Abstract
We report evidence for the existence of a putative ABC transporter for corrinoid utilization in the extremely halophilic archaeon Halobacterium sp. strain NRC-1. Results from genetic and nutritional analyses of Halobacterium showed that mutants with lesions in open reading frames (ORFs) Vng1370G, Vng1371Gm, and Vng1369G required a 105-fold higher concentration of cobalamin for growth than the wild-type or parent strain. The data support the conclusion that these ORFs encode orthologs of the bacterial cobalamin ABC transporter permease (btuC; Vng1370G), ATPase (btuD; Vng1371Gm), and substrate-binding protein (btuF; Vng1369G) components. Mutations in the Vng1370G, Vng1371Gm, and Vng1369G genes were epistatic, consistent with the hypothesis that their products work together to accomplish the same function. Extracts of btuF mutant strains grown in the presence of cobalamin did not contain any cobalamin molecules detectable by a sensitive bioassay, whereas btuCD mutant strain extracts did. The data are consistent with the hypothesis that the BtuF protein is exported to the extracellular side of the cell membrane, where it can bind cobalamin in the absence of BtuC and BtuD. Our data also provide evidence for the regulation of corrinoid transport and biosynthesis. Halobacterium synthesized cobalamin in a chemically defined medium lacking corrinoid precursors. To the best of our knowledge, this is the first genetic analysis of an archaeal corrinoid transport system.
Corrinoids belong to the family of cyclic tetrapyrroles that includes hemes, chlorophylls, and coenzyme F430 (16, 48). A complete corrinoid (also called cobamide) has upper and lower ligands that play important biochemical roles (16). The upper ligand forms a labile, covalent bond with the cobalt ion of the corrin ring (Co-C), while the lower ligand interacts with the cobalt ion via a coordination bond. The best-known cobamide is cobalamin (Cbl), which in its biologically active form has a 5′-deoxyadenosyl group as an upper ligand, hence the name adenosylcobalamin or coenzyme B12. Cobamides are distinguished from one another by the nature of the lower ligand nucleotide base (39), which is 5,6-dimethylbenzimidazole (DMB) in the case of Cbl.
Because of the complex structure of corrinoids, biosynthesis of the complete Cbl molecule requires at least 24 genes (48). Only prokaryotes synthesize corrinoids, although many eukaryotes, including humans, require corrinoids for their metabolism (39, 40, 48). Active transport of corrinoids is a process found in both prokaryotes and eukaryotes. Because the levels of corrinoids in the environment are low, transport of corrinoids requires specific systems with high affinity. In prokaryotes, most of the work on corrinoid transport has been performed with the gram-negative bacteria Escherichia coli and Salmonella enterica (9, 13, 37, 40, 47). Corrinoid transport is a special problem to these bacteria because the molecule must pass through both an outer and an inner membrane and the periplasm (13). Transport across the outer membrane requires both the BtuB and TonB proteins (18, 36). Active transport across the inner membrane is achieved via an ATP-binding cassette (ABC) transport system encoded by the btuC, btuD, and btuF genes, which encode the membrane permease, ATPase, and periplasmic-binding protein components, respectively (4, 9, 12, 47). ABC transporters are widely distributed in all domains of life and drive the translocation of substrates across membranes by the hydrolysis of ATP.
No corrinoid transport systems have been described for archaea, although some archaea synthesize and require corrinoids for survival. For example, methanogenic archaea require cobamides for methanogenesis from H2 and CO2, acetate, or methanol (14). Active cobamide-dependent (class II) ribonucleotide reductases have been purified from both Thermoplasma acidophilum (45) and Pyrococcus furiosus (38), suggesting cobamides are used by these organisms. We recently showed that the extremely halophilic archaeon Halobacterium sp. strain NRC-1 requires corrinoids under certain growth conditions, although the reasons for their corrinoid requirement remain unknown (50). We also showed that Halobacterium salvages Cbl and several of its precursors when present in the medium at subnanomolar concentrations, suggesting that this archaeon possesses a high-affinity corrinoid transport system (51).
Based on genome sequence analyses, ABC transporters appear to be as ubiquitous in archaea as in bacteria (2). Therefore, we hypothesized that, like bacteria, archaea use an ABC transporter for the utilization of corrinoids. Substrate uptake systems of this type have been studied in Sulfolobus solfataricus, P. furiosus, and Thermococcus litoralis and have been shown to be composed of a permease, an ATPase, and an extracellular substrate-binding protein that is anchored to the cell membrane (1, 3, 15, 20, 22, 52). Presumably because archaea only have a single membrane and no periplasm, no orthologs of outer membrane transporters have been found.
Using Halobacterium sp. strain NRC-1 as a model system, we report genetic evidence of an ABC-type corrinoid transporter in archaea. The Vng1370G, Vng1370Gm, and Vng1369G genes were predicted to encode the archaeal orthologs of the bacterial BtuC, BtuD, and BtuF proteins, respectively. These functions were required to salvage low nanomolar levels of corrinoids from the environment of this archaeon. We also report evidence for the regulation of this transport system and demonstrate that Halobacterium synthesizes Cbl (with the lower ligand DMB) de novo.
MATERIALS AND METHODS
Strains and plasmids.
The genotypes of the Halobacterium and S. enterica strains and plasmids used in this work are described in Table 1.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Marker(s)a | Relevant genotype | Relevant characteristics and use | Reference or sourceb |
|---|---|---|---|---|
| Halobacterium strains | ||||
| MPK414 | Δura3 | Able to synthesize Cbl de novo | 50 | |
| JE6738 | Δura3 ΔcbiP | Unable to synthesize cobamides de novo | 51 | |
| JE7084 | Δura3 ΔcbiP ΔbtuCD | In-frame deletion of btuCD; unable to synthesize cobamides de novo | ||
| JE7108 | Δura3 ΔbtuCD | In-frame deletion of btuCD | ||
| JE7494 | Δura3 ΔcbiP ΔbtuCD ura3::cbiP+ | Complementation of de novo pathway | ||
| JE7495 | Δura3 ΔcbiP ΔbtuCD ura3::btuCD+ | Complementation of btuCD | ||
| JE7681 | Δura3 ΔbtuF | In-frame deletion of btuF | ||
| JE7682 | Δura3 ΔcbiP ΔbtuF | In-frame deletion of btuF; unable to synthesize cobamides de novo | ||
| JE7683 | Δura3 ΔcbiP ΔbtuF ΔbtuCD | In-frame deletions of btuF and btuCD; unable to synthesize cobamides de novo | ||
| JE7684 | Δura3 ΔcbiP ΔbtuCD ura3::btuC+ | Complementation of btuC | ||
| JE7685 | Δura3 ΔcbiP ΔbtuCD ura3::btuD+ | Complementation of btuD | ||
| JE7796 | Δura3 ΔcbiP ΔbtuF ura3::cbiP+ | Complementation of de novo pathway in btuF mutant | ||
| JE7797 | Δura3 ΔcbiP ΔbtuF ura3::btuF+ | Complementation of btuF | ||
| S. enterica strains | ||||
| JE873 | metE205 ara-9 Δcob272 (cobUST) | Cobamide bioassay | Laboratory collection | |
| JE2243 | metE205 ara-9 btuB101::MudJc | Cobamide bioassay | Laboratory collection | |
| Plasmids | ||||
| pMPK428 | 5-FOAs Mevr | ura3+ | Generation of in-frame deletions of targeted genes | 32 |
| pMPK424 | 5-FOAs Mevr | ura3+ | Recombination at ura3 locus | 31 |
| pCBIP7 | 5-FOAs Mevr | ΔcbiP | Generates cbiP deletion | 51 |
| pVNG1370-2 | 5-FOAs Mevr | ura3+ ΔbtuCD | Generates btuCD deletion | |
| pVNG1370-4 | 5-FOAs Mevr | ura3+btuCD+ | Recombination of btuCD into ura3 locus | |
| pHsBTUC5 | 5-FOAs Mevr | ura3+btuC+ | Recombination of btuC into ura3 locus | |
| pHsBTUD1 | 5-FOAs Mevr | ura3+btuD+ | Recombination of btuD into ura3 locus | |
| pHsBTUF5 | 5-FOAs Mevr | ura3+ ΔbtuF | Generates btuF deletion | |
| pHsBTUF6 | 5-FOAs Mevr | ura3+btuF+ | Recombination of btuF into ura3 locus |
Abbreviations: Mevr, resistance to mevinolin; 5-FOAs, sensitivity to 5-FOA.
Unless otherwise stated, strains and plasmids were constructed during the course of this study.
Abbreviation of Mu dI1734 (10).
Chemicals.
Unless otherwise stated, all chemicals used in this work were commercially available, high-purity compounds. All corrinoids were added in their cyano-liganded form. Cobinamide dicyanide (Cbi) and cyanocobalamin were purchased from Sigma (St. Louis, MO). Cbi-GDP dicyanide (Cbi-GDP) was synthesized as previously described (46). Cobyric acid dicyanide (Cby) was a gift from Paul Renz (Universität Hohenheim, Stuttgart, Germany), 5-fluoroorotic acid (5-FOA) was purchased from Zymo Research (Orange, CA), and mevinolin was purchased from LKT Laboratories, Inc. (St. Paul, MN).
Halobacterium growth studies.
Strains were grown in liquid rich peptone (RP) medium (Oxoid, Hampshire, England) (28) lacking trace metals. Halobacterium cultures were grown for 4 days to stationary phase at 37°C with shaking. Cells were added to 5 ml chemically defined (CD) medium (17) at a dilution of 1:100, and cultures were grown at 37°C with shaking. Briefly, the CD medium contains the amino acids A, R, C, E, G, I, L, K, M, F, P, S, T, Y, and V; 11 mM glycerol; salts; and trace metals. Growth was monitored every 24 h for 6 days by measuring the absorbance of the culture at 650 nm with a Spectronic 20D spectrophotometer (Milton Roy, Rochester, NY). To determine cell viability (calculated as CFU), cells were plated onto solid RP medium (6.6% [wt/vol] agar). In all cases, media were supplemented with uracil (450 μM).
Halobacterium plasmid constructions.
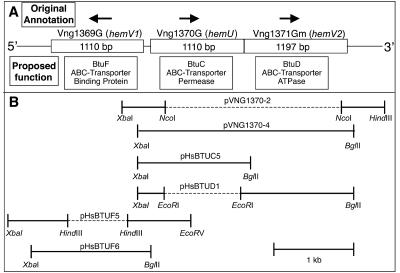
Plasmids were propagated in E. coli strain DH5α, except where noted otherwise. Unless stated otherwise, Halobacterium strain MPK414 (Δura3) genomic DNA was used as the template for PCR and was prepared as previously described (50). The high-fidelity enzyme Pfu (Stratagene) was used for PCR amplification. All DNA fragments were digested with the appropriate restriction enzymes (indicated by the underlined portion in the name of the primers) and then gel purified using a QIA quick gel extraction kit (QIAGEN). Plasmid pMPK424 was prepared from the E. coli dam mutant strain GM2163 (New England Biolabs). All primers were purchased from Integrated DNA Technologies. Underlined portions of the primer sequences (see below) indicate introduced restriction sites. All plasmids were subsequently sequenced for verification. A diagram of the Halobacterium sp. strain NRC-1 DNA included in the most relevant plasmids is included in Fig. 1.
FIG. 1.
Putative gene cluster in Halobacterium sp. strain NRC-1 indicating putative btuF (Vng1369G), btuC (Vng1370G), and btuD (Vng1371Gm) genes and plasmid constructions. (A) The reported ORF designation and original gene annotation are shown above each gene. Proposed functions are shown below each gene. The reported length (base pairs) of each gene is indicated. Arrows indicate directions of transcription. (B) Brackets connected by solid lines indicate the regions of DNA that were included in the indicated plasmids (pVNG1370-2, pVNG1370-4, pHsBTUC5, pHsBTUD1, pHsBTUF5, and pHsBTUF6). Dashed lines indicate regions that were not included in the plasmids. DNA restriction enzyme sites used for cloning are indicated below the brackets.
Plasmid pVNG1370-2.
The primer sets VNG1370DelXbaI5′ (TCTAGATCTAGACGTCGGCAGCGATGTTGTGG)-VNG1370DelNcoI3′ (CCATGGCCATGGGCGCAGCGTGATCGGTTCC) and VNG1370DelNcoI5′ (CCATGGCCATGGGCTGTCGTGTCCGCAGTCG)-VNG1370DelHindIII3′ (AAGCTTAAGCTTACGAGCGTGATGGTCTGTCC) were used to PCR amplify 890-bp and 760-bp fragments, respectively. The former fragment was cloned into the XbaI/NcoI restriction sites of plasmid pMPK428 (32). The second fragment was then cloned into the NcoI/HindIII restriction sites of the resulting plasmid to create plasmid pVNG1370-2, which contains an in-frame deletion of btuC and btuD replacing bases 112 to 1110 of btuC and bases 1 to 1143 of btuD with a 6-bp NcoI restriction site. The gene product of this construct should be a nonfunctional peptide with amino acid residues 1 to 37 of BtuC fused to amino acid resides 382 to 398 of BtuD.
Plasmid VNG1370-4.
The primer set VNG1370-Comp-XbaI-5′ (TCTAGTTCTAGATGTGATCGCGGTGTTGCTGG)-VNG1371-Comp-BglII-3′ (AGATCAAGATCTTGGCTGCCGTGCGACCCATG) was used to PCR amplify a 2,620-bp fragment that was cloned into the XbaI/BglII restriction site of plasmid pT7-7 (44). This cloned insert was excised with an XbaI/BglII restriction enzyme digest from a plasmid prepared from strain GM2163. The DNA fragment was then cloned into the XbaI/BglII sites of plasmid pMPK424 (31). The resulting plasmid, pVNG1370-4, contains the putative operon containing Vng1370G and Vng1371Gm. In addition to the two open reading frames (ORFs), 225 bp of genetic material 5′ of Vng1370G and 70 bp 3′ of Vng1371Gm were included to preserve any transcriptional regulation.
Plasmid pHsBTUC5.
The primer set VNG1370-Comp-XbaI-5′-Hs-BTUC-Comp-BglII-3′ (ACATCAAGATCTAAAAGCCGCGCCGGTTGCCAACTCCACGTCGAGG) was used to PCR amplify a 1,370-bp fragment that was cloned into the XbaI/BglII sites of pMPK424 (31). The resulting plasmid contains the entire btuC ORF, 225 bp 5′ of btuC to preserve transcriptional regulation, and a 16-bp sequence derived from the bop transcriptional terminator (11) (included in the 3′ reverse primer) to ensure termination of the btuC mRNA transcript.
Plasmid pHsBTUD1.
The primer sets VNG1370-Comp-XbaI-5′-Hs-BtuD-Comp-EcoRI-3′ (TGTTCTGAATTCAACGGTGCGCAGCGTGATC) and Hs-BtuD-Comp-EcoRI-5′ (TGTTCAGAATTCATCATCACCGCCCTGATCG)-Hs-BtuD-Comp-BglII-3′ (ACATCAAGATCTAAAAGCCGCGCCGGTTGTCCACGTAATACGTTCC) were used to PCR amplify 340-bp and 1,310-bp DNA fragments, respectively. Both DNA products were cut with EcoRI restriction enzyme and ligated together with T4 ligase (MBI Fermentas, Amherst, NY). Using the ligated DNA as the template, the primer set VNG1370-Comp-XbaI-5′-Hs-BtuD-Comp-BglII-3′ was used to PCR amplify a 1,650-bp DNA fragment, which was cloned into the XbaI/BglII sites of plasmid pMPK424 (31). The resulting plasmid contains a wild-type allele of btuD, as well as the 225 bp 5′ of btuC to include the transcription start site. To include this sequence, as well as 70 bp 5′ of the btuD ORF (to include the ribosome-binding site), part of btuC was included but as an in-frame deletion. A 6-bp EcoRI restriction site replaced bases 118 to 1041 of btuC. This construct should not encode a functional BtuC peptide but should ensure the production of a btuD mRNA transcript. As described for plasmid pHsBTUC5, a transcriptional terminator sequence was included 3′ of the btuD ORF.
Plasmid pHsBTUF5.
The primer sets VNG1369-Del-XbaI-5′ (GATATCTCTAGATGCCCATCAGCCAGTACATC)-VNG1369-Del-HindIII-3′ (AGATCTAAGCTTGAGTGTGATCGCGGTGTTGC) and VNG1369-Del-HindIII-5′ (TCTAGAAAGCTTAACACCACCATCAACACGACG)-VNG1369-Del-EcoRV-3′ (TCTAGAGATATCACTTGGACGACGACGAACAG) were used to PCR amplify 920-bp and 840-bp DNA fragments, respectively. The former fragment was first cloned into the XbaI/HindIII sites of pMPK428 (32). The second fragment was cloned into the constructed plasmid to create plasmid psBTUF5. The resulting plasmid contains an in-frame deletion of btuF, which replaces bases 169 to 948 with a 6-bp HindIII site, thus removing 260 of the 369 amino acid residues of the resulting peptide.
Plasmid pHsBTUF6.
The primer set HsBTUF-Comp-XbaI-5′ (TGAAGATCTAGAGTGCGCAGCGTGATCGGTTC)-HsBTUF-Comp-BglII-3′ (ACTACTAGATCTAAAAGCCGCGCCGGTTGAGGAATGAAACGGTGTCG) was used to PCR amplify a 130-bp DNA fragment that was cloned into the XbaI/BglII sites of the pMPK424 (31). The resulting plasmid, pHsBTUF6, contains the entire btuF ORF, 170 bp 5′ of the start site to preserve transcriptional regulation, and the same 16-bp terminator sequence included in plasmid pHsBTUC5.
Halobacterium strain constructions. (i) In-frame deletion mutants.
In-frame deletions of the btuCD and btuF loci were generated using previously described methodology (30). Briefly, deletion strains were constructed by transforming the desired Halobacterium sp. strain NRC-1 derivative of MPK414 (ura3) with a pMPK428-derived plasmid containing a deletion of the desired gene as described previously (25). Flanking sequences around the deletion of over 700 bp allowed efficient recombination of the fragment into the chromosome. Mevinolin-resistant mutants were selected as previously described (25) and replated on medium containing 5-FOA to select for loss of the plasmid (30). Colonies resistant to 5-FOA were screened by PCR to identify desired recombinants. DNA sequencing was used to confirm the presence of an in-frame deletion. Plasmid pVNG1370-2 (ΔbtuCD) was transformed into MPK414 and JE6738 (ΔcbiP) to generate strains JE7108 (ΔbtuCD) and JE7084 (ΔcbiP ΔbtuCD), respectively. Plasmid pHsBTUF5 (ΔbtuF) was transformed into MPK414, JE6738 (ΔcbiP), and 7084 (ΔcbiP ΔbtuCD) to generate strains JE7861 (ΔbtuF), JE7862 (ΔcbiP ΔbtuF), and JE7683 (ΔcbiP ΔbtuCD ΔbtuF), respectively.
Construction of complementation strains.
Complementation studies were performed with a single copy of the appropriate wild-type gene(s) in question placed at the ura3 locus. The same ura3-based gene replacement method for the isolation of deleted genes was used. PCR and DNA sequencing were used to confirm the presence of the correct gene at the ura3 locus. Plasmid pCBIP7 (cbiP+) was transformed into JE7084 (ΔcbiP ΔbtuCD) and JE7682 (ΔcbiP ΔbtuF) to generate strains JE7494 (ΔcbiP ΔbtuCD ura3::cbiP+) and JE7796 (ΔcbiP ΔbtuF ura3:: cbiP+), respectively. Plasmids pVNG1370-4 (btuCD+), pHsBTUC5 (btuC+), and pHsBTUD1 (btuD+) were transformed into JE7084 (ΔcbiP ΔbtuCD) to generate JE7495 (ΔcbiP ΔbtuCD ura3::btuCD+), JE7684 (ΔcbiP ΔbtuCD ura3::btuC+), and JE7685 (ΔcbiP ΔbtuCD ura3::btuD+), respectively. Plasmid pHsBTUF6 (btuF+) was transformed into JE7682 (ΔcbiP ΔbtuF) to generate strain JE7797 (ΔcbiP ΔbtuF ura3::btuF+).
Halobacterium corrinoid extraction assays.
Ten milliliters of dense Halobacterium culture was used to inoculate 1 liter of liquid RP or CD medium supplemented with various concentrations of Cbl. The cultures were grown to full density (4 days in RP medium and 6 days in CD medium) at 37°C with shaking at 180 rpm. Serial dilutions of the cells were plated on solid medium to calculate total CFU. Cells were harvested at 4,300 × g for 10 min in a Beckman-Coulter J21 centrifuge, washed by gently resuspending them in 200 ml of medium salts (4.3 M NaCl, 81 mM MgSO4, 27 mM KCl, 14 mM sodium citrate), and pelleted again. This was repeated twice, and the cell pellet was resuspended in 25 ml of methanol and incubated for 2 h at 65°C with gentle shaking. The suspension was cleared by centrifugation at 40,000 × g for 2 h in a Beckman-Coulter J25-I centrifuge. The supernatant was then dried under vacuum by using a Savant concentrator, and the sample was resuspended in 1 ml of buffer (100 mM phosphate buffer [pH 6.5], 10 mM KCN) and incubated under light for 10 min to derivatize any corrinoids to their cyano form. Total cell protein was determined by the Bio-Rad (Hercules, CA) Bradford protein assay. Samples were prepared by resuspending pelleted cells in 5 M NaOH.
Detection of corrinoids.
The presence of Cbl or other corrinoids was assessed by means of a bioassay. For this purpose, S. enterica strains JE873 (metE cobUST) and JE2243 (metE btuB) were used as indicator strains in an overlay on minimal no-carbon E medium (5) supplemented with glycerol and MgSO4. Two microliters of Halobacterium corrinoid extract or 2 pmol of authentic Cbl was spotted onto the agar overlay. The inoculated plates were incubated aerobically at 37°C for 24 h. The last step of cobamide biosynthesis in strain JE873 is blocked, making growth dependent on complete cobamides. Cell growth around the application site on overlays containing strain JE873 would indicate the presence of Cbl or another cobamide in the extract. Strain JE2243 was used as a negative control because it cannot transport Cbl (due to a lesion in the gene encoding the outer membrane corrinoid transporter BtuB) and will not respond to its presence in the extracts.
High-performance liquid chromatography (HPLC) analysis of corrinoids.
Halobacterium corrinoid extracts were filtered using Corning Spin-X centrifuge filters. Corrinoids were separated by using a Beckman-Coulter HPLC system equipped with a Luna (Phenomenex) 5-μm C18 column (150 by 4.6 mm) developed with a modification of the system reported elsewhere (6) at a flow rate of 1 ml/min. The column was equilibrated with a buffer system containing 98% A and 2% B. For quantification of Cbl in the extracts, 2 min after injection, the column was developed for 10 min with a linear gradient until the final composition reached 100% B. For the purification of corrinoids for mass spectrometry analysis, the column was developed for 55 min to 100% B 5 min after injection. The solvents used were as follows: A, 100 mM phosphate buffer (pH 6.5)-10 mM KCN; B, 100 mM phosphate buffer (pH 8.0)-10 mM KCN-acetonitrile (1:1). Corrinoids were detected using a Beckman-Coulter photodiode array detector. Authentic Cbl was used as the standard.
Mass spectrometry.
The HPLC-purified corrinoid in Halobacterium extracts was prepared for mass spectrometry analysis as previously described (49). This sample, as well as authentic Cbl, was submitted for analysis to the mass spectrometry facility at the University of Wisconsin—Madison Biotechnology Center. The mass spectrum was obtained using a Bruker Daltronics (Billerica, MA) BILFLEX III matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometer.
RESULTS AND DISCUSSION
Halobacterium synthesizes Cbl de novo.
To identify the cobamide synthesized by Halobacterium, we extracted corrinoids from strain JE7681 (ΔbtuF cbiP+) grown in CD medium. Strain JE7681 synthesized corrinoids de novo but did not salvage exogenous corrinoids due to the lack of BtuF function (see below). Corrinoids were resolved using HPLC as previously described (see Materials and Methods). A peak with the diagnostic UV-visible spectrum of corrinoids eluted 35.1 min after injection, the same retention time as authentic Cbl (data not shown). The material under this peak was used in bioassays.
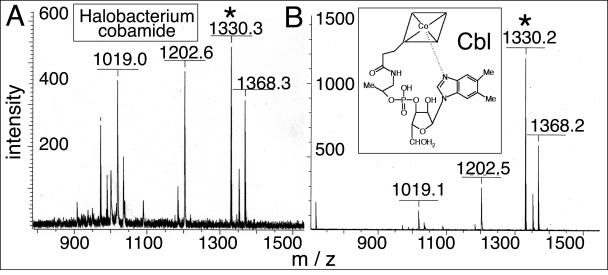
S. enterica strain JE873 (metE ΔcobUST) was the indicator strain used in bioassays to detect the presence of cobamides in Halobacterium corrinoid extracts (26). S. enterica strain JE2243 (metE btuB) lacking the outer membrane corrinoid transporter BtuB protein (19) was used as a negative control. A fraction containing the putative Halobacterium cobamide supported the growth of strain JE873, but not JE2243, consistent with the presence of a cobamide (data not shown). The MALDI-TOF mass spectrum of the Halobacterium cobamide contained a molecular ion signal with an m/z of 1,330.3 that was consistent with Cbl lacking an upper ligand. Authentic Cbl was used as a control, and its spectrum was strikingly similar to that of the Halobacterium cobamide (Fig. 2A). Other peaks were observed, but these were also present in the mass spectrometry profile of authentic Cbl (Fig. 2B). On the basis of these results, we concluded that Halobacterium sp. strain NRC-1 synthesizes a cobamide with DMB as the lower α ligand (i.e., Cbl).
FIG. 2.
Mass spectrometry analysis of the de novo synthesized cobamide extracted from Halobacterium. Shown is the MALDI-TOF mass spectrometry analysis of the HPLC-purified cobamide extracted from Halobacterium cells producing corrinoids de novo (A) and authentic Cbl (B). The signals with m/z values of 1,330.3 and 1,330.2 (indicated by asterisks) were consistent with the molecular mass of Cbl (without the upper cyano ligand), where z = +1. No significant signals were detected above an m/z value of 1,500 in either case. Me, methyl.
Methanothermobacter marburgensis strain Marburg is the only other archaeon shown to produce Cbl, but it only did so when DMB was supplied in the medium (43). Cobamides have been isolated with the lower ligand 5-methylbenzimidazole from Archaeoglobus fulgidus and T. acidophilum (23), adenine from Methanosarcina barkeri (35), and 5-hydroxybenzimidazole from M. marburgensis strain Marburg (24). It appears that the differences between the cobamides made by different species of prokaryotes correlate not with the biological functions of the cobamide but with the metabolic conditions of the organism in its natural habitat (23).
Halobacterium has an efficient corrinoid transport system.
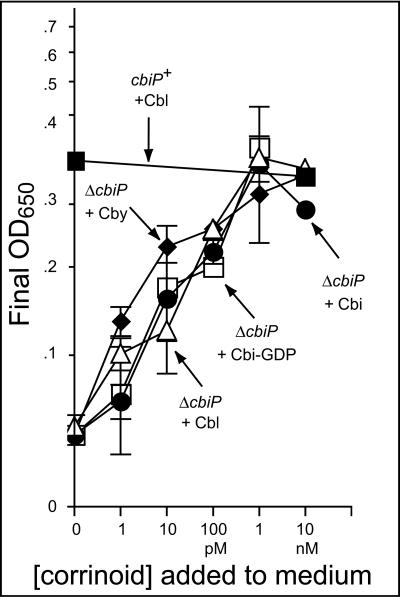
Strain JE6738 (ΔcbiP) was used to assess the ability of Halobacterium to assimilate low concentrations of various corrinoids. Strain JE6738 cannot synthesize corrinoids de novo due to the lack of CbiP (Cby synthase enzyme) and is dependent on exogenous cobamides or corrinoid precursors for growth (50). Strain JE6738 did not grow without added corrinoids, as opposed to strain JE6735 (cbiP+), which did not require corrinoids to grow (Fig. 3). The growth response of strain JE6738 was assessed as a function of the concentration of incomplete cobamides (i.e., Cby, Cbi, Cbi-GDP) and Cbl. The growth responses of strain JE6738 to all corrinoids tested were very similar. At least 1 nM corrinoid was required for growth equivalent to a functional de novo pathway, while a 100 pM concentration of every corrinoid tested allowed intermediate growth. Concentrations of Cbl of up to 100 μM did not significantly increase growth any further and may have a slight inhibitory effect (Fig. 4A, inverted solid triangles). The concentration of corrinoid needed to support optimal growth of Halobacterium was very similar to the one needed for S. enterica and E. coli (4, 29). This result strongly suggested the existence of a transport system for corrinoids in Halobacterium.
FIG. 3.
Corrinoid transport efficiency of Halobacterium strains. Corrinoid-dependent growth of Halobacterium sp. strain NRC-1 in CD liquid medium with various concentrations of corrinoids at 37°C is reported as the absorbance at 650 nm (OD650) after 6 days of growth. Separate panels include the effects of a ΔbtuCD mutation (A), complementation of ΔbtuCD mutant strains (B), a ΔbtuF mutation (C), and complementation of ΔbtuF mutant strains (D). After 6 days, cells reached maximum density, and this value is indicative of the ability to grow in the given medium. The mean cell densities of duplicated experiments are reported ± the standard deviations. Strains are identified by their genotypes. The corrinoids added to the medium are indicated next to the genotypes. The strains used were MPK414 (cbiP+) and JE6738 (ΔcbiP).
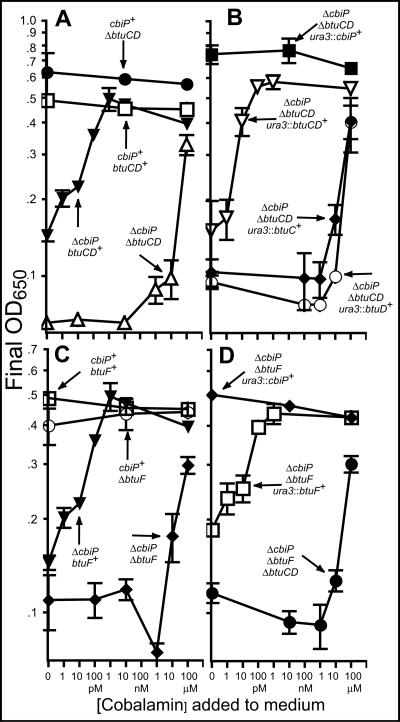
FIG. 4.
Nutritional studies of Halobacterium sp. strain NRC-1 btu mutants. Cbl-dependent growth of Halobacterium sp. strain NRC-1 mutants in CD liquid medium with the indicated concentrations of Cbl at 37°C is reported as the absorbance at 650 nm (OD650) after 6 days of growth. After 6 days, cells reach maximum density, and this value is indicative of the ability to grow in the given medium. The mean cell densities of duplicate experiments are reported ± the standard deviations. The strains are indicated by their genotypes. The strains used were MPK414 (cbiP+ btuCD+ btuF+), JE6738 (ΔcbiP btuCD+ btuF+), JE7084 (ΔcbiP ΔbtuCD btuF+), JE 7108 (cbiP+ ΔbtuCD btuF+), JE7494 (ΔcbiP ΔbtuCD btuF+ ura3::cbiP+), JE7495 (ΔcbiP ΔbtuCD btuF+ ura3::btuCD+), JE7684 (ΔcbiP ΔbtuCD btuF+ ura3::btuC+), JE7685 (ΔcbiP ΔbtuCD btuF+ ura3::btuD+), JE7681 (cbiP+ ΔbtuF btuCD+), JE7682 (ΔcbiP ΔbtuF btuCD+), JE7683 (ΔcbiP ΔbtuCD ΔbtuF), JE7796 (ΔcbiP ΔbtuF ura3::cbiP+ btuCD+), and JE7797 (ΔcbiP ΔbtuF btuCD+ ura3::btuF+).
Bioinformatic analysis of the B12 utilization (btu) genes of Halobacterium.
In bacteria, the btuC, btuD, and btuF genes encode the corrinoid ABC transporter permease, ATPase, and corrinoid-binding periplasmic protein, respectively.
BtuC.
ORF Vng1370G (gi: 1570394) shared 39% identity and 58% similarity with the BtuC protein of E. coli (7). We predict the Vng1370G gene is cotranscribed with the predicted btuD gene ortholog (Vng1371Gm [gi: 16554494]).
BtuD.
The putative Vng1371Gm protein shared 29% identity and 45% similarity with the E. coli BtuD protein.
BtuF.
ORF Vng1369G is encoded divergently from the putative btuCD operon and had 28% identity and 44% similarity to the E. coli BtuF protein. The btuF gene did not appear to be part of an operon. The Halobacterium BtuF protein is predicted to have an N-terminal signal peptide that directs it to the extracellular side of the cellular membrane via the Sec pathway (33, 34, 53). The signal (residues 1 to 21) is predicted to be cleaved after residue Ala24 (8). The BtuF protein has a C-terminal hydrophobic domain (amino acid residues 348 to 367) preceding a stretch of six hydroxylated amino acid residues, which may indicate anchoring to the extracellular side of the cell membrane (2).
A structure-based sequence alignment between Halobacterium BtuF and E. coli BtuF (the latter's crystal structure bound to B12 has been solved [21]) was used to identify possible B12-biding residues. Of the 11 residues in E. coli BtuF that make direct contacts with the B12 molecule, Halobacterium BtuF has three that are identical (E. coli BtuF residues S8, P9, and A10) and two are similar substituted hydrophobic residues (Y28F and W174Y). Based on this comparison, it is unclear if Halobacterium BtuF plays the same role or binds B12 like E. coli BtuF.
btuC (Vng1370G), btuD (Vng1371Gm), and btuF (Vng1369G) of Halobacterium are required for corrinoid utilization.
Strains JE7084 (ΔcbiP ΔbtuCD) and JE7862 (ΔcbiP ΔbtuF) were used to determine if Halobacterium btuC, btuD, and btuF functions are required for corrinoid utilization. In these strains, the lesion in cbiP blocks de novo cobamide synthesis, thus rendering cell growth dependent on corrinoid transport (50). Strains JE7084 and JE7682 were grown in CD liquid medium with various concentrations of Cbl. These strains failed to grow when provided with 10 nM Cbl, a concentration that was sufficient for growth of JE6738 (ΔcbiP btuCD+ btuF+) (Fig. 4A [open triangles versus inverted closed triangles] and C [closed diamonds versus closed triangles]). No significant growth was observed until the medium was supplemented with 100 μM Cbl. Because cells are unlikely to encounter 100 μM corrinoid in nature, this observed growth was most likely due to nonspecific transport. These strains were also tested for the ability to assimilate incomplete cobamides. At 10 nM, Cby, Cbi, and Cbi-GDP did not support the growth of either strain, while 1 μM Cbl supported wild-type growth (data not shown).
The observed block of corrinoid transport in strains JE7084 and JE7682 was corrected when wild-type alleles of Halobacterium btuC and btuD or btuF were introduced into the chromosome. Strains JE7495 (ΔcbiP ΔbtuCD ura3::btuCD+) (Fig. 4B, inverted open triangles) and JE7797 (ΔcbiP ΔbtuF ura3::btuF+) (Fig. 4D, open squares) grew when 1 nM Cbl was added.
As expected, a lesion in the btuCD or btuF locus in a cbiP+ strain did not interfere with growth under the conditions tested. Strains JE7108 (cbiP+ ΔbtuCD), JE7494 (ΔcbiP ΔbtuCD ura3::cbiP+), JE7681 (cbiP+ ΔbtuF), and JE7796 (ΔcbiP ΔbtuF ura3::cbiP+) grew without corrinoid supplementation (Fig. 4A [closed circles], B [closed squares], C [open squares], and D [closed diamonds], respectively).
btuC, btuD, and btuF mutations are epistatic.
If the products of the btuC, btuD, and btuF genes work together as a transport system, the phenotypes caused by any combination of the mutations would be the same, i.e., would be epistatic. To test this idea, strains JE7683 (ΔcbiP ΔbtuCD ΔbtuF), JE7684 (ΔcbiP ΔbtuCD ura3::btuC+), and JE7685 (ΔcbiP ΔbtuCD ura3::btuD+) were tested for the ability to utilize Cbl. When growing in CD liquid medium, all three strains displayed the same phenotype and did not grow unless ≥100 μM Cbl was added to the medium (Fig. 4B and D). These data suggested that the products of these three genes work together to transport Cbl.
Absence of BtuF but not BtuCD functions prevents Cbl-cell association in Halobacterium.
To test if BtuC, BtuD, and BtuF are required for the assimilation of exogenous Cbl, Halobacterium ΔcbiP mutants were grown in liquid RP medium with and without 100 nM Cbl. RP medium allows cobamide-independent growth, and all of the strains in these studies grew at similar rates in this medium regardless of exogenous corrinoids (data not shown). Possible reasons for why corrinoid auxotrophs grew in RP medium are discussed below.
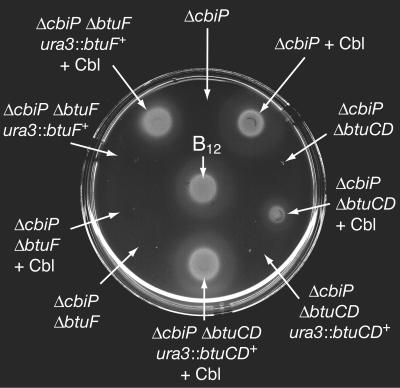
S. enterica strain JE873 (metE ΔcobUST) was used to test for Cbl presence in Halobacterium cells. None of the corrinoid extracts from Halobacterium cells grown without Cbl supported the growth of strain JE873 (Fig. 5), indicating that the de novo corrin ring biosynthetic pathway was not functional and that there was no contaminating Cbl in the RP medium. When 100 nM Cbl was added to the medium, Cbl was found in the extracts of strains JE6738 (ΔcbiP), JE7084 (ΔcbiP ΔbtuCD), JE7495 (ΔcbiP ΔbtuCD ura3::btuCD+), and JE7797 (ΔcbiP ΔbtuF ura3::btuF+) but not in strain JE7682 (ΔcbiP ΔbtuF) (Fig. 5). Collectively, these results suggested that the btuF gene is required for the presence of Cbl in extracts but that the btuCD genes are not. This phenotype of strain JE7682 was corrected by reintroduction of a wild-type copy of the btuF gene into the chromosome (Fig. 5). None of the spotted extracts supported the growth of S. enterica strain JE2243 (metE btuB), indicating that the growth of JE873 was due specifically to Cbl (results not shown).
FIG. 5.
Bioassay for the detection of Cbl extracted from Halobacterium cells. Shown is the response of S. enterica indicator strain JE873 (metE205 ΔcobUST) to 2 μl of Halobacterium corrinoid extracts and 2 pmol of authentic Cbl. Growth around the area of application indicated the presence of Cbl in the extract. Arrows indicate the genotype of the strain from which the extract was obtained. All Halobacterium strains were grown in liquid RP medium with or without 100 nM Cbl (also indicated by arrows) prior to Cbl extraction.
Quantification of Cbl in Halobacterium.
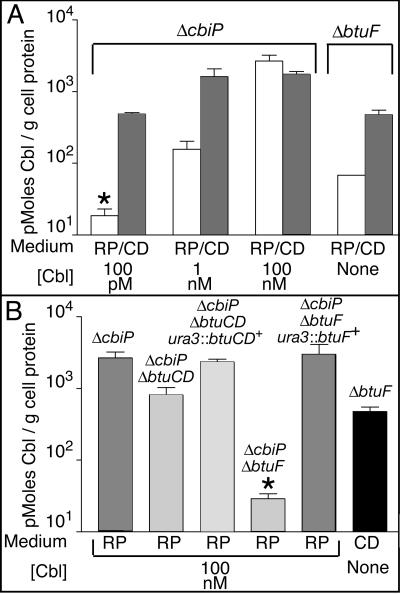
The Cbl associated with cell extract was quantified and expressed as picomoles of Cbl per gram of total cell protein. In RP medium, no Cbl was detected in strain JE6738 grown in medium supplemented with 100 pM Cbl (<20 pmol Cbl per g protein), but 2,680 pmol Cbl per g protein (1,176 molecules of Cbl per CFU) accumulated in the same strain when the medium contained 100 nM Cbl (Fig. 6A). When CD medium was supplemented with 100 pM Cbl, cultures reached 79% of the cell density (2.0 × 108 CFU/ml) of a culture grown in RP medium and they accumulated 490 pmol Cbl per g protein (Fig. 6A). Cbl-cell association reached a maximum level when CD medium was supplemented with 1 nM Cbl (1,620 pmol Cbl per g protein), consistent with growth data that showed maximum growth at this concentration (Fig. 4, open triangles). The relationship between CFU and total cell protein did not vary significantly between growth media, suggesting that levels of Cbl can be compared. Strain JE7681 (cbiP+ ΔbtuF) was used to determine how much Cbl was synthesized de novo by Halobacterium. When grown in RP medium, strain JE7681 synthesized 70 pmol Cbl per g protein compared to 480 pmol Cbl per g protein when grown in CD medium (Fig. 6A). These data suggest regulation of the corrinoid transport system as a function of nutrient availability. At this point, no specific nutrient(s) to which the cells may respond has been identified. Additionally, no obvious transcriptional regulatory sequences in the DNA sequences 5′ of either the Cbl biosynthetic or transport genes have been identified (data not shown).
FIG. 6.
Quantification of Cbl in Halobacterium cells. Shown is the amount of Cbl extracted from Halobacterium cells indicated as picomoles of Cbl per gram of total cell protein. The genotype of the strain from which Cbl was extracted is indicated above each column. Under each column, the type of liquid medium (RP, RP medium; CD, CD medium) and the concentration of Cbl added are indicated. Asterisks above a column indicate that the values are below the detection limit of the assay. The mean values of duplicated experiments are reported ± the standard deviations. The strains used were JE6738 (ΔcbiP btuCD+ btuF+), JE7084 (ΔcbiP ΔbtuCD btuF+), JE7495 (ΔcbiP ΔbtuCD btuF+ ura3::btuCD+), JE7681 (cbiP+ ΔbtuF btuCD+), JE7682 (ΔcbiP ΔbtuF btuCD+), and JE7797 (ΔcbiP ΔbtuF btuCD+ ura3::btuF+).
To determine how much a lesion in the btuCD or btuF locus would affect the assimilation of Cbl, strains JE7084 (ΔcbiP ΔbtuCD) and JE7682 (ΔcbiP ΔbtuF) were tested. These strains were grown in RP medium supplemented with 100 nM Cbl. Compared to strain JE6738 (ΔcbiP), the presence of Cbl in strain JE7084 was reduced 69% to 820 pmol Cbl per g protein, whereas no Cbl was detected in strain JE7682 extract (<30 pmol Cbl per g protein) (Fig. 6B). The phenotypes of strains JE7084 and JE7682 were corrected by reintroduction of the wild-type alleles of btuCD and btuF, respectively (Fig. 6B). During the quantification studies, Cbl was the only corrinoid detected by HPLC in strains lacking de novo capabilities, suggesting that the Cbl molecules did not have to be modified for usage.
It is possible that in the btuCD mutant strain, Cbl is associated with the cells but is inaccessible to metabolism. The Cbl molecules may still be associated with the BtuF protein, which is predicted to be anchored to the extracellular side of the membrane by its C-terminal hydrophobic domain. Without the BtuC and BtuD proteins in the membrane, BtuF may be binding Cbl molecules but not releasing them. In vitro binding studies with Halobacterium BtuF and Cbl, as well as localization studies, are needed to test if BtuF may be binding Cbl on the outer surface of the cell membrane.
Conclusions.
We have identified a corrinoid transport system in the hyperhalophilic archaeon Halobacterium sp. strain NRC-1. Genes encoding this system were annotated as hemU, hemV2, and hemV1 (27). We suggest a change in their nomenclature to btuC, btuD, and btuF, respectively, to reflect their role in corrinoid transport.
Most other available archaeal genome sequences are predicted to contain orthologs to the btuC, btuD, and btuF genes. Two notable exceptions lacking btuC, btuD, and btuF were Methanothermobacter thermautotrophicus strain ΔH (42) and Methanopyrus kandleri AV19 (41). Both of these archaea appear to contain genetic information for an entire cobamide de novo biosynthetic pathway. The latter may have evolved to rely on endogenously synthesized corrinoids. However, M. marburgensis strain Marburg, a close relative of M. thermautotrophicus strain ΔH, has been shown to assimilate exogenous corrinoids (43), suggesting that a nonorthologous transport system exists in this archaeon and thus may exist in other archaea.
Identification of btuC, btuD, and btuF orthologs in other archaea based on sequence analysis alone may be problematic. Corrinoid transport systems have amino acid sequences very similar to ABC-type Fe3+, siderophore, and heme transport systems. Many archaea have several putative orthologs to these systems, and they are not always encoded in close proximity to each other or cobamide biosynthetic genes, making it difficult to identify or match the components of transport systems. Identification of these transport systems may have to rely on more classical genetic and biochemical approaches, like the ones used in the work reported here.
Putative Cbl-dependent enzymes in Halobacterium.
It is unknown why Halobacterium requires corrinoids to grow in CD medium. The ability of RP medium to allow growth of Halobacterium corrinoid mutants suggests that these strains are auxotrophic for a nutrient present in this medium. Analysis of the genome sequence predicts that Halobacterium synthesizes at least three cobamide-dependent enzymes, methylmalonyl-coenzyme A mutase (encoded by ORFs Vng0481G, Vng0653G, and Vng0673G), glutamate mutase (encoded by ORFs Vng2286G and Vng2288G), and class II ribonucleotide reductase (encoded by ORF Vng1644G) (27). These enzymes would likely require adenosylcobalamin as the coenzyme; thus, Cbl would have to be adenosylated after transport by an ATP:co(I)rrinoid adenosyltransferase (CobA in S. enterica). Halobacterium contains a putative cobA ortholog (Vng1574G in Halobacterium), but its function has not been demonstrated experimentally. Nutritional analyses of mutants defective for these enzymes may determine if growth in CD medium requires Cbl.
Acknowledgments
This work was supported by NIH grant GM40313 to J.C.E.-S. J.D.W. was supported in part by the Ira L. Baldwin and Jerome J. Stefaniak predoctoral fellowships.
We thank P. Renz for the gift of Cby.
Footnotes
We dedicate this work to the memory of Robert Kadner, a pioneer in the field of corrinoid transport, a good friend and colleague.
REFERENCES
- 1.Albers, S. V., M. G. Elferink, R. L. Charlebois, C. W. Sensen, A. J. Driessen, and W. N. Konings. 1999. Glucose transport in the extremely thermoacidophilic Sulfolobus solfataricus involves a high-affinity membrane-integrated binding protein. J. Bacteriol. 181:4285-4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albers, S. V., S. M. Koning, W. N. Konings, and A. J. Driessen. 2004. Insights into ABC transport in archaea. J. Bioenerg Biomembr. 36:5-15. [DOI] [PubMed] [Google Scholar]
- 3.Albers, S. V., W. N. Konings, and A. J. Driessen. 1999. A unique short signal sequence in membrane-anchored proteins of Archaea. Mol. Microbiol. 31:1595-1596. [DOI] [PubMed] [Google Scholar]
- 4.Bassford, P. J., Jr., and R. J. Kadner. 1977. Genetic analysis of components involved in vitamin B12 uptake in Escherichia coli. J. Bacteriol. 132:796-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berkowitz, D., J. M. Hushon, H. J. Whitfield, J. Roth, and B. N. Ames. 1968. Procedure for identifying nonsense mutations. J. Bacteriol. 96:215-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanche, F., D. Thibaut, M. Couder, and J.-C. Muller. 1990. Identification and quantitation of corrinoid precursors of cobalamin from Pseudomonas denitrificans by high-performance liquid chromatography. Anal. Biochem. 189:24-29. [DOI] [PubMed] [Google Scholar]
- 7.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 8.Bolhuis, A. 2002. Protein transport in the halophilic archaeon Halobacterium sp. NRC-1: a major role for the twin-arginine translocation pathway? Microbiology 148:3335-3346. [DOI] [PubMed] [Google Scholar]
- 9.Cadieux, N., C. Bradbeer, E. Reeger-Schneider, W. Koster, A. K. Mohanty, M. C. Wiener, and R. J. Kadner. 2002. Identification of the periplasmic cobalamin-binding protein BtuF of Escherichia coli. J. Bacteriol. 184:706-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castilho, B. A., P. Olfson, and M. J. Casadaban. 1984. Plasmid insertion mutagenesis and lac gene fusions with mini-Mu bacteriophage transposons. J. Bacteriol. 158:488-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DasSarma, S., U. L. RajBhandary, and H. G. Khorana. 1984. Bacterio-opsin mRNA in wild-type and bacterio-opsin-deficient Halobacterium halobium strains. Proc. Natl. Acad. Sci. USA 81:125-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeVeaux, L. C., D. S. Clevenson, C. Bradbeer, and R. J. Kadner. 1986. Identification of the BtuCED polypeptides and evidence for their role in vitamin B12 transport in Escherichia coli. J. Bacteriol. 167:920-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeVeaux, L. C., and R. J. Kadner. 1985. Transport of vitamin B12 in Escherichia coli: cloning of the btuCD region. J. Bacteriol. 162:888-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiMarco, A. A., T. A. Bobik, and R. S. Wolfe. 1990. Unusual coenzymes of methanogenesis. Annu. Rev. Biochem. 59:355-394. [DOI] [PubMed] [Google Scholar]
- 15.Elferink, M. G., S. V. Albers, W. N. Konings, and A. J. Driessen. 2001. Sugar transport in Sulfolobus solfataricus is mediated by two families of binding protein-dependent ABC transporters. Mol. Microbiol. 39:1494-1503. [DOI] [PubMed] [Google Scholar]
- 16.Friedmann, H. C., and R. K. Thauer (ed.). 1992. Macrocyclic tetrapyrrole biosynthesis in bacteria, vol. 3. Academic Press, Inc., New York, N.Y.
- 17.Grey, V. L., and P. S. Fitt. 1976. An improved synthetic growth medium for Halobacterium cutirubrum. Can. J. Microbiol. 22:440-442. [DOI] [PubMed] [Google Scholar]
- 18.Gudmundsdottir, A., P. E. Bell, M. D. Lundrigan, C. Bradbeer, and R. J. Kadner. 1989. Point mutations in a conserved region (TonB box) of Escherichia coli outer membrane protein BtuB affect vitamin B12 transport. J. Bacteriol. 171:6526-6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heller, K., B. J. Mann, and R. J. Kadner. 1985. Cloning and expression of the gene for the vitamin B12 receptor protein in the outer membrane of Escherichia coli. J. Bacteriol. 161:896-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horlacher, R., K. B. Xavier, H. Santos, J. DiRuggiero, M. Kossmann, and W. Boos. 1998. Archaeal binding protein-dependent ABC transporter: molecular and biochemical analysis of the trehalose/maltose transport system of the hyperthermophilic archaeon Thermococcus litoralis. J. Bacteriol. 180:680-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karpowich, N. K., H. H. Huang, P. C. Smith, and J. F. Hunt. 2003. Crystal structures of the BtuF periplasmic-binding protein for vitamin B12 suggest a functionally important reduction in protein mobility upon ligand binding. J. Biol. Chem. 278:8429-8434. [DOI] [PubMed] [Google Scholar]
- 22.Koning, S. M., W. N. Konings, and A. J. Driessen. 2002. Biochemical evidence for the presence of two alpha-glucoside ABC-transport systems in the hyperthermophilic archaeon Pyrococcus furiosus. Archaea 1:19-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kräutler, B., H. P. E. Kohler, and E. Stupperich. 1988. 5′-Methylbenzimidazolyl-cobamides are the corrinoids from some sulfate-reducing and sulfur-metabolizing bacteria. Eur. J. Biochem. 176:461-469. [DOI] [PubMed] [Google Scholar]
- 24.Kräutler, B., J. Moll, and R. K. Thauer. 1987. The corrinoid from Methanobacterium thermautotrophicum (Marburg strain). Spectroscopic structure analysis and identification as Cob-cyano-5′-hydroxybenzimidazolyl-cobamide (factor III). Eur. J. Biochem. 162:275-278. [DOI] [PubMed] [Google Scholar]
- 25.Krebs, M. P., R. Mollaaghababa, and H. G. Khorana. 1993. Gene replacement in Halobacterium halobium and expression of bacteriorhodopsin mutants. Proc. Natl. Acad. Sci. USA 90:1987-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maggio-Hall, L. A., and J. C. Escalante-Semerena. 1999. In vitro synthesis of the nucleotide loop of cobalamin by Salmonella typhimurium enzymes. Proc. Natl. Acad. Sci. USA 96:11798-11803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng, W. V., S. P. Kennedy, G. G. Mahairas, B. Berquist, M. Pan, H. D. Shukla, S. R. Lasky, N. S. Baliga, V. Thorsson, J. Sbrogna, S. Swartzell, D. Weir, J. Hall, T. A. Dahl, R. Welti, Y. A. Goo, B. Leithauser, K. Keller, R. Cruz, M. J. Danson, D. W. Hough, D. G. Maddocks, P. E. Jablonski, M. P. Krebs, C. M. Angevine, H. Dale, T. A. Isenbarger, R. F. Peck, M. Pohlschroder, J. L. Spudich, K. W. Jung, M. Alam, T. Freitas, S. Hou, C. J. Daniels, P. P. Dennis, A. D. Omer, H. Ebhardt, T. M. Lowe, P. Liang, M. Riley, L. Hood, and S. DasSarma. 2000. Genome sequence of Halobacterium species NRC-1. Proc. Natl. Acad. Sci. USA 97:12176-12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oesterhelt, D., and W. Stoeckenius. 1974. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 31:667-678. [DOI] [PubMed] [Google Scholar]
- 29.O'Toole, G. A., M. R. Rondon, and J. C. Escalante-Semerena. 1993. Analysis of mutants of Salmonella typhimurium defective in the synthesis of the nucleotide loop of cobalamin. J. Bacteriol. 175:3317-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peck, R. F., S. Dassarma, and M. P. Krebs. 2000. Homologous gene knockout in the archaeon Halobacterium salinarum with ura3 as a counterselectable marker. Mol. Microbiol. 35:667-676. [DOI] [PubMed] [Google Scholar]
- 31.Peck, R. F., C. Echavarri-Erasun, E. A. Johnson, W. V. Ng, S. P. Kennedy, L. Hood, S. DasSarma, and M. P. Krebs. 2001. brp and blh are required for synthesis of the retinal cofactor of bacteriorhodopsin in Halobacterium salinarum. J. Biol. Chem. 276:5739-5744. [DOI] [PubMed] [Google Scholar]
- 32.Peck, R. F., E. A. Johnson, and M. P. Krebs. 2002. Identification of a lycopene beta-cyclase required for bacteriorhodopsin biogenesis in the archaeon Halobacterium salinarum. J. Bacteriol. 184:2889-2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pogliano, J. A., and J. Beckwith. 1994. SecD and SecF facilitate protein export in Escherichia coli. EMBO J. 13:554-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pogliano, K. J., and J. Beckwith. 1994. Genetic and molecular characterization of the Escherichia coli secD operon and its products. J. Bacteriol. 176:804-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pol, A., C. Van der Drift, and G. D. Vogels. 1982. Corrinoids from Methanosarcina barkeri: structure of the a-ligand. Biochem. Biophys. Res. Commun. 108:731-737. [DOI] [PubMed] [Google Scholar]
- 36.Postle, K., and R. J. Kadner. 2003. Touch and go: tying TonB to transport. Mol. Microbiol. 49:869-882. [DOI] [PubMed] [Google Scholar]
- 37.Reynolds, P. R., G. P. Mottur, and C. Bradbeer. 1980. Transport of vitamin B12 in Escherichia coli. Some observations on the roles of the gene products of btuC and tonB. J. Biol. Chem. 255:4313-4319. [PubMed] [Google Scholar]
- 38.Riera, J., F. T. Robb, R. Weiss, and M. Fontecave. 1997. Ribonucleotide reductase in the archaeon Pyrococcus furiosus: a critical enzyme in the evolution of DNA genomes? Proc. Natl. Acad. Sci. USA 94:475-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rondon, M. R., J. R. Trzebiatowski, and J. C. Escalante-Semerena. 1997. Biochemistry and molecular genetics of cobalamin biosynthesis. Prog. Nucleic Acid Res. Mol. Biol. 56:347-384. [DOI] [PubMed] [Google Scholar]
- 40.Roth, J. R., J. G. Lawrence, and T. A. Bobik. 1996. Cobalamin (coenzyme B12): synthesis and biological significance. Annu. Rev. Microbiol. 50:137-181. [DOI] [PubMed] [Google Scholar]
- 41.Slesarev, A. I., K. V. Mezhevaya, K. S. Makarova, N. N. Polushin, O. V. Shcherbinina, V. V. Shakhova, G. I. Belova, L. Aravind, D. A. Natale, I. B. Rogozin, R. L. Tatusov, Y. I. Wolf, K. O. Stetter, A. G. Malykh, E. V. Koonin, and S. A. Kozyavkin. 2002. The complete genome of hyperthermophile Methanopyrus kandleri AV19 and monophyly of archaeal methanogens. Proc. Natl. Acad. Sci. USA 99:4644-4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith, D. R., L. A. Doucette-Stamm, C. Deloughery, L. Hongmei, J. Dubois, T. Aldredge, R. Bashirzadeh, D. Blakely, R. Cook, K. Gilbert, D. Harrison, L. Hoang, P. Keagle, W. Lumm, B. Pothier, D. Qiu, R. Spadafora, R. Vicaire, Y. Wang, J. Wierzbowski, R. Gibson, N. Jiwani, A. Caruso, D. Bush, H. Safer, D. Patwell, S. Prabhakar, S. McDougall, S. G., A. Goyal, S. Pietrokovski, G. M. Church, C. H. Daniels, J.-I. Mao, P. Rice, J. Nölling, and J. N. Reeve. 1997. Complete genome sequence of Methanobacterium thermautotrophicum DH: functional analysis and comparative genomics. J. Bacteriol. 179:7135-7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stupperich, E., I. Steiner, and H. J. Eisinger. 1987. Substitution of Coa-(5-hydroxybenzimidazolyl)cobamide (factor III) by vitamin B12 in Methanobacterium thermautotrophicum. J. Bacteriol. 169:3076-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tabor, S. (ed.). 1990. Current protocols in molecular biology. Greene Publishing Associates and Wiley-Interscience, New York, N.Y.
- 45.Tauer, A., and S. A. Benner. 1997. The B12-dependent ribonucleotide reductase from the archaebacterium Thermoplasma acidophila: an evolutionary solution to the ribonucleotide reductase conundrum. Proc. Natl. Acad. Sci. USA 94:53-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas, M. G., T. B. Thompson, I. Rayment, and J. C. Escalante-Semerena. 2000. Analysis of the adenosylcobinamide kinase/adenosylcobinamide phosphate guanylyltransferase (CobU) enzyme of Salmonella typhimurium LT2. Identification of residue H46 as the site of guanylylation. J. Biol. Chem. 275:27376-27386. [DOI] [PubMed] [Google Scholar]
- 47.Van Bibber, M., C. Bradbeer, N. Clark, and J. R. Roth. 1999. A new class of cobalamin transport mutants (btuF) provides genetic evidence for a periplasmic binding protein in Salmonella typhimurium. J. Bacteriol. 181:5539-5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Warren, M. J., E. Raux, H. L. Schubert, and J. C. Escalante-Semerena. 2002. The biosynthesis of adenosylcobalamin (vitamin B12). Nat. Prod. Rep. 19:390-412. [DOI] [PubMed] [Google Scholar]
- 49.Woodson, J. D., and J. C. Escalante-Semerena. 2004. CbiZ, an amidohydrolase enzyme required for salvaging the coenzyme B12 precursor cobinamide in archaea. Proc. Natl. Acad. Sci. USA 101:3591-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woodson, J. D., R. F. Peck, M. P. Krebs, and J. C. Escalante-Semerena. 2003. The cobY gene of the archaeon Halobacterium sp. strain NRC-1 is required for de novo cobamide synthesis. J. Bacteriol. 185:311-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woodson, J. D., C. L. Zayas, and J. C. Escalante-Semerena. 2003. A new pathway for salvaging the coenzyme B12 precursor cobinamide in archaea requires cobinamide-phosphate synthase (CbiB) enzyme activity. J. Bacteriol. 185:7193-7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xavier, K. B., L. O. Martins, R. Peist, M. Kossmann, W. Boos, and H. Santos. 1996. High-affinity maltose/trehalose transport system in the hyperthermophilic archaeon Thermococcus litoralis. J. Bacteriol. 178:4773-4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yi, L., F. Jiang, M. Chen, B. Cain, A. Bolhuis, and R. E. Dalbey. 2003. YidC is strictly required for membrane insertion of subunits a and c of the F1F0 ATP synthase and SecE of the SecYEG translocase. Biochemistry 42:10537-10544. [DOI] [PubMed] [Google Scholar]