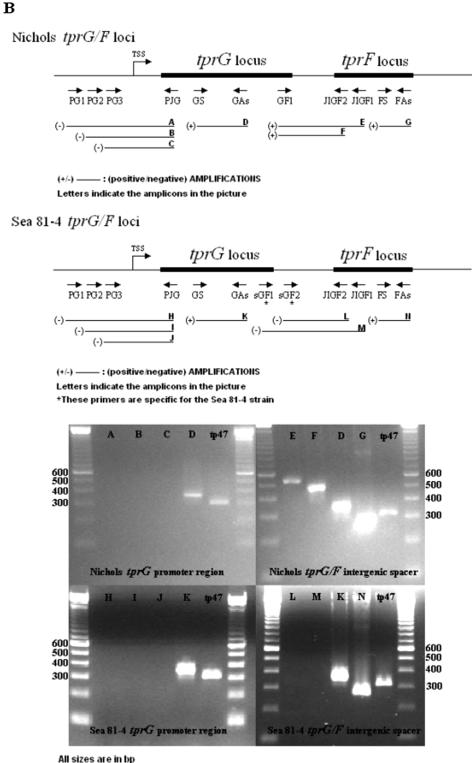
All primers used in this study are listed in Table 1. Three sets of primers were used to determine the presence of mRNA spanning the intergenic spacers between tprJ and tprI in the Nichols strain (JI1 plus JIGF1, JI2 plus JIGF1, and JI2 plus JIGF2) as well as in the Sea 81-4 strain (JI1 plus JIGF1, JI1 plus JIGF2, and JI2 plus JIGF2), two sets of primers for the Nichols tprG-tprF intergenic region (GF1 plus JIGF1 and GF2 plus JIGF2), and two sets for the Sea 81-4 tprG-tprF spacer (sGF1 plus JIGF1 and sGF2 plus JIGF2). To investigate the presence of transcript upstream of tprJ and tprG in the Nichols and Sea 81-4 strains and as a first approach to localize possible transcriptional start sites (TSSs), a total of six primer combinations were used in the 5′-flanking regions of tprJ (PJ1 plus PJG, PJ2 plus PJG, and PJ3 plus PJG) and tprG (PG1 plus PJG, PG2 plus PJG, and PG3 plus PJG). The PJG antisense primer sequence is shared by both tprJ and tprG. Specific primers targeting the central regions of tprJ, tprI, tprG, and tprF were used to assess the presence of gene-specific mRNA (listed as reverse transcription-PCR [RT-PCR] control primers in Table 1). Primers that amplify tp47 were used as a positive control. Primer sequences and optimal annealing temperatures for each primer set are listed in Table 1; the primer locations are shown schematically in Fig. 3, below.
FIG. 3.
Transcriptional analysis of Nichols and Sea 81-4 tprJ-tprI and tprG-tprF loci. Primer positions used for the RT-PCR analysis are schematically shown along with the amplification result (+ or −) for each reaction. Expected amplicons are named A through P. Pictures show only results obtained from cDNA. DNase I-treated RNA, DNase I-untreated RNA, and DNA were used as negative or positive controls (not shown). (A) The tprJ-tprI intergenic spacer is shown to be transcribed in both strains, indicating cotranscription of these two genes. No amplification of the tprJ 5′-flanking region was detectable in either strain, due to lack of transcript encompassing this region. (B) tprG and tprF are shown to be cotranscribed only in the Nichols strain. No amplification of the Sea 81-4 tprG-F intergenic spacer was seen. tprG 5′-flanking region amplification in both strains was also negative. In both panels A and B, lengths of ORFs, intergenic regions, flanking regions, and amplicons are not reported on scale, to facilitate comprehension of primer positions.
cDNA synthesis from DNase I-treated T. pallidum RNA was performed as described elsewhere (1). The same primers were used in both strains, except when otherwise specified. PCR amplifications were performed in a 50-μl final volume, containing 200 μM dNTPs, 50 mM Tris-HCl (pH 9.0 at 20°C), 1.5 mM MgCl2, 20 mM NH4SO4, and 2.5 U of Taq polymerase (Promega, Madison, WI). The cycling conditions were denaturation at 95°C for 2 min, followed by 95°C for 1 min, annealing (at temperatures reported in Table 1) for 1 min, and extension at 72°C for 1 min for 45 cycles. Final extension was 72°C for 10 min. DNase I-treated RNA, DNase I-untreated RNA, and DNA were used as amplification controls. All PCR products were separated in 2% agarose gels.
In order to assess sequence identity and primer specificity, the amplicons relative to the promoter regions and intergenic spacers from the Sea 81-4 and Nichols strains obtained from both cDNA (when a positive signal could be retrieved) and DNA (used as positive controls in the reactions) were cloned into the TOPO-TA cloning vector (Invitrogen). Plasmids were purified from the clones containing inserts using the QIAGEN Plasmid Mini kit (QIAGEN). Sequencing was performed as described above. Cloned amplicons from the promoter regions in both strains were generated with the primers PJ2 and PG2 in combination with PGJ (Table 1). The products cloned from the tprJ-tprI intergenic spacer were obtained with the JI2 and JIGF1 primers (Table 1), also common to both strains. In contrast, cloned amplicons from the tprG-tprF intergenic region were obtained with specific primers GF 1 and JIGF 1 for the Nichols strain and sGF 1 with JIGF 1 for the Sea 81-4 strain (Table 1). An average of seven different clones were sequenced and analyzed for each region.
IVT assay.
Initial computer analysis (using the Neural Network Promoter Prediction program for prokaryotes, available at http://www.fruitfly.org/seq_tools/promoter.html) predicted the presence of putative sigma 70-like promoters in the 5′-flanking regions of tprG, tprJ, and tprF/I (sequences are identical for tprF and tprI in this region) in both strains. The regions upstream of these four tpr genes were amplified only from the Nichols strain, because they are identical in both strains (primers in Table 1). They were initially cloned into the TOPO-TA vector. The inserts were sequenced to verify sequence identity and the absence of mutations. The inserts were subsequently excised using the EcoRI and PstI restriction sites and subcloned into the pSA509 vector (kindly provided by D. J. Jin, Laboratory of Molecular Biology, National Cancer Institute, National Institutes of Health, Bethesda, Md.), specifically designed for in vitro transcription (IVT) assays (4). Plasmid-containing inserts were purified using the QIAgen Plasmid Mini kit (QIAGEN) and brought to a final concentration of 1 μg/ml with diethyl pyrocarbonate (DEPC)-treated water.
One microgram of pSA509 plasmid carrying the putative promoter sequence of tprJ, tprG, or tprF/I was then mixed with 2 μl of 5× transcription buffer (0.2 M Tris-HCl, pH 7.5, 0.75 M KCl, 50 mM MgCl2, 0.05% Triton X-100, 0.5 M dithiothreitol), 1 U of Escherichia coli RNA polymerase holoenzyme (Epicenter, Madison, WI), and DEPC-treated water to a final volume of 9 μl. After preincubation at 37°C for 15 min, 1 μl of 10× biotin RNA labeling mix (10 mM ATP, 10 mM CTP, 10 mM GTP, 6.5 mM UTP, 3.5 mM biotin-16-UTP, pH 7.5 [F. Hoffmann-La Roche Ltd., Basel, Switzerland]) was added to the reaction mixture and then incubated for 10 min at 37°C. The products obtained from the IVT assay were collected by brief centrifugation and treated with molecular-grade DNase I (Invitrogen). Five microliters of 10× DNase I buffer, 5 U of DNase I, and DEPC-treated water to a 50-μl final volume were added to the reaction mixture and then incubated at room temperature for 15 min. The reaction was stopped by adding 5 μl of 25 mM EDTA to the mixture followed by a 10-min incubation at 65°C. The IVT products were then purified by phenol-chloroform extraction (15) and analyzed by using a 5-′ rapid amplification of cDNA ends (5′-RACE) system.
5′-RACE.
The 5′-RACE system (Invitrogen) was used to determine TSSs in the 5′-flanking regions of tprF, tprI, tprJ, and tprG. 5′-RACE analysis was performed on two different templates: T. pallidum total RNA (tprJ and tprG as well as tprF and tprI promoters of the Nichols and Sea 81-4 isolates [Table 2]) and RNA obtained from the IVT assays (tprF/I, tprG, and tprJ promoters of both isolates [Table 2]) as described above. 5′-RACE analysis was performed following the manufacturer's instructions on RNA obtained from IVT assays. When 5′-RACE was applied to total RNA, we followed the manufacturer's protocol except that the SuperScript system for cDNA synthesis was replaced by the ThermoScript RT kit (Invitrogen) to increase yield due to the high GC content (52.8%) of the T. pallidum genome. A 2.5-pmol aliquot of gene-specific primer and 2 μg of sample RNA were used in each reaction mixture. After the reaction was terminated, 1 μl of RNase H was added to the tube and the cDNA was incubated for 20 min at 37°C. Primers used in this set of experiments are listed in Table 1. Primers J/G-RACE 1 and F/I-RACE 1, used for initial retrotranscription from total T. pallidum RNA, are tprJ/G and tprI/F specific, respectively, due to sequence identity between these pairs of genes in the region where the primer was designed. All PCR amplification reactions were performed using 5 μl of dC-tailed cDNA in a 50-μl final volume containing 200 μM of each dNTP, 20 mM Tris-HCl (pH 8.4), 1.5 mM MgCl2, 50 mM KCl, 400 nM of each primer, and 2.5 U of Taq DNA polymerase (Invitrogen). For nested PCR, 1 μl of the original amplicon of a 1/500 dilution was used. Cycling parameters were denaturation for 2 min at 94°C, followed by 1 min at 94°C, annealing for 1 min at 63°C, and extension for 1 min at 72°C. Final extension was 10 min at 72°C. Thirty-five cycles were required for amplification of IVT-generated transcripts, 45 cycles for when 5′-RACE was used on total RNA, and 35 cycles for nested PCR to identify the Sea 81-4 tprF TSS. PCR products were separated in 2% agarose gels, gel purified, cloned, and sequenced as described above.
TABLE 2.
5′-RACE and 5′-RACE-IVT reactions.
| Gene(s) | 5′ RACE on total RNA | 5′-RACE on IVT RNA |
|---|---|---|
| tprJ | Performed on Nichols and Sea 81-4 strains; primer J/G-RACE-1 is shared by both tprJ and tprG; it is located at position +282 in the ORF. | Performed on the tprJ putative promoter sequence cloned from the Nichols straina with the J/G-RACE-1 primer |
| tprG | Same as for tprJ | Performed on the tprG putative promoter sequence cloned from the Nichols straina with the J/G-RACE-1 primer |
| tprI/tprF | Performed on Nichols and Sea 81-4 strains; primer F/I-RACE-1 is shared by both tprF and tprI; it is located at position +295 in the ORF. | Performed on the tprF/Ib putative promoter sequence cloned from the Nichols straina with the F/I-RACE-1 primer |
This sequence is identical in both the Sea 81-4 and Nichols strains of T. pallidum.
tprI and tprF share an identical sequence in this region.
RESULTS
Sequence organization of the Sea 81-4 tprJ/I and tprG/F ORFs.
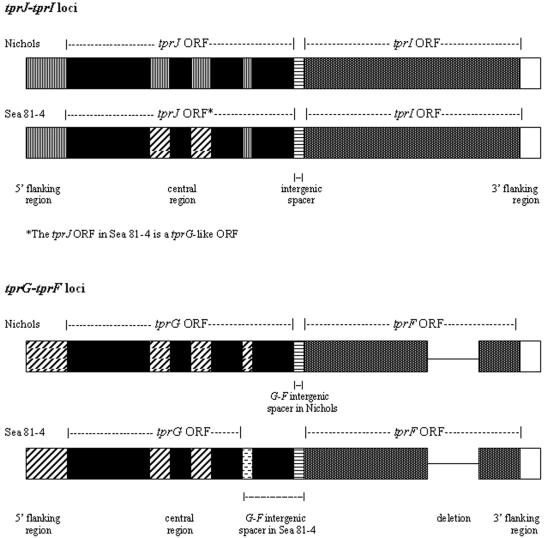
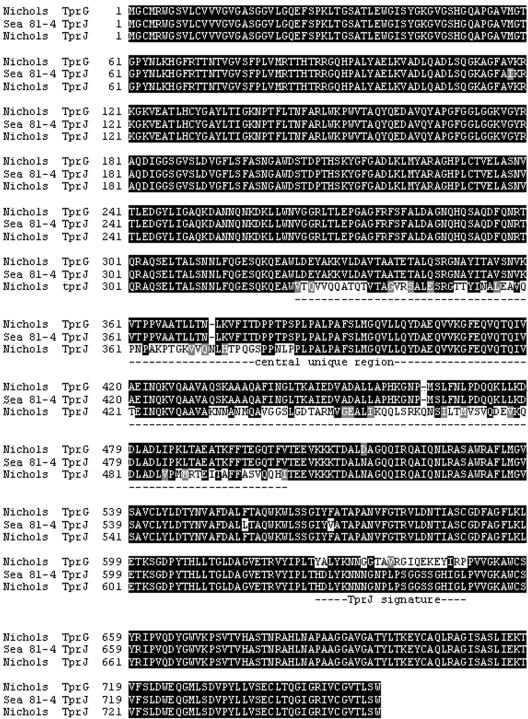
Long-range PCR amplification using primers (Table 1) that bind upstream of the start codons of tprJ and tprG in combination with primers binding the regions downstream of the stop codons of tprI and tprF, respectively, yielded in the Sea 81-4 isolate two amplicons of 5,205 and 4,903 bp in length (Fig. 1; see also Fig. S1A and S1B in the supplemental material). After cloning and sequencing these amplicons, comparative analysis of the Sea 81-4 sequences with the corresponding regions of the Nichols isolate showed an overall similar gene organization with two significant differences: (i) the tprJ locus in Sea 81-4 contains a tprG-like ORF, almost identical to tprG except for a tprJ nucleotide signature of 49 bases near the 3′ end (Fig. 2; see also Fig. S1A and S2 in the supplemental material); and (ii) the predicted TprG amino acid sequence in Sea 81-4 is 124 amino acids shorter than its homolog in Nichols (632 amino acids instead of 756 amino acids), due to an extra adenosine in the 3′ end (see Fig. S1A in the supplemental material), which introduces a frameshift in the coding sequence and an earlier stop codon. Due to this C-terminal truncation of the TprG protein, the Sea 81-4 tprG-tprF intergenic spacer is 422 nucleotides long, instead of 56 bp as in Nichols (see Fig. S1B in the supplemental material). No other sequence changes were observed between the Sea 81-4 and Nichols TprG ORFs, except for two single amino acid changes upstream of the frameshift (see Fig. S3 in the supplemental material). The TprF sequences between the two strains show two amino acid changes upstream of the central nonconserved region in Sea 81-4 (see Fig. S4 in the supplemental material). A trinucleotide insertion and a single base change differentiated the tprG 5′-flanking regions of Sea 81-4 from the Nichols strain sequence, and a single nucleotide change was seen in the 3′-flanking region of tprF (see Fig. S1B in the supplemental material).
FIG. 1.
Schematic representation of the tprF, tprG, tprI, and tprJ loci in the Nichols and Sea 81-4 strains. Similar patterns indicate sequence identity. The Sea 81-4 strain tprG-like ORF central region is identical to the Sea 81-4 and Nichols tprG central regions. The Sea 81-4 TprG is shorter than its homolog in Nichols due to a premature stop generating a longer tprG-tprF spacer. tprI and tprF loci are virtually identical in both isolates. A few base pair changes are described in the text.
FIG. 2.
Alignment of the deduced protein sequences of the Sea 81-4 tprJ and Nichols tprG and tprJ ORFs. Identical residues are indicated by black shading, and nonsynonymous changes are indicated in gray or no shading. The tprJ locus in Sea 81-4 contains a tprG-like ORF. The Sea 81-4 TprJ central region (amino acid positions 326 to 502) has been replaced by the corresponding Nichols/Sea 81-4 TprG sequence; the COOH terminus is identical to the Nichols TprJ (amino acid positions 627 to 649).
Transcriptional analysis of the tpr 5′-flanking and intergenic regions.
RT-PCR analysis was performed to determine whether tprJ and tprI as well as tprG and tprF are cotranscribed and to roughly define the boundaries of the 5′ ends of the tprJ and tprG putative promoter regions. cDNA from both T. pallidum strains consistently gave PCR amplicons for the tprJ-I intergenic spacer with all primer sets used, indicating that these two genes are cotranscribed in both strains (Fig. 3A ). In contrast, transcript spanning the tprG-tprF intergenic region was detected only in the Nichols strain; cDNA from the Sea 81-4 isolate gave no signal (Fig. 3B) with any of the relevant primer sets used. However, a positive RT-PCR using the F-S- and F-As-specific primers, which target a specific central region of tprF, indicated transcription of tprF and, by inference, the presence of a promoter in its 5′-flanking region able to drive transcription of this gene (Fig. 3B). Negative controls (DNase I-treated RNA and H2O) always gave no amplification, ruling out DNA contamination.
None of our primer combinations gave a positive signal from cDNA when the regions upstream of tprG and tprJ ORFs containing the putative promoter regions were analyzed by RT-PCR. This suggests the presence of a TSS within the first 115 bp upstream of the tprG putative start codon in both strains and within the first 99 bp and 98 bp upstream of the predicted tprJ start codon in the Nichols and Sea 81-4 isolates, respectively (Fig. 3B).
Specific mRNA for tprG was consistently detected in cDNA from the Nichols and Sea 81-4 strains and for tprJ in cDNA from the Nichols isolate. No transcript was detected for tprJ in the Sea 81-4 strain (Table 1) due to lack of the target sequence in this locus (the tprJ locus in the Sea 81-4 strain contains a tprG-like ORF instead) or elsewhere in the chromosome. Transcript for the tp47 gene was always present in both strains, indicating the presence of T. pallidum RNA in all samples. DNase I-untreated RNA and DNA always gave positive amplification. DNase I-treated RNA controls without the reverse transcription step were always negative (data not shown).
Identification of transcriptional start sites and homopolymeric repeats of variable lengths in 5′-flanking regions.
To define the TSSs of the tprJ and tprG genes in the Nichols and Sea 81-4 strains, we used 5′-RACE alone on total T. pallidum RNA; results were then confirmed using IVT followed by 5′-RACE assays on cloned promoter regions of these genes. TSSs for tprG and tprJ were identified 9 bases upstream of the start codons described in the Nichols genome sequence (Fig. 4A and B).
FIG. 4.
tprJ, tprG, and tprF and tprI TSSs identified using IVT in combination with 5′-RACE and 5′-RACE applied to RNA extracted from both strains. +1, transcriptional start site; Met, translational start according to the Nichols genome sequence (8). Bold lowercase sequences represent the homopolymeric repeats. Boxed codons represent alternative predicted translational start sites. Bold italic lowercase sequences represent putative ribosomal binding sites. *, The number of Gs in the homopolymeric repeat preceding the TSSs varied in the different clones.
We used the same approach to determine the presence of alternative TSSs upstream of tprF and tprI in both isolates. Alternative tprF and tprI TSSs were found in both strains, 29 bases upstream of their predicted start codon (Fig. 4C). Remarkably, all TSSs identified are located immediately downstream of G polynucleotide repeats.
Initial alignments of the promoter regions of all four genes in the published Nichols genome sequence revealed the presence of G polynucleotide repeats. Sequencing of the cloned amplicons from the transcriptional analysis not only confirmed the specificity of the amplifications but also revealed variability in length of the G homopolymeric tracts in clones from the Sea 81-4 and Nichols strains. The lengths of the tprJ and tprG G repeats in the Sea 81-4 strain vary from 7 to 11 nucleotides and from 8 to 11 nucleotides, respectively. In the Nichols isolate these repeats contain 9 to 11 nucleotides in both the tprJ and tprG promoter regions. The upstream regions of tprF and tprI (intergenic spacers) contain repeats that are 8 to 10 nucleotides long (tprJ-tprI intergenic regions) and 8 to 9 nucleotides long (tprG-tprF spacers) in both strains.
DISCUSSION
The organization of the tprJ-tprI and tprG-tprF loci in the Sea 81-4 strain is similar to the gene organization reported for the Nichols strain. The Sea 81-4 tprF and tprI genes show only a few single-nucleotide differences compared to their homologs in the Nichols strain and six other syphilis isolates (20), suggesting functional conservation and, although still undetermined, an important role common to all T. pallidum strains. The presence of the tprG-like ORF in the tprJ locus of the Sea 81-4 strain is intriguing in that this is the only syphilis isolate known to date to carry such a gene in this locus; at least seven other syphilis isolates appear to have the Nichols tprJ allele as determined by PCR analysis with tprJ-specific primers (data not shown). However, the Sea 81-4 tprJ ORF is nearly identical to the tprJ locus ORF in the Gauthier isolate of Treponema pallidum subsp. pertenue (>98% identity) (19) and the Treponema paraluiscuniculi tprG1 and tprG2 genes (>96% identity) (9). The high degree of sequence conservation of this tprG-like ORF among species suggests an ancient origin of this gene, and the presence of syphilis isolates with tpr genes such as the Sea 81-4 tprG-like ORF in other subspecies or species suggests different evolutionary pathways among syphilis strains from a common predecessor. It is unlikely that the Sea 81-4 isolate could mutate the tprG-like sequence to generate a Nichols tprJ-like sequence (the Nichols tprG and tprJ sequences differ in 605 nucleotides) unless lateral gene transfer between an isolate carrying the tprJ ORF and the Sea 81-4 strain occurred. An alternative explanation for this unique sequence composition may be geographical distribution, although the absence of this tprG-like ORF in other strains isolated from the same region (Seattle) in the same year (1981) speaks against this possibility.
Because T. pallidum cannot be cultured in vitro continuously, only limiting amounts of RNA can be obtained. For this reason, we chose the 5′-RACE assays alone and in combination with IVT assays to identify the TSSs of tprF, tprI, tprG, and tprJ. Sometimes, different assays may result in the identification of slightly different transcriptional start sites, usually within a few bases of each other. In our case, both approaches identified the same transcriptional starts in both the Nichols and the Sea 81-4 strains. The positions of all four TSSs are similar in that they are located immediately downstream of a G homopolymeric repeat. This unique architecture has been previously described in T. pallidum by Weigel et al. (24) for the tp47 gene. The tp47 transcriptional start site is also located immediately downstream of a G homopolymeric repeat, as determined by both primer extension and with the E. coli transcription machinery in vivo. It is of interest that the tpr TSSs are all Ts, while in E. coli, the majority of experimentally demonstrated TSSs are usually Gs or As. However, several other E. coli genes have been also shown to have TSSs containing pyrimidines (13). To date, it is unknown what the biological significance of this phenomenon is and what proportion of TSSs in T. pallidum start with purines or pyrimidines. In T. pallidum, only a few TSSs have been deduced experimentally to date (10, 11, 24).
A predicted sigma 70 ORF is described in the published Nichols genome sequence. The ability of the 5′-flanking regions of tprJ, tprI, tprG, and tprF to drive transcription using the E. coli sigma 70 holoenzyme indicates the presence of sigma 70-like promoters within these regions. However, the absence of typical E. coli −35 and −10 consensus sequences is interesting. This finding may represent unique promoters in T. pallidum. Lack of −35 and −10 typical E. coli sigma 70 signatures has been already reported in the promoter region of the tp47 gene of T. pallidum (24), indicating that this is not unique to the tpr genes.
RT-PCR analysis showed cotranscription of tprG and tprF as well as of tprJ and tprI in the Nichols strain. Of interest is the observation that 5′-RACE plus IVT performed on the tprF/I 5′-flanking region also showed the ability of these regions to drive transcription. Similarly, the transcription of tprF in the Sea 81-4 strain independently from tprG is interesting, indicating the presence of a TSS able to generate suboperonic transcripts for tprF/I in both strains. Suboperonic transcription in a rather larger polycistronic transcript has been already described in bacterial organisms, including spirochetes (17, 6, 7). This takes place when one or more genes from the largest polycistronic transcript are transcribed independently from the others to fulfill a different function, providing an organism with the ability to respond to particular needs or host signals.
Homopolymeric repeats in promoter regions have been shown to regulate virulence gene expression in several bacterial pathogens. Two classic examples are the variable expression of the PorA antigen of Neisseria meningitidis (23) and the Vlp variable lipoproteins of Mycoplasma hyorhinis (5). These organisms control transcription of these genes by altering the length of the poly(G) and poly(A) repeats in their promoters, respectively. The homopolymeric G repeats located immediately upstream of the TSSs of the tprF, tprI, tprG, and tprJ loci vary in length in individual bacteria and between the Nichols and Sea 81-4 strains in DNA and mRNA samples and are likely to be involved in regulation of the transcription levels of these genes by modulating promoter strength and inducing differential expression of these genes among T. pallidum strains over the course of infection. The difference in the number of G residues is not due to PCR or sequencing artifacts. This is supported by the fact that homopolymeric repeats in the promoter region of the Nichols tprK (another tpr homolog) always show the same number of nucleotides in clones obtained from different DNA batches, PCRs, and sequencing reactions.
There are several issues that still remain unanswered, including promoter strength, levels of transcription over time, transcript length, correlation between levels of mRNA and protein synthesis, and patterns of expression in response to microenvironmental changes during infection. Nevertheless, the differences in sequence organization and cotranscription in these paralogous genes in the Nichols and Sea 81-4 strains suggest that they may be useful models for the study of gene regulation in this bacterium.
Supplementary Material
Acknowledgments
We are grateful to Sheila Lukehart for critical comments that improved the manuscript and to Heidi Pecoraro for manuscript preparation.
This work was supported by NIH grant AI42143 (A.C.L. and L.G.).
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Centurion-Lara, A., C. Castro, L. Barrett, C. Cameron, M. Mostowfi, W. C. Van Voorhis, and S. A. Lukehart. 1999. Treponema pallidum major sheath protein homologue TprK is a target of opsonic antibody and the protective immune response. J. Exp. Med. 189:647-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centurion-Lara, A., C. Castro, R. Castillo, J. M. Shaffer, W. C. Van Voorhis, and S. A. Lukehart. 1998. The flanking region sequences of the 15-kDa lipoprotein gene differentiate pathogenic treponemes. J. Infect. Dis. 177:1036-1040. [DOI] [PubMed] [Google Scholar]
- 3.Centurion-Lara, A., C. Castro, W. C. Van Voorhis, and S. A. Lukehart. 1996. Two 16S-23S ribosomal DNA intergenic regions in different Treponema pallidum subspecies contain tRNA genes. FEMS Microbiol. Lett. 143:235-240. [DOI] [PubMed] [Google Scholar]
- 4.Choy, H. E., and S. Adhya. 1992. Control of gal transcription through DNA looping: inhibition of the initial transcribing complex. Proc. Natl. Acad. Sci. USA 89:11264-11268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Citti, C., and K. S. Wise. 1995. Mycoplasma hyorhinis vlp gene transcription: critical role in phase variation and expression of surface lipoproteins. Mol. Microbiol. 18:649-660. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham, L., and J. R. Guest. 1998. Transcription and transcript processing in the sdhCDAB-sucABCD operon of Escherichia coli. Microbiology 144:2113-2123. [DOI] [PubMed] [Google Scholar]
- 7.Dobrikova, E. Y., J. Bugrysheva, and F. C. Cabello. 2001. Two independent transcriptional units control the complex and simultaneous expression of the bmp paralogous chromosomal gene family in Borrelia burgdorferi. Mol. Microbiol. 39:370-378. [DOI] [PubMed] [Google Scholar]
- 8.Fraser, C. M., S. J. Norris, G. M. Weinstock, O. White, G. G. Sutton, R. Dodson, M. Gwinn, E. K. Hickey, R. Clayton, K. A. Ketchum, E. Sodergren, J. M. Hardham, M. P. McLeod, S. Salzberg, J. Peterson, H. Khalak, D. Richardson, J. K. Howell, M. Chidambaram, T. Utterback, L. McDonald, P. Artiach, C. Bowman, M. D. Cotton, J. C. Venter, et al. 1998. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science 281:375-388. [DOI] [PubMed] [Google Scholar]
- 9.Giacani, L., E. S. Sun, K. Hevner, B. J. Molini, W. C. Van Voorhis, S. A. Lukehart, and A. Centurion-Lara. 2004. Tpr homologs in Treponema paraluiscuniculi Cuniculi A strain. Infect. Immun. 72:6561-6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hazlett, K. R. O., T. J. Sellati, T. T. Nguyen, D. L. Cox, M. L. Clawson, M. J. Caimano, and J. D. Radolf. 2001. The TprK protein of Treponema pallidum is periplasmic and is not a target of opsonic antibody or protective immunity. J. Exp. Med. 193:1015-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isaacs, R. D., and J. D. Radolf. 1990. Expression in Escherichia coli of the 37-kilodalton endoflagellar sheath protein of Treponema pallidum by use of the polymerase chain reaction and a T7 expression system. Infect. Immun. 58:2025-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leader, B. T., K. Hevner, B. J. Molini, L. K. Barrett, W. C. Van Voorhis, and S. A. Lukehart. 2003. Antibody responses elicited against the Treponema pallidum repeat proteins differ during infection with different isolates of Treponema pallidum subsp. pallidum. Infect. Immun. 71:6054-6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lisser, S., and H. Margalit. 1993. Compilation of E. coli mRNA promoter sequences. Nucleic Acids Res. 21:1507-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lukehart, S. A., S. A. Baker-Zander, and S. Sell. 1980. Characterization of lymphocyte responsiveness in early experimental syphilis. I. In vitro response to mitogens and Treponema pallidum antigens. J. Immunol. 124:454-460. [PubMed] [Google Scholar]
- 15.Maniatis, T., J. Sambrook, and E. F. Fritsch. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 16.Nichols, H. J., and W. H. Hough. 1913. Demonstration of Spirochaeta pallida in the cerebrospinal fluid. JAMA 60:108-110. [Google Scholar]
- 17.Schmid, M. B., and J. R. Roth. 1983. Internal promoters of the his operon in Salmonella typhimurium. J. Bacteriol. 153:1114-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smajs, D., M. McKevitt, J. K. Howell, S. J. Norris, W. W. Cai, T. Palzkill, and G. M. Weinstock. 2005. Transcriptome of Treponema pallidum: gene expression profile during experimental rabbit infection. J. Bacteriol. 187:1866-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stamm, L. V., S. R. Greene, H. L. Bergen, J. M. Hardham, and N. Y. Barnes. 1998. Identification and sequence analysis of Treponema pallidum tprJ, a member of a polymorphic multigene family. FEMS Microbiol. Lett. 169:155-163. [DOI] [PubMed] [Google Scholar]
- 20.Sun, E. S., B. J. Molini, L. K. Barrett, A. Centurion-Lara, S. A. Lukehart, and W. C. Van Voorhis. 2004. Subfamily I Treponema pallidum repeat protein family: sequence variation and immunity. Microbes Infect. 6:725-737. [DOI] [PubMed] [Google Scholar]
- 21.Tantalo, L. C., S. A. Lukehart, and C. M. Marra. 2005. Treponema pallidum strain-specific differences in neuroinvasion and clinical phenotype in a rabbit model. Infect. Dis. 191:75-80. [DOI] [PubMed] [Google Scholar]
- 22.Turner, T. B., and D. H. Hollander. 1957. Biology of the treponematoses. World Health Organization, Geneva, Switzerland.
- 23.van der Ende, A., C. T. Hopman, S. Zaat, B. B. Essink, B. Berkhout, and J. Dankert. 1995. Variable expression of class 1 outer membrane protein in Neisseria meningitidis is caused by variation in the spacing between the −10 and −35 regions of the promoter. J. Bacteriol. 177:2475-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weigel, L. M., M. E. Brandt, and M. V. Norgard. 1992. Analysis of the N-terminal region of the 47-kilodalton integral membrane lipoprotein of Treponema pallidum. Infect. Immun. 60:1568-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.