Abstract
Thyroid transcription factor-1 (TTF-1) is required for maximal expression of thyrotropin receptor (TSHR) in the thyroid. Extrathyroidal TSHR expression is detectable in normal orbital adipose tissues, with increased levels found in orbital tissues from patients with Graves’ ophthalmopathy (GO), and in orbital preadipocyte cultures following differentiation. In order to determine whether TTF-1 might be involved in orbital TSHR expression, we used quantitative real-time polymerase chain reaction (PCR) to assess relative expression of this and other thyroid-associated transcription factors (TTF-2 and Pax-8) in GO orbital tissue specimens (n = 28) and cultures (n = 3), and in normal orbital tissues (n = 19) and cultures (n = 3). We detected TTF-1 and TTF-2 mRNA in GO and normal orbital tissue samples, with no difference in levels noted between the tissues. In the GO orbital cultures, TTF-1 mRNA was higher in differentiated than in control (undifferentiated) cultures (p < 0.05), while TTF-2 was unchanged. In the normal cultures, neither TTF-1 nor TTF-2 mRNA levels increased in differentiated cultures. Pax8 was undetectable in all orbital tissues and cell cultures. The presence of mRNA encoding TTF-1 in orbital tissues and cultures suggest that this transcription factor may play an important role in extrathyroidal, as it does in thyroidal, TSHR expression.
Introduction
Graves’ ophthalmopathy (GO) is an autoimmune inflammatory disorder characterized by an increase in the volume of the orbital fatty/connective tissues and extraocular muscles within the orbit. Because the autoantigen involved in the hyperthyroidism of Graves’ disease is the thyrotropin receptor (TSHR) (1–3), investigators have long been interested in whether GO orbital tissues might express this receptor and thus be subject to the ongoing autoimmune process. In recent years, TSHR expression has been demonstrated at both the mRNA and protein levels in orbital tissues and cultured orbital fibroblasts obtained from patients with GO and normals (4–6), and has been shown to increase with differentiation of preadipocytes into mature adipocytes (7,8). In addition, higher TSHR mRNA levels have been demonstrated in orbital adipose tissue samples from patients with GO than in normal orbital specimens (9,10). These findings suggest that enhanced TSHR expression in the orbit in the setting of Graves’ disease may play a role in GO pathogenesis.
A set of transcriptional regulators, including thyroid transcription factor-1 (TTF-1), TTF-2, and Pax-8 is coordinately responsible for the expression of the thyroglobulin, thyroperoxidase, sodium iodide symporter, and thyrotropin receptor (TSHR) genes within the thyroid gland (11). The combined expression of these genes ultimately controls both the synthesis of thyroid hormone and the differentiation of thyroid cells (12–14). In contrast, the expression of TTF-1 alone appears to be required for maximal expression of the TSHR gene in thyrocytes (15,16). Because orbital preadipocytes increase their expression of TSHR with differentiation, we undertook the current studies to determine whether TTF-1 and other thyroid-related transcription factors (TTF-2 and Pax-8) are detectable in orbital adipose tissues and whether their expression is impacted by differentiation of orbital cells.
Materials and Methods
Patients
Orbital adipose/connective tissue specimens were obtained from patients who underwent orbital decompression surgery for severe GO (n = 28 for orbital tissue studies and n = 3 for in vitro studies) and from individuals with no history of Graves’ disease (n = 19 for orbital tissue studies and n = 3 for in vitro studies). The number of orbital tissue specimens actually used to study each of the genes varied to some degree and is specified for each experiment).
Cell cultures
Tissues were either frozen immediately and stored at −70°C or minced and placed in plastic culture dishes, allowing preadipocyte fibroblasts to proliferate in medium 199 containing 20% fetal bovine serum (FBS; Hyclone Laboratories, Logan, UT), penicillin (100 U/mL), and gentamicin (20 μg/mL) in a humidified 5% CO2 incubator at 37°C.
To initiate adipocyte differentiation, orbital cells were grown to confluence in 6-well plates in medium 199 with 10% FBS. Cultures were then changed to serum-free Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s F-12 (1:1; Sigma, St. Louis, MO) supplemented with biotin (33 μM), pantothenic acid (17 μM), transferrin (10 μg/mL), triiodothyronine (T3) (0.2 nM), insulin (1 μM), carbaprostacyclin (cPGI2; 0.2 μM; Calbiochem, San Diego, CA); and, for the first 4 days only, dexamethasone (1 μM) and isobutylmethylxanthine (IBMX; 0.1 mM). The differentiation protocol was continued for 10 days, during which time the medium was replaced every 3–4 days. Control (undifferentiated) cultures were grown to confluence as above, then maintained in medium lacking several of the components necessary for complete adipocyte differentiation (i.e., cPGI2, dexamethasone, and IBMX).
Quantitative real-time PCR
Total RNA was isolated either from frozen tissues or cultured cells using the RNeasy Midi kit (Qiagen, Valencia, CA). cDNA was synthesized using 100 ng of total RNA incubated with random hexamers, followed by a 100 μL reverse transcription reaction with 125 units of Multiscribe Reverse Transcriptase (Applied Biosystems, Foster City, CA). Conditions used were 25°C for 10 minutes, 48°C for 30 minutes, and 95°C for 5 minutes.
Oligonucleotide primers and Taqman probes (5′-56FAM and 3′BHQ1) for TTF-1, TTF-2, Pax8, and TSHR were designed using the computer program Primer Express (Applied Biosystems; Table 1). PCR reactions were done in a 96-well optical reaction plate. Amplification reactions contained cDNA equivalent of 3 ng total RNA for orbital tissues and 2 ng total RNA for orbital cultures, 900 nM of the forward and reverse primers, 250 nM of the probes and the Universal Taqman 1 × PCR mastermix (Applied Biosystems) in a total volume of 25 μL. The thermal cycling conditions used were: 2 minutes at 50°C, 10 minutes at 95°C, followed by 40 cycles at 95°C for 15 seconds and 60°C for 1 minute. The target genes and 18S rRNA were amplified in separate wells. All reactions were performed in duplicate in the ABI PRISM® 7700 Sequence Detector (Applied Biosystems) and the data pooled. Thyroid tissue was used as the positive control for each gene. Reaction mixture, without the cDNA, was used as the negative control in each run.
Table 1.
Primers and Probes Used for Quantitative Real-Time Polymerase Chain Reaction
| TTF-1 |
| Forward primer (TTF1-F1): 5′-AAG TTC TCA CAG ATC AGC AGT CAA AG-3′ |
| Reverse primer (TTF1-R1): 5′-TTC TTC GAC AGC CTT GAT TAG TTT C-3′ |
| Probe (TTF1-P1): 5′-/56-FAM/GGG TTT CAG ACT TAC TCC AAG CAC CAC CAC GAT/3BHQ_1/-3′ |
| TTF-2 |
| Forward primer (TTF2-F1): 5′-CCA GCA CCG TCC ACA TAC TGT-3′ |
| Reverse primer (TTF2-R1): 5′-CCA GGG CCG ACT TCA GTA AAG-3′ |
| Probe (TTF2-P1): 5′-/56-FAM/CCA GTT GCT GAG ACT CCG CCA GTG TT/3BHQ_1/-3′ |
| Pax8A |
| Forward primer (Pax8A-F1): 5′-CAG CCT GGC AGC GAC AA-3′ |
| Reverse primer (Pax8A-R1): 5′-GCT GCT CTG TGA GTC AAT GCT TAG-3′ |
| Probe (Pax8A-P1): 5′-/56-FAM/CAG CTA TCC TGA TCA CTG TCA TCC ATT TTC CT/3BHQ_1/-3′ |
| TSHR |
| Forward primer (TSHR-F1): 5′-GAC CCT GAT GCC CTC AAA GA-3′ |
| Reverse primer (TSHR-R1): 5′-TCA GGG AAC ATT TTA AGT CCA GTG T-3′ |
| Probe (TSHR-P1): 5′-/56-FAM/CTC CCC CTC CTA AAG TTC CTT GGC ATT T/3BHQ_1/-3′ |
TTF, thyroid transcription factor; TSHR, thyrotropin receptor.
The standard curve method was used to quantify the expression of the various genes and 18S rRNA in each sample. Normalized results were expressed as the ratio of RNA (pg) of the target gene to RNA (pg) of 18S rRNA. For each experimental sample, a gene was considered to be expressed if amplification was detected by cycle 36 and 18S rRNA was amplified by cycle 20. Samples having genes amplified after cycle 36, but in which 18S rRNA was amplified by cycle 20 were included considered truly “undetectable” and were included in the calculations as having a value of “0”. Samples in which 18S rRNA was not amplified by cycle 20 were considered to be inadequate for analysis and not included in the calculations.
Statistical analyses
The Mann-Whitney rank sum test was used to assess statistically significant differences between groups. Additional analyses were done using Spearman rank order correlation to study correlations between expression levels of TSHR and TTF-1 or TTF-2 on patient samples.
Results
Orbital tissues
We documented the presence of TTF-1 mRNA in 9 of 11 normal orbital tissue samples and in 16 of 18 orbital tissue samples from patients with GO. There was no difference between the mean values for normal (0.278) and Graves’ (0.328) TTF-1 mRNA levels (Fig. 1). Similarly, TTF-2 was detected in 9 of 10 normal orbital tissue samples and in 16 of 16 GO samples, with no difference between the mean values (0.785 versus 0.702, respectively; Fig. 2). Pax-8 was not detected in any normal or GO tissue specimen studied, although this gene was detected in the positive control thyroid tissues included in the analyses. In contrast, as we previously published using other orbital tissue samples, the mean TSHR/18S value was significantly higher in GO orbital tissues (0.064; n = 28) than in normal orbital tissues (0.007; n = 19; p < 0.05; Fig. 3). Finally, we found no correlation between TTF-1 or TTF-2 and TSHR mRNA levels in any of the tissues (data not shown).
FIG. 1.
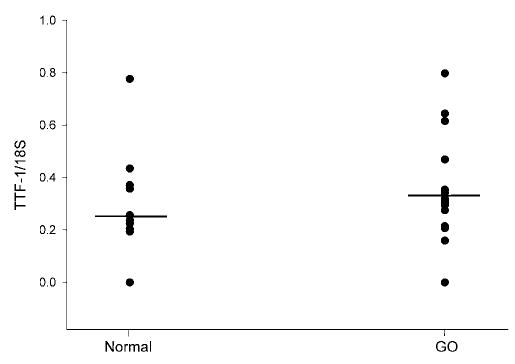
Relative levels of thyroid transcription factor-1 (TTF-1) mRNA (normalized to 18S RNA) in orbital adipose tissue samples obtained from normal individuals (n = 11) and patients with Graves’ ophthalmopathy (GO; n = 18).
FIG. 2.
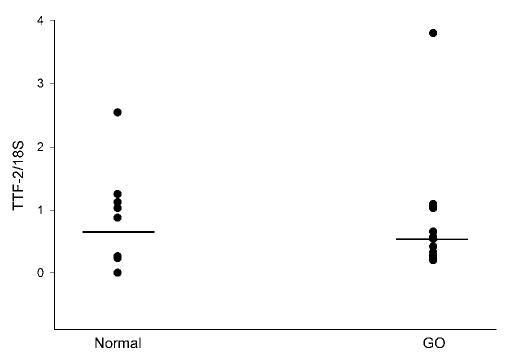
Relative levels of thyroid transcription factor-2 (TTF-2) mRNA (normalized to 18S RNA) in orbital adipose tissue samples obtained from normal individuals (n = 10) and patients with Graves’ ophthalmopathy (GO; n = 16).
FIG. 3.
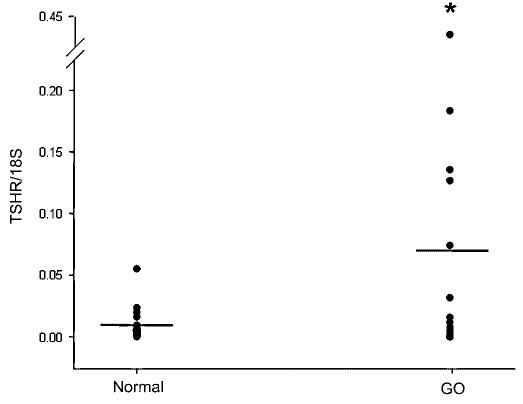
Relative levels of thyrotropin receptor (TSHR) mRNA (normalized to 18S RNA) in orbital adipose tissue samples obtained from normal individuals (n = 19) and patients with Graves’ ophthalmopathy (GO; n = 28). *p < 0.05 between groups.
Orbital preadipocyte cultures
TSHR, TTF-1, and TTF-2 mRNA was detectable at baseline in all normal and GO orbital cell cultures, while Pax-8 mRNA was undetectable in all cultures. Levels of TTF-1 and TTF-2 mRNA (normalized to 18S rRNA) did not differ between the normal and GO cultures at baseline. Differentiation of GO orbital preadipocyte cultures increased TTF-1 mRNA levels in 3 of 3 cultures (mean = 2.0 fold; p < 0.05), and TSHR mRNA levels in 2 of 3 cultures (mean = 4.5 fold; p < 0.05; Fig. 4). While TTF-2 mRNA levels increased in 2 of 3 GO cultures, the mean increase did not reach statistical significance. In the normal orbital cultures, while mRNA levels corresponding to each of the 3 genes tended to increase following differentiation, none of the mean changes reached statistical significance (data not shown).
FIG. 4.
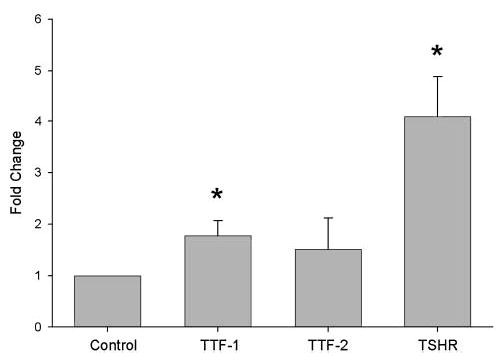
Fold change (mean ± standard error of the mean [SEM]) in levels of thyroid transcription factor (TTF)-1, TTF-2, and thyrotropin receptor (TSHR) mRNA (normalized to 18S rRNA) in differentiated orbital preadipocyte cultures from patients with Graves’ ophthalmopathy (GO; n = 3). Results are expressed relative to parallel control (undifferentiated) orbital cultures from the same individuals. *p < 0.05 compared to control values.
Discussion
The thyroid-associated transcription factor TTF-1 is a 38-kd DNA binding protein belonging to the homeobox–containing gene family (17). This protein contains 378 amino acids and is encoded by a mRNA species of 2.4–2.7 kb (13). TTF-1 is required for maximal expression of the TSHR gene in thyroid cells (11), and is essential for morphogenesis of the thyroid, lung and ventral forebrain (13,15,17,18). TTF-1 knockout mice (TTF1−/−) lacking these organs are carried to term but are born dead (18). TTF1+/− mice survive, but have poor coordination, increased serum TSH concentration, and slightly lower thyroxine (T4) values compared to TTF1+/+ mice (18). Similarly, humans expressing only one allele of the TTF-1 gene have neurologic symptoms, increased serum TSH with variable degrees of hypothyroidism, and significantly lower levels of both TSHR and thyroglobulin mRNAs (19).
The expression of TTF-1 is thought to be restricted to thyroid, brain, and lung (18). In the lung, it is detectable in both alveolar type II cells and bronchiolar epithelial cells (20). Our studies show for the first time the expression of TTF-1 in orbital adipose tissues and preadipocyte cultures. These findings differ somewhat from those of another group who were unable to detect TTF-1 mRNA in rat adipose tissue or in mouse 3T3-L1 adipogenic cells by Northern analysis (8). These differences may be explained either by the greater sensitivity of our quantitative real-time PCR studies or by species differences. Our finding that TTF-1 is expressed in these extrathyroidal tissues and cells also known to express TSHR suggests that TTF-1 might be involved in maximal expression of TSHR in orbit, as it is in the thyroid.
The TSHR, thyroglobulin, and thyroperoxidase genes all have TTF-1 binding sites in both their enhancer and promoter regions (11). However, only the latter two genes have additional sites for TTF-2 and Pax-8 binding within their promoter sequences (11). The early increase in TSHR gene expression seen in thyrocytes after TSH stimulation is associated with increased TTF-1 binding to the TSHR promoter (16,21,22). This appears to be mediated by cyclic adenosine monophosphate (cAMP)-dependant activation of protein kinase A (PKA) with subsequent phosphorylation of TTF-1 (16,22). In our studies, although TSHR mRNA levels were higher in GO than in normal orbital tissues, we found no difference in mean TTF-1 mRNA levels between GO and normal tissues and no correlation between expression levels of these two genes in individual tissues. However, this does not preclude the presence of enhanced TTF-1 binding to the TSHR gene promoter in GO orbital tissues, resulting in increased orbital TSHR expression. This might stem from activation of cAMP-dependent protein kinase A (PKA) by thyroid-stimulating antibodies (TSAb) within the GO orbital tissues, with subsequent phosphorylation of TTF-1. We also found TTF-2 to be expressed in orbital adipose tissue and derivative cells. The role of this transcription factor within the orbit is unknown, but not likely related to orbital TSHR expression.
In studies of cultured orbital preadipocytes, we found increased TTF-1 and TSHR mRNA in GO orbital preadipocyte cultures following differentiation, while no change in mean levels of either TTF-1 or TSHR mRNA was observed in the normal cultures. It is possible that increased binding of TTF-1 to the TSHR promoter in the GO cultures (not present in the normal cultures) stimulated increased production of TTF-1 and TSHR mRNA. Perhaps previous exposure of the GO cells to TSAb led to cAMP/PKA-dependent phosphorylation of TTF-1 with subsequent enhanced binding to the TSHR promoter. If so, the reason that increased TTF-1 mRNA was not detectable in the GO tissues may have been because the effect was of small magnitude in these heterogeneous tissue specimens.
In conclusion, we report the presence of TTF-1 and TTF-2 mRNA in both GO and normal orbital tissues and derivative preadipocyte cultures. The importance of these findings is twofold: first, this study represents the first demonstration of TTF-1 and TTF-2 expression in adipose tissue and derivative cell cultures. Secondays, these results suggest that TTF-1 may play a role in TSHR expression within the orbit, as it does within the thyroid. Whether TSAb in Graves’ disease (or other factors present within the orbit in GO) stimulate increased binding of TTF-1 to the TSHR promoter, with subsequent enhanced orbital TSHR expression, awaits further study.
Acknowledgments
This work was supported in part by National Institutes of Health EYO8819 (to R.S.B.) from the National Eye Institute.
References
- 1.Adams DD. Thyroid-stimulating autoantibodies. Vitam Horm. 1980;38:119–203. doi: 10.1016/s0083-6729(08)60485-9. [DOI] [PubMed] [Google Scholar]
- 2.Rees Smith B, McLachlan SM, Furmaniak J. Autoantibodies to the thyrotropin receptor. Endocr Rev. 1988;9:106–120. doi: 10.1210/edrv-9-1-106. [DOI] [PubMed] [Google Scholar]
- 3.Perros P, Kendall-Taylor P. Thyroid-associated opthalmopathy: pathogenesis and clinical management. Clin Endocrinol Metab. 1995;9:115–135. doi: 10.1016/s0950-351x(95)80867-1. [DOI] [PubMed] [Google Scholar]
- 4.Feliciello A, Porcellini A, Ciullo I, Bonalonta G, Avvedimento VE, Fenzi GF. Expression of thyrotropin-receptor specific mRNA in healthy and Graves’ disease retroorbital tissue. Lancet. 1993;342:337–338. doi: 10.1016/0140-6736(93)91475-2. [DOI] [PubMed] [Google Scholar]
- 5.Heufelder AE, Dutton CM, Sarkar G, Donovan KA, Bahn RS. Detection of TSH receptor RNA in cultured fibroblasts from patients with Graves’ opthalmopathy and pretibial dermopathy. Thyroid. 1993;3:297–300. doi: 10.1089/thy.1993.3.297. [DOI] [PubMed] [Google Scholar]
- 6.Burch HB, Sellitti D, Barnes SG, Nagy EV, Bahn RS, Burman KD. TSH receptor antisera for the detection of immunoreactive protein species in retroocular fibroblasts obtained from patient with Graves’ opthalmopathy. J Clin Endocrinol Metab. 1994;78:1384–1391. doi: 10.1210/jcem.78.6.8200941. [DOI] [PubMed] [Google Scholar]
- 7.Valyasevi RW, Erikson DZ, Harteneck DA, Dutton CM, Heufelder AE, Jyonouchi SC, Bahn RS. Differentiation of human orbital preadipocyte fibroblasts induces expression of functional thyrotropin receptor. J Clin Endocrinol Metab. 1999;84:2257–2562. doi: 10.1210/jcem.84.7.5838. [DOI] [PubMed] [Google Scholar]
- 8.Shimura H, Haraguchi K, Endo T, Onaya T. Regulation of thyrotropin receptor gene expression in 3T3–L1 adipose cells is distinct from its regulation in FRTL-5 thyroid cells. Endocrinology. 1997;138:1483–1490. doi: 10.1210/endo.138.4.5048. [DOI] [PubMed] [Google Scholar]
- 9.Crisp M, Lane C, Halliwell M, Wynford-Thomas days, Ludgate M. Thyrotropin receptor transcripts in human adipose tissue. J Clin Endocrinol Metab. 1997;82:2003–2005. [PubMed] [Google Scholar]
- 10.Bahn RS, Dutton CM, Natt N, Joba W, Spitzweg C, Heufelder AE. Thyrotropin receptor expression in Graves’ orbital adipose/connective tissues: Potential autoantigen in Graves’ opthalmopathy. J Clin Endocrinol Metab. 1998;83:998–1002. doi: 10.1210/jcem.83.3.4676. [DOI] [PubMed] [Google Scholar]
- 11.Kambe F, Seo H. Thyroid-specific transcription factors. Endocrin J. 1997;44:775–784. doi: 10.1507/endocrj.44.775. [DOI] [PubMed] [Google Scholar]
- 12.Damante G, Tell G, DiLauro R. A unique combination of transcription factors controls differentiation of thyroid cells. Prog Nucl Res Mol Biol. 2000;66:307–356. doi: 10.1016/s0079-6603(00)66033-6. [DOI] [PubMed] [Google Scholar]
- 13.Di Lauro R, Damante G, De Felice M, Arnone MI, Sato K. Molecular events in the differentiation of the thyroid gland. J Endocrinol Invest. 1995;18:117–119. doi: 10.1007/BF03349716. [DOI] [PubMed] [Google Scholar]
- 14.Missero C, Cobellis G, De Felice M, Di Lauro R. Molecular events involved in differentiation of thyroid follicular cells. Mol Cell Endocrinol. 1998;140:37–43. doi: 10.1016/s0303-7207(98)00027-6. [DOI] [PubMed] [Google Scholar]
- 15.Civitareale days, Castelli MP, Falasca P, Saiardi A. Thyroid transcription factor1 activates the promoter of the thyrotropin receptor gene. Mol Endocrinol. 1993;7:1589–1595. doi: 10.1210/mend.7.12.8145764. [DOI] [PubMed] [Google Scholar]
- 16.Shimura H, Okjima F, Ikuyama S, Shimura Y, Kimura S, Saji M, Kohn LD. Thyroid-specific expression and cyclic adenosine 3′,5′-monophosphate autoregilation of the thyrotropin receptor gene involves thyroid transcription factor-1. Mol Endocrinol. 1994;8:1049–1069. doi: 10.1210/mend.8.8.7997232. [DOI] [PubMed] [Google Scholar]
- 17.Lau SK, Luthringer DJ, Eisen RN. Thyroid transcription factor-1: A review. Appl Immunocytochem Mol Morphol. 2002;10:97–102. doi: 10.1097/00129039-200206000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Bingle CD. Thyroid transcription factor-1. Int J Biochem Cell Biol. 1997;29:1471–1473. doi: 10.1016/s1357-2725(97)00007-1. [DOI] [PubMed] [Google Scholar]
- 19.Moeller LC, Kimura S, Kusakabe T, Liao X, Sande JV, Refetoff S. Hypothyroidism in thyroid transcription factor 1 haploinsufficiency is caused by reduced expression of the thyroid-stimulating hormone receptor. Mol Endocrinol. 2003;17:2295–2302. doi: 10.1210/me.2003-0175. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda K, Clark JC, Shaw-White JR, Stahlman MT, Boutell CJ, Whitsett JA. Gene structure and expression of human thyroid transcription factor-1 in respiratory epithelial cells. J Biol Chem. 1995;270:8108–8114. doi: 10.1074/jbc.270.14.8108. [DOI] [PubMed] [Google Scholar]
- 21.Shimura H, Shimura Y, Ohmori M, Ikuyama S, Kohn LD. Single strand DNA-binding proteins and thyroid transcription factor-1 conjointly regulate thyrotropin receptor gene expression. Mol Endocrinol. 1995;9:527–539. doi: 10.1210/mend.9.5.7565801. [DOI] [PubMed] [Google Scholar]
- 22.Ohmori M, Shimura H, Shimura Y, Kohn LD. Characterization of an up-stream thyroid transcription factor-1-binding site in the thyrotropin receptor promoter. Endocrinology. 1995;136:269–282. doi: 10.1210/endo.136.1.7828540. [DOI] [PubMed] [Google Scholar]


