Abstract
Objectives:
Tc2 cells, a subset of CD8+ T cells, are able to facilitate engraftment in a murine model of postnatal allogeneic bone marrow transplantation. The purpose of this study was to evaluate whether Tc2 cells could improve engraftment in fetal transplantation.
Methods:
Gestational day 13 C57BL/6 (H-2b) fetal mice were used as recipients, adult B6D2F1 mice (C57BL/6 × DBA/2,H-2b/d) as donors, and splenocytes from B6C3F1 (C57BL/6 × C3H/He, H-2b/k) mice were used as stimulators in cultures used to generate the Tc2 cells from B6D2F1 mice Peripheral blood chimerism was examined monthly for 3 months. Thereafter, recipients were sacrificed to evaluate the levels of peritoneal, splenic and bone marrow chimerism. The T-cell responses of recipient splenocytes to cells of host origin were measured as a proliferative response in mixed lymphocyte cultures.
Results:
Low levels of peripheral blood cell chimerism (<0.3%) were observed at 1 month of age, which declined further by 3 months of age. The levels of donor cells in the spleen, bone marrow and peritoneal cavity were usually not more than 0.05%. The peritoneal cavity tended to have higher levels of donor cells with 1 recipient sustaining as high as 25.03% at the age of 3 months. Higher peritoneal chimerism correlated with a lower donor-specific T-cell response.
Conclusions:
Transplantation of Tc2 cells was insufficient to improve bone marrow engraftment in utero, suggesting that graft rejection is not the major barrier to successful in utero transplantation. Donor cells can persist in the peritoneal cavity and might play an important role in inducing immune tolerance in fetuses.
Keywords: Cytotoxic T cells, Fetal transplantation, Peritoneal chimerism, Immune tolerance
Introduction
Despite considerable promise as an approach for the management of congenital hematological and metabolic disorders, in utero transplantation (IUT) of hematopoietic stem cells (HSCs) faces obstacles that limit the effectiveness of this form of therapy [1–3]. Although IUT has produced notable levels of hematopoietic chimerism in a sheep model [4], minimal or no engraftment has been observed in most murine models [5–9]. Moreover, clinical experience with IUT has yielded little or no engraftment with the exception of transplants made in fetuses suffering from certain immunodeficiencies [1, 2]. A number of lines of evidence point toward a lack of space in the hematopoietic tissues for donor HSCs to engraft as one possible reason for poor outcomes [1, 10]. Despite the immaturity of the fetal immune system, NK cells and even T cells are present in the late first trimester fetus and may further prevent donor cell engraftment [11]. Better defining the barriers to HSC engraftment in utero is the key to improving IUT, to treat a wider range of birth defects.
In postnatal bone marrow transplantation (BMT), T lymphocytes present in the marrow inoculum can abrogate graft rejection but often at the expense of graft-versus-host disease (GVHD) [12–16]. Donor T cells have also been found to facilitate IUT [17–19]. It has been suggested that the graft-versus-host effect of donor T cells can help to generate homing spaces so that donor HSCs stand a better chance to engraft [19]. However, the transplantation of alloreactive T cells also presents the risk of GVHD [18]. Further study has indicated that the CD8+ subset of T cells appear to be responsible for abrogating graft rejection as well as generating GVHD [20–24]. It is recognized that cytotoxic CD8+ T cells exist in polarized cytokine secreting subsets, defined as Tc1 and Tc2 cells [25, 26]. The Tc1 subset secretes interleukin (IL)-2 and interferon-γ (IFN-γ), whereas the Tc2 subset secretes IL-4, IL-5 and IL-10. The type I cytokines induce a pro-inflammatory immune reaction, whereas the type II cytokines exert an anti-inflammatory effect [27]. The balance between the two subsets may play an important role in determining the nature of the systemic immune response to a particular pathogen. Recently, it has been recognized that Tc2 cells are able to dramatically facilitate engraftment with reduced GVHD in a murine model of postnatal allogeneic BMT [27–30]. The current study was aimed at evaluating whether IUT of the Tc2 subset could facilitate HSC engraftment in mice.
Materials and Methods
Mice Used
The same F1-into-parent transplantation model was used as reported by Fowler et al. [28] in their study of the role of Tc2 cells in adult allogeneic transplantation: C57BL/6 (H-2b) fetal mice were used as recipients, B6D2F1 (C57BL/6 × DBA/2, H-2b/d) as donors, and splenocytes from B6C3F1 (C57BL/6 × C3H/He, H-2b/k) mice were used as stimulators in cultures used for generating the Tc2 cells. C57BL/6 and B6C3F1 (8 weeks of age) mice were purchased from Charles River (Wilmington, Mass., USA) or Simonsen Laboratory(Gilroy, Calif., USA). B6D2F1 (8–20 weeks of age) mice were either purchased, from the above companies, or obtained from a breeding program at Howard Hughes Medical Institute at University of California, San Francisco (UCSF). All animals were housed in the Animal Care Facility at UCSF in accordance with federal guidelines and with the approval of the UCSF Committee on Animal Research.
Generation of Tc2 Cells
Donor (B6D2F1) splenocytes were harvested by passage through 70-μm cell strainers (Becton Dickinson & Co., Franklin Lakes, N.J., USA) and depleted of red cells using ACK buffer, pH 7.2–7.4, consisting of 0.15 M NH4Cl, 1.0 mM KHCO3 and 0.1 mM Na2EDTA (Sigma Chemical Co., St. Louis, Mo., USA). The splenic leukocytes were treated with biotinylated anti-CD4, anti-CD19, anti-CD24, anti-MHC class II (I-A/I-E) and anti-Gr1 monoclonal antibodies (BD Biosciences, San Diego, Calif., USA). CD8+ T cells were then enriched by negative depletion using streptavidin-coated magnetic Dynabeads (Dynal Biotech Inc., Lake Success, N.Y., USA).
Tc2 cultures were performed as described by Fowler et al. [28] with minor modifications as suggested by Dr. Fowler [pers. commun., December 11, 2000]. Culture media consisted of RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (Hyclone, Logan, Utah, USA), sodium pyruvate (1%), non-essential amino acids (1%), L-glutamate (0.5%), 2-mercaptoethanol (5 × 10−5), penicillin (50 U/ml) and streptomycin (50 μg/ml) and N-acetylcysteine (Sigma Chemical Co.; 10 mmol/l, pH adjusted to 7.2). The enriched CD8+ cells were suspended at 1 × 106 cells/ml and cocultured, at a ratio of 1:5, with 3,000 cGy irradiated whole splenocytes from B6C3F1 mice. These Tc2 cultures received recombinant human (rh) IL-2 at 1,000 U/ml (R&D Systems, Inc., Minneapolis, Minn., USA), rhIL-7 at 20 ng/ml (R&D Systems) and recombinant murine IL-4 at 1,000 U/ml (PeproTech, Rocky Hill, N.J., USA) on day 0. On day 2, they were supplemented with rhIL-2 (40 U/ml) and rhIL-7 (20 ng/ml). On day 4 of culture, Tc2 flasks were harvested, brought to a concentration of 5 × 105 cells/ml, supplemented with rhIL-2 (40 U/ml) and rhIL-7 (20 ng/ml), and stimulated again with 3,000 cGy irradiated B6C3F1 whole splenocytes at 1:5 ratio. On day 7, Tc2 cultures were harvested and centrifuged over a layer of NycoPrep 1.077A (Nycomed, Pharma AS, Oslo, Norway) to remove dead cells. The light-density cells were carefully transferred and resuspended in RPMI 1640 for flow cytometric evaluation and in utero injection.
For the comparison of secreted-cytokine profiles, Tc1 cells were generated using recombinant murine IL-12 (PeproTech) at 20 U/ml and recombinant human transforming growth factor-β1 (R&D Systems) at 10 ng/ml, instead of IL-4, on day 0 [28].
Flow Cytometric and Enzyme-Linked Immunosorbent Assay (ELISA) Evaluation of Tc2 Cells
Aliquots of light-density Tc2 cells were suspended in culture supernatant, containing 0.01% NaN3, from the hybridoma clone 2.4G2 (American Type Culture Collection, Manassas, Va., USA) that produces monoclonal antibody (mAb) against FcγII/FcγIII receptors. This supernatant was used to block non-antigen-specific binding of immunoglobulins. Cells were incubated with anti-CD4 FITC (Caltag Laboratories, Burlingame, Calif., USA)/anti-CD8 PE (Caltag Laboratories) at 4°C for 30 min. Cells were also stained with anti-CD8 FITC (Caltag Laboratories)/anti-H-2Kd PE (BD Biosciences) and anti-CD8 FITC/anti-CD69 PE (BD Biosciences). Three-color flow cytometry was performed on a FACScan (BD Biosciences) using propidium iodide (Molecular Probes, Eugene, Oreg., USA) staining to identify and exclude dead cells from the analysis. 2 × 104 live cells were acquired for analysis using CellQuest software (BD Biosciences).
For analysis of cytokine-secretion profiles, aliquots of light-density cells from Tc2 and Tc1 cultures were brought to a concentration of 5 × 105 cells/ml in 24-well plates (Costar, Cambridge, Mass., USA) and stimulated with 3,000 cGy irradiated whole splenocytes of B6D2F1 (syngeneic) mice and B6C3F1 (haplogeneic) mice, respectively, at a ratio of 1:5. Supernatants were harvested after 24 h. These supernatants and those from day 7 cultures were tested for the presence of IFN-γ IL-2, IL-4, IL-5 and IL-10 by ELISA as recommended by the supplier, BD Biosciences/PharMingen.
In utero Transplantation
C57BL/6 females were caged with males in the afternoon and checked for vaginal plugs the following morning. The day when the plug was observed was designated as day 0 of the pregnancy.
Adult bone marrow cells (BMCs) from B6D2F1 mice were harvested by flushing the tibias and femurs with phosphate buffer saline containing 0.3% bovine serum albumin (PBS/BSA) using a 26-gauge needle. Light-density BMCs were obtained by layering them over NycoPrep 1.077A and centrifuging at 600 g for 25 min. Light-density BMCs was then depleted magnetically of CD3+ cells using biotinylated anti-CD3 mAb (BD Biosciences) and streptavidin-coated Dynabeads. All the donor cells were freshly injected within 3 h after preparation.
Time-dated pregnant mice (day 13) were subcutaneously anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg). After vertical laparotomy to expose uteri, 1 × 106 T-depleted BMCs and 3.5–5.0 × 105 Tc2 cells in 5 μl of RPMI 1640 were injected into the peritoneum of each fetus of a litter using a 60-μm glass micropipette with a beveled tip. The abdomen was then closed using two layers of 4-O silk suture. After the operation, all mice were housed in an undisturbed room without bedding changes until the pups were 1 week old. Pups were weaned at 3 weeks of age.
Analyses of Chimerism
Peripheral blood was taken from a small incision at the tail tip at the ages of 1, 2 and 3 months. Recipients were euthanized at the age of 3–5 months for obtaining splenocytes, BMCs and peritoneal cells. Peritoneal cells were first harvested by flushing the peritoneal cavity with 10 ml PBS/BSA using a syringe with an 18-gauge needle. Subsequently, BMCs were harvested by flushing the tibias and femurs with PBS/BSA. At last, spleens were removed under sterile conditions, washed with PBS/BSA and dissociated by passage through a 70-μm cell strainers. Samples were depleted of red cells using ACK buffer.
Prior to staining, cells were first incubated with culture supernatant containing anti-mouse FcγII/FcγIII antibody, and then stained with anti-H-2Kb FITC (BD Biosciences) and anti-H-2Kd PE. A negative control for each sample consisted of anti-H-2Kb FITC and mouse IgG2a PE (BD Biosciences) to define background staining. In some cases, cells were further stained with anti-H-2Kd PE and either anti-CD4 FITC, anti-CD8 FITC, biotinylated anti-Ter119, biotinylated anti-CD19, biotinylated anti-Gr1 or biotinylated anti-F4/80 (Caltag Laboratories). FITC-conjugated streptavidin (Caltag Laboratories) was used in conjunction with the biotinylated mAbs. 1 × 105 events were acquired for analysis after gating out dead cells using propidium iodide. The levels of chimerism were derived by subtracting the percentage of background staining, defined by events positive for anti-H-2Kb FITC and mouse IgG2a PE, from the percentage of donor cells defined by events positive for anti-H-2Kb FITC and anti-H-2Kd PE.
Mixed Lymphocyte Reaction (MLR)
T-cell tolerance was evaluated by measuring MLRs using a flow cytometric method recently described [31]. Briefly, responder cells were red cell-depleted splenocytes from the recipients and stimulator cells were irradiated splenocytes from C57BL/6 (syngeneic), B6D2F1(donor strain) and FVB/N (third-party) mice. Splenocytes from untransplanted C57BL/6 mice were used as the control responders. Mixed lymphocyte cultures were harvested after 6 days. The number of reactive daughter CD3+ T cells generated was measured and compared for the experimental (B6D2F1), third-party (FVB/N) and control (C57BL/6) groups. A relative simulation index (SI) was calculated as follows: SI = (MEBDF1 − MEC57BL/6)/(MEFVB/N − MEC57BL/6). In this equation, MEBDF1, MEC57BL/6 and MEFVB/N represent the mean number of events of recipient daughter T cells responsive to donor B6D2F1, syngeneic C57BL/6 and third-party FVB/N stimulators, respectively.
Statistical Methods
The non-parametric Wilcoxon signed ranks test was used to measure the significance of differences between two related samples of the peripheral blood. The independent-samples t test was used to compare proliferation of CD3+ T cells in MLR stimulated by the different stimulator cells. Spearman correlation coefficient was used to test bivariate correlations between SI and donor cell levels in peripheral blood, spleen, bone marrow or peritoneal cavity. Results were considered significantly different when p < 0.05.
Results
Characteristics of the Donor T Cells
Cultured CD8+ T cells were shown to be enriched for Tc2 cells based on their cytokine secretion profile, which was compared to that of cultures optimized for Tc1 production (table 1). The Tc2 cell preparations contained more than 90% CD8+ cells and less than 0.1% of CD4+ cells. The activation status of Tc2 cells was assessed by CD69 expression. Before culture, <3% of CD8+ cells were CD69+ but after 7 days in culture >90% of the T cells were CD69+ (data not shown).
Table 1.
Cytokine secretion pattern of in vitro generated Tc1 and Tc2 T cells
| IFN-γ | IL-2 | IL-4 | IL-5 | IL-10 | |
|---|---|---|---|---|---|
| Tc2 supernatant | |||||
| Day 7 | 3,731.2 | ND | 58.6 | 471.0 | 1,109.3 |
| Syngeneic re-stimulation | ND | ND | ND | ND | ND |
| Allogeneic re-stimulation | 4,190.4 | ND | 13.3 | 96.7 | 73.3 |
| Tc1 supernatant | |||||
| Day 7 | 6,328.6 | ND | ND | ND | 181.4 |
| Syngeneic re-stimulation | ND | ND | ND | ND | ND |
| Allogeneic re-stimulation | 13,117.6 | 5.5 | ND | ND | ND |
Unit = pg/ml; ND = none detected. Threshold values: IFN-γ 31.3, IL-2: 3.1, IL-4: 7.8, IL-5: 15.6, IL-10: 31.3.
Survival and Chimerism of the IUT Recipients
Ninety-three fetuses from 10 pregnant mice were transplanted in utero with T-depleted BMCs and Tc2 cells. Ten recipients survived more than 1 month. However, 1 mouse died by the age of 2 months without any signs of GVHD such as changes in the appearance of the skin, weight loss or runting.
Donor cells were readily detected in the peripheral blood at 1 month after IUT (fig. 1a). Owing to the small sample sizes, the lineages represented by the donor cells were not analyzed. However, the light scatter properties of the donor cells suggested that the donor cells were comprised of both lymphoid and myeloid cells (fig. 1b). Chimerism levels in the blood were usually < 0.3% (fig. 2). However, 1 recipient had 4.56% of donor cells in the blood at 1 month of age, but this engraftment was not durable. It dropped to 0.1% by the age of 2 months and then 0.01% at 3 months. Moreover, the median level of chimerism decreased by 2 months of age to near undetectable levels. The level of chimerism at 1 month of age was not significantly different from that at 2 months (p = 0.109), but was significantly higher than that at 3 months (p = 0.008). These results indicate a transient engraftment by donor cells.
Fig. 1.
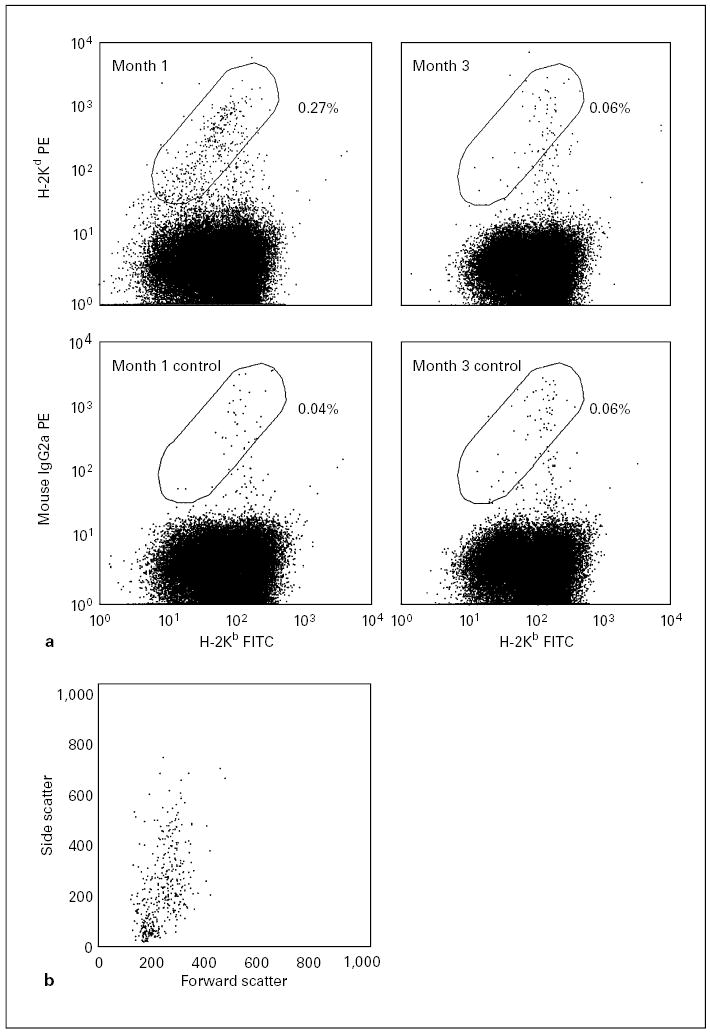
Flow cytometric analyses of chimerism levels in the peripheral blood in 1 recipient at 1 and 3 months of age. a These representative analyses include control samples stained with isotype-matched non-specific antibodies (bottom row) used to determine the levels of background staining. The levels of chimerism are determined by subtracting the frequency of non-specific events measured in the elliptic region from the frequency of specific events (top row). Accordingly, 0.23% chimerism was detected at 1 month of age and no evidence of chimerism was apparent by the 3rd month of age. b The dot plot of forward scatter vs. side scatter is gated by the live donor cells at 1 month of age in a. It shows that the donor cells comprise lymphoid and myeloid lineages.
Fig. 2.
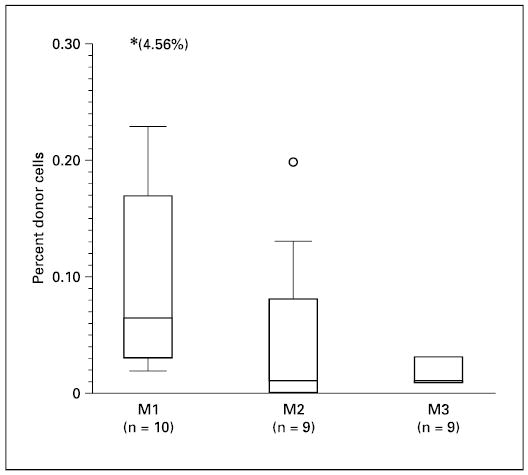
Levels of donor cells in peripheral blood after IUT at the ages of 1, 2 and 3 months are shown. The presence of donor cells in the peripheral blood of recipients was measured by flow cytometry. Results are shown using box plots. The circle in the box plots indicates an outlying data point between 1.5 and 3 box lengths from the upper edge of the box. Another data point that falls beyond the scale is shown in parentheses.
The recipients were sacrificed after 3–5 months for an analysis of chimerism in the various hematopoietic tissues and in the peritoneal cavity, the site of transplantation. The levels of donor cells in the spleen, bone marrow and peritoneal cavity were usually ≤0.05% (table 2). Remarkably, 1 mouse had 25.03% donor cells in its peritoneal cavity (fig. 3). This recipient also had higher levels of splenic chimerism (0.15%). Additional examination of the peritoneal cells and splenocytes was performed for this recipient. The engrafted lineages included B cells (CD19+), myeloid cells (Gr1+) and macrophages (F4/80+). The Gr1+ cells were mostly located in the area of low forward and side light scatter, suggesting that they might be immature progenitors or macrophages rather than granulocytes [32]. There was no evidence that CD8+ donor cells were present in either the spleen or peritoneal cavity at the time the animals were sacrificed.
Table 2.
Chimerism levels in the spleens, bone marrow and peritoneal cavities of recipients sacrificed at 3–5 months of age
| Mouse No.
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Spleen | 0.04 | ND | 0.02 | 0.15 | ND | ND | ND | 0.02 | 0.02 |
| BM | 0.01 | ND | ND | ND | 0.05 | 0.03 | 0.03 | 0.01 | ND |
| Peritoneum | 0.60 | 0.03 | 0.05 | 25.03 | ND | 0.04 | ND | ND | ND |
| SI | −0.11 | 0.34 | 0.51 | 0.43 | 1.07 | 0.71 | 1.52 | 4.35 | 3.69 |
ND = None detected.
Fig. 3.
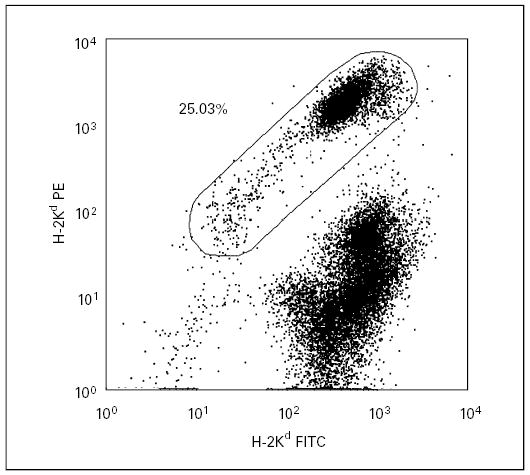
Flow cytometric analyses of chimerism levels in the peritoneum of a transplanted mouse. The dot plot shows a chimerism level of 25.03% in the peritoneal cavity at the age of 3 months.
Analysis of T-Cell Tolerance
At the time of sacrifice, the responses of T cells from the recipient mice were analyzed in mixed lymphocyte cultures (fig. 4). T-cell responses to splenocytes of donor, syngeneic and third-party origin were assessed. One recipient (No. 1) displayed T-cell-specific tolerance to donor cells. Its T cells proliferated in response to third-party stimulators but did not respond to donor stimulators. Three mice (No. 2–4) were hypo-responsive to donor cells, exhibiting significantly more T-cell proliferation to donor stimulators than to syngeneic stimulators but significantly less than to third-party stimulators. In 3 recipients (No. 5–7 as well as a control), T-cell proliferation to donor stimulators was not significantly different from that to third-party stimulators. These mice were considered to have a normal response comparable to untransplanted C57BL/6 mice. Two mice (No. 8, 9) had a hyper-response to donor stimulators. Their T cells proliferated more vigorously to the donor stimulators than to the third-party stimulators.
Fig. 4.
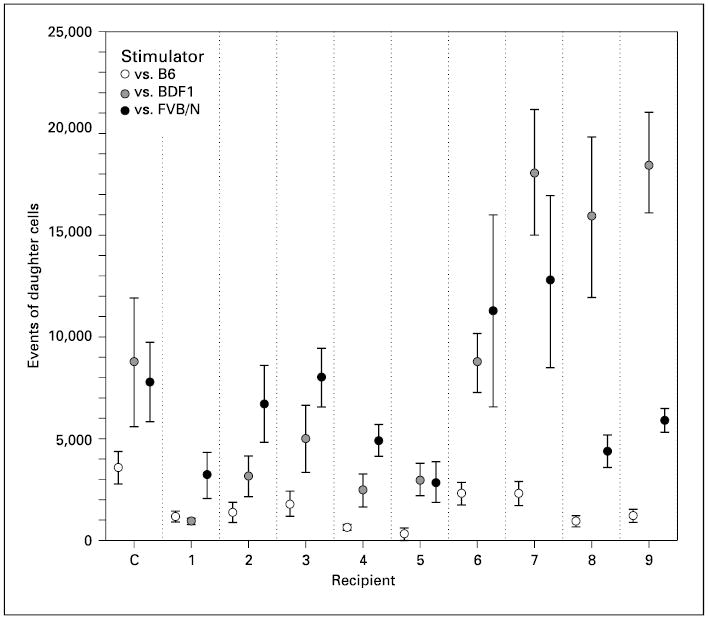
Proliferative responses of recipient T cells in MLRs. The number of daughter CD3+ T cells generated in response to syngeneic (C57BL/6), donor (B6D2F1) and third-party allogeneic (FVB/N) stimulators is shown. Recipient (C) is an untransplanted C57BL/6 mouse used as a control. One of 10 recipients died by the age of 2 months and its MLR could not be measured. Data are shown as the mean ± 2.0 SEM.
As the transplanted mice displayed a wide range of responses from tolerance to hyper-responsiveness, a link between tolerance and chimerism levels was sought by comparing the levels of chimerism in the different tissues between tolerant and non-tolerant mice (table 2). For simplicity, the T-cell responses in the mixed lymphocyte cultures are represented by a SI, which is calculated as described in the Materials and Methods. For these analyses, tolerant animals included those animals that were deemed completely tolerant or hypo-responsive as described above. Non-tolerant mice included the remaining animals that had a normal response or were hyper-responsive. The tolerant mice had a significantly higher level of donor cells in the peritoneal cavity (p = 0.021). A bivariate correlation between SI and the level of peritoneal chimerism reached statistical significance by Spearman correlation coefficient (p = 0.007), indicating that a higher level of peritoneal chimerism was related to a weaker T-cell response to donor cells (lower SI).
Discussion
Successful induction of immunological tolerance to allogeneic donor cells by means of IUT would provide a new means of treatment for a potentially large number of birth defects. Some success has been reported using this approach, but results have not always been consistently favorable. Although the murine fetus before gestational day 17 is unable to mount an effective immunological attack against an allograft [33], a recent study has disclosed the possibility of in utero immunization to donor cells in mice [34]. Graft rejection was shown to occur following IUT as evidenced by accelerated skin graft rejection. It is possible that persistence of donor cells during the development of fetal immune system can sensitize the developing immune system to foreign cells rather than induce tolerance. Thus, graft rejection should be taken into consideration as one possible reason for graft failure following IUT. In this study, we tested if addition of Tc2 cells to bone marrow grafts can facilitate induction of donor-specific tolerance and/or engraftment. This study was modeled after a previous study demonstrating that Tc2 cells can dramatically facilitate engraftment following allogeneic postnatal BMT [28]. Our findings were mixed with tolerance induction occurring in only some instances. Although no clinically significant levels of bone marrow chimerism were observed, notable levels of chimerism were observed in the peritoneum, which correlated with the induction donor-specific T-cell tolerance.
Host T-cell responses to donor cells were found to be variable, ranging from complete tolerance to hyper-responsiveness. The most interesting finding was that tolerant or hypo-responsive recipients had significantly higher levels of donor cells in their peritoneal cavities. This was most clearly indicated by the inverse relationship between the SI and the level of peritoneal chimerism. What remains to be determined is whether the T-cell tolerance observed was the result of lasting peritoneal chimerism or whether the peritoneal chimerism was the result of tolerance established early in development.
Chimerism in the spleen and bone marrow was very low or undetectable, even in the mice that were tolerant to donor cells. This observation suggests two conclusions. The first is that the donor cells in the peritoneal cavity are probably the result of local expansion of injected donor BMCs, rather than coming from the egress of donor cells from the bone marrow as a result of stem cell engraftment. This was most clearly indicated in the animal with the highest observed peritoneal chimerism of 25.03%. Splenic chimerism in this animal was a much lower 0.15% and bone marrow chimerism was not detected. The second conclusion that can be drawn from our findings is that HSC engraftment in the bone marrow is prevented by more than just immunological rejection. This supports a favored hypothesis that HSC engraftment in fetuses, as in adults, is limited by the presence of the host’s own hematopoietic cells [1, 10].
A graft-versus-host effect provided by donor T cells was reported to improve engraftment following IUT by generating available homing spaces [19]. Irradiation can also generate available homing spaces in the bone marrow, but is not a viable option for human prenatal transplantation. A notable difference between this study and the previous study of the effects of Tc2 cells on adult BMT was the use of a sublethal dose of irradiation prior to transplant [28]. Facilitation of engraftment by Tc2 cells has been attributed to a veto effect [35]. It relies upon that host precursor cytotoxic T lymphocytes, which are capable of mediating rejection, are deleted by the donor Tc2 cells. Thus, it is presumed that the veto effect of donor Tc2 cells aids in abrogating rejection, which in association with irradiation that further enables HSC engraftment, leads to a dramatic increase in chimerism. We only found a transient wave of donor cells in the peripheral blood at 1 month of age with a subsequent drop of donor-cell levels to an insignificant level by the age of 3 months. This result is on a par with past reports [5–9]. Since the F1-to-parent model we used does not permit a graft-versus-host effect by donor T cells, we can conclude that abrogating or alleviating rejection towards a graft alone is insufficient to increase HSC engraftment in the IUT setting. A recent report that used attenuated donor T cells was successful in increasing engraftment with minimal GVHD [19]. Further studies of Tc2 cells in an allogeneic transplant model using methods to limit their graft-versus-host effect are warranted. The dose of Tc2 cells transplanted also needs further evaluation, since higher numbers of Tc2 cells may improve the incidence of tolerance inductions. There also remains the possibility for synergism between hematopoietic engraftment and transplanted Tc2 cells in promoting tolerance induction. Selected subsets of T cells may yet provide a means to both improve engraftment as well as induce tolerance in the prenatal transplant setting.
Acknowledgments
We thank Rong-Hua Lu for his technical support in fetal injection, and Drs. Alicia Bárcena, Michael R. Harrison and Yuet-Wai Kan for their suggestions, helpful discussions and support. We also wish to thank Paul Dazin for assistance with flow cytometry. Thanks are further given to Drs. Akihiko Hara, KuoJen Tsao and Linda Flebbe-Rehwaldt for providing us with assistance in the care and breeding of mice.
This work was supported by grant NSC 93-2314-B-782A-086 (J.-C.C.) from the National Science Council, Taiwan and by NIH grant DK59301 (M.O.M.).
References
- 1.Flake AW, Zanjani ED. In utero hematopoietic stem cell transplantation. A status report JAMA. 1997;278:932–937. [PubMed] [Google Scholar]
- 2.Flake AW, Roncarolo MG, Puck JM, Almeida-Porada G, Evans MI, Johnson MP, Abella EM, Harrison DD, Zanjani ED. Treatment of X-linked severe combined immunodeficiency by in utero transplantation of paternal bone marrow. N Engl J Med. 1996;335:1806–1810. doi: 10.1056/NEJM199612123352404. [DOI] [PubMed] [Google Scholar]
- 3.Muench MO, Bárcena A. Stem cell transplantation in the fetus. Cancer Control. 2004;11:105–118. doi: 10.1177/107327480401100217. [DOI] [PubMed] [Google Scholar]
- 4.Zanjani ED, Pallavicini MG, Ascensao JL, Flake AW, Langlois RG, Reitsma M, MacKintosh FR, Stutes D, Harrison MR, Tavassoli M. Engraftment and long-term expression of human fetal hemopoietic stem cells in sheep following transplantation in utero. J Clin Invest. 1992;89:1178–1188. doi: 10.1172/JCI115701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carrier E, Lee TH, Busch MP, Cowan MJ. Induction of tolerance in nondefective mice after in utero transplantation of major histocompatibility complex-mismatched fetal hematopoietic stem cells. Blood. 1995;86:4681–4690. [PubMed] [Google Scholar]
- 6.Hajdu K, Tanigawara S, McLean LK, Cowan MJ, Golbus MS. In utero allogeneic hematopoietic stem cell transplantation to induce tolerance. Fetal Diagn Ther. 1996;11:241–248. doi: 10.1159/000264309. [DOI] [PubMed] [Google Scholar]
- 7.Milner R, Shaaban A, Kim HB, Fichter C, Flake AW. Postnatal booster injections increase engraftment after in utero stem cell transplantation. J Surg Res. 1999;83:44–47. doi: 10.1006/jsre.1998.5558. [DOI] [PubMed] [Google Scholar]
- 8.Kim HB, Shaaban AF, Yang EY, Liechty KW, Flake AW. Microchimerism and tolerance after in utero bone marrow transplantation in mice. J Surg Res. 1998;77:1–5. doi: 10.1006/jsre.1997.5255. [DOI] [PubMed] [Google Scholar]
- 9.Carrier E, Lee TH, Busch MP, Cowan MJ. Recruitment of engrafted donor cells postnatally into the blood with cytokines after in utero transplantation in mice. Transplantation. 1997;64:627–633. doi: 10.1097/00007890-199708270-00014. [DOI] [PubMed] [Google Scholar]
- 10.Flake AW, Zanjani ED. In utero hematopoietic stem cell transplantation: Ontogenic opportunities and biologic barriers. Blood. 1999;94:2179–2191. [PubMed] [Google Scholar]
- 11.Muench MO, Rae J, Bárcena A, Leemhuis T, Farrell J, Humeau L, Maxwell-Wiggins JR, Capper J, Mychaliska GB, Albanese CT, Martin T, Tsukamoto A, Curnutte J, Harrison MR. Transplantation of a fetus with paternal Thy-1+CD34+ cells for chronic granulomatous disease. Bone Marrow Transplant. 2001;27:355–364. doi: 10.1038/sj.bmt.1702798. [DOI] [PubMed] [Google Scholar]
- 12.Martin PJ. Determinants of engraftment after allogeneic marrow transplantation. Blood. 1992;79:1647–1650. [PubMed] [Google Scholar]
- 13.Martin PJ, Hansen JA, Torok-Storb B, Durnam D, Przepiorka D, O’Quigley J, Sanders J, Sullivan KM, Witherspoon RP, Deeg HJ, et al. Graft failure in patients receiving T cell-depleted HLA-identical allogeneic marrow transplants. Bone Marrow Transplant. 1988;3:445–456. [PubMed] [Google Scholar]
- 14.Martin PJ, Hansen JA, Buckner CD, Sanders JE, Deeg HJ, Stewart P, Appelbaum FR, Clift R, Fefer A, Witherspoon RP, et al. Effects of in vitro depletion of T cells in HLA-identical allogeneic marrow grafts. Blood. 1985;66:664–672. [PubMed] [Google Scholar]
- 15.Kernan NA, Collins NH, Juliano L, Cartagena T, Dupont B, O’Reilly RJ. Clonable T lymphocytes in T cell-depleted bone marrow transplants correlate with development of graft-vs-host disease . Blood. 1986;68:770–773. [PubMed] [Google Scholar]
- 16.Korngold R, Sprent J. Surface markers of T cells causing lethal graft-vs.-host disease to class I vsclass II H-2 differences. J Immunol. 1985;135:3004–3010. [PubMed] [Google Scholar]
- 17.Shields LE, Andrews RG. In utero stem cell transplantation in non-human primates: The role of T-cells number (abstract) Am J Obstet Gynecol. 2001;184:S2. [Google Scholar]
- 18.Crombleholme TM, Harrison MR, Zanjani ED. In utero transplantation of hematopoietic stem cells in sheep: The role of T cells in engraftment and graft-versus-host disease. J Pediatr Surg. 1990;25:885–892. doi: 10.1016/0022-3468(90)90197-h. [DOI] [PubMed] [Google Scholar]
- 19.Bhattacharyya S, Chawla A, Smith K, Zhou Y, Talib S, Wardwell B, Cowan MJ. Multilineage engraftment with minimal graft-versus-host disease following in utero transplantation of s-59 psoralen/ultraviolet a light-treated, sensitized T cells and adult T cell-depleted bone marrow in fetal mice. J Immunol. 2002;169:6133–6140. doi: 10.4049/jimmunol.169.11.6133. [DOI] [PubMed] [Google Scholar]
- 20.Martin PJ. Donor CD8 cells prevent allogeneic marrow graft rejection in mice: Potential implications for marrow transplantation in humans. J Exp Med. 1993;178:703–712. doi: 10.1084/jem.178.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palathumpat V, Dejbakhsh-Jones S, Strober S. The role of purified CD8+ T cells in graft-versus-leukemia activity and engraftment after allogeneic bone marrow transplantation. Transplantation. 1995;60:355–361. doi: 10.1097/00007890-199508270-00010. [DOI] [PubMed] [Google Scholar]
- 22.Martin PJ, Rowley SD, Anasetti C, Chauncey TR, Gooley T, Petersdorf EW, van Burik JA, Flowers ME, Storb R, Appelbaum FR, Hansen JA. A phase I–II clinical trial to evaluate removal of CD4 cells and partial depletion of CD8 cells from donor marrow for HLA-mismatched unrelated recipients. Blood. 1999;94:2192–2199. [PubMed] [Google Scholar]
- 23.Martin PJ, Akatsuka Y, Hahne M, Sale G. Involvement of donor T-cell cytotoxic effector mechanisms in preventing allogeneic marrow graft rejection. Blood. 1998;92:2177–2181. [PubMed] [Google Scholar]
- 24.Gandy KL, Domen J, Aguila H, Weissman IL. CD8+TCR+ and CD8+TCR– cells in whole bone marrow facilitate the engraftment of hematopoietic stem cells across allogeneic barriers. Immunity. 1999;11:579–590. doi: 10.1016/s1074-7613(00)80133-8. [DOI] [PubMed] [Google Scholar]
- 25.Sad S, Marcotte R, Mosmann TR. Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity. 1995;2:271–279. doi: 10.1016/1074-7613(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 26.Croft M, Carter L, Swain SL, Dutton RW. Generation of polarized antigen-specific CD8 effector populations: Reciprocal action of interleukin (IL)-4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J Exp Med. 1994;180:1715–1728. doi: 10.1084/jem.180.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fowler DH, Gress RE. CD8+ T cells of Tc2 phenotype mediate a GVL effect and prevent marrow rejection. Vox Sang. 1998;74:331–340. doi: 10.1111/j.1423-0410.1998.tb05439.x. [DOI] [PubMed] [Google Scholar]
- 28.Fowler DH, Whitfield B, Livingston M, Chrobak P, Gress RE. Non-host-reactive donor CD8+ T cells of Tc2 phenotype potently inhibit marrow graft rejection. Blood. 1998;91:4045–4050. [PubMed] [Google Scholar]
- 29.Fowler DH, Breglio J, Nagel G, Eckhaus MA, Gress RE. Allospecific CD8+ Tc1 and Tc2 populations in graft-versus-leukemia effect and graft-versus-host disease. J Immunol. 1996;157:4811–4821. [PubMed] [Google Scholar]
- 30.Fowler DH, Gress RE. Th2 and Tc2 cells in the regulation of GVHD, GVL, and graft rejection: Considerations for the allogeneic transplantation therapy of leukemia and lymphoma. Leuk Lymphoma. 2000;38:221–234. doi: 10.3109/10428190009087014. [DOI] [PubMed] [Google Scholar]
- 31.Chen JC, Chang ML, Muench MO. A kinetic study of the murine mixed lymphocyte reaction by 5,6-carboxyfluorescein diacetate succinimidyl ester labeling. J Immunol Meth. 2003;279:123–133. doi: 10.1016/s0022-1759(03)00236-9. [DOI] [PubMed] [Google Scholar]
- 32.Hestdal K, Ruscetti FW, Ihle JN, Jacobsen SEW, Dubois CM, Kopp WC, Longo DL, Keller JR. Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. J Immunol. 1991;147:22–28. [PubMed] [Google Scholar]
- 33.Defresne MP, Humblet C, Deman J, et al. Ontogeny of T-cell surface molecules and receptors in the thymus. Prog Histochem Cytochem. 1992;26:194–200. doi: 10.1016/s0079-6336(11)80095-1. [DOI] [PubMed] [Google Scholar]
- 34.Donahue J, Gilpin E, Lee TH, Busch MP, Croft M, Carrier E. Microchimerism does not induce tolerance and sustains immunity after in utero transplantation. Transplantation. 2001;71:359–368. doi: 10.1097/00007890-200102150-00004. [DOI] [PubMed] [Google Scholar]
- 35.Hiruma K, Nakamura H, Henkart PA, Gress RE. Clonal deletion of postthymic T cells: Veto cells kill precursor cytotoxic T lymphocytes. J Exp Med. 1992;175:863–868. doi: 10.1084/jem.175.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]


