Abstract
The cyclic dinucleotide 3′,5′-cyclic diguanylic acid (c-di-GMP) is a naturally occurring small molecule that regulates important signaling systems in bacteria. We have recently shown that c-di-GMP inhibits Staphylococcus aureus biofilm formation in vitro and its adherence to HeLa cells. We now report that c-di-GMP treatment has an antimicrobial and antipathogenic activity in vivo and reduces, in a dose-dependent manner, bacterial colonization by biofilm-forming S. aureus strains in a mouse model of mastitis infection. Intramammary injections of 5 and 50 nmol of c-di-GMP decreased colonization (bacterial CFU per gram of gland) by 0.79 (P > 0.05) and 1.44 (P < 0.01) logs, respectively, whereas 200-nmol doses allowed clearance of the bacteria below the detection limit with a reduction of more than 4 logs (P < 0.001) compared to the untreated control groups. These results indicate that cyclic dinucleotides potentially represent an attractive and novel drug platform which could be used alone or in combination with other agents or drugs in the prevention, treatment, or control of infection.
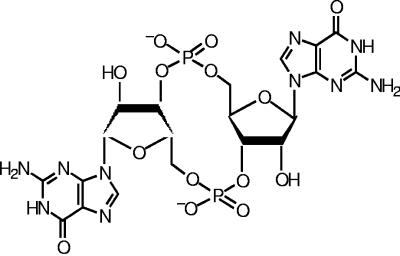
The cyclic dinucleotide 3′,5′-cyclic diguanylic acid (cyclic diguanylate, c-di-GMP, or cGpGp) is a small novel naturally occurring signaling molecule composed of two cGMP molecules linked by a 3′,5′ phosphodiester bond (12, 15, 25) (Fig. 1). The c-di-GMP molecule was first identified in the bacterial species Gluconacetobacter xylinus (Acetobacter xylinum) (27). The intracellular levels of c-di-GMP regulate cellulose production through interaction with the cellulose synthase BcsB (1, 20, 25, 26). The level of c-di-GMP in the cell is modulated by the opposing effects of proteins with GGDEF and EAL domains, the c-di-GMP phosphodiesterase and the diguanylate cyclase, which cleave the c-di-GMP molecule or catalyze its formation, respectively (30). Studies by us and others have shown that proteins with GGDEF and/or EAL domains are found in many bacterial species and are increasingly being associated with the differential expression of certain phenotypes such as biofilm formation (3, 11, 16, 23, 24), further suggesting that c-di-GMP is a widespread modulator of several cellular processes in prokaryotes (12, 15).
FIG. 1.
Molecular structure of c-di-GMP.
Supporting a role in pathogenesis, we have recently shown that exogenous c-di-GMP treatment, in contrast to other guanosine nucleotide analogs, specifically inhibits Staphylococcus aureus cell-cell interactions, biofilm formation, and adherence to HeLa epithelial cells in vitro (18). In that same study, we reported that search of the COG database shows that S. aureus has only one protein (SA0701, COG2199) with a C-terminal GGDEF domain and another protein (SA0013, COG3887) with a modified GGDEF domain (31). According to a Pfam analysis, SA0701 is predicted to be a diguanylate cyclase (18). Unfortunately, the role of these putative signal transduction proteins in S. aureus and whether they are potentially linked to c-di-GMP in the cell, whether c-di-GMP is made intracellularly by S. aureus, and the regulatory effects of intracellular c-di-GMP are not yet known.
S. aureus is an important human and animal pathogen causing a variety of infections that are often difficult to manage clinically (2, 6). Cow mastitis caused by S. aureus, a costly disease for dairy producers, is frequently chronic and hard to treat with currently available antimicrobial therapies (28, 29). The intercellular adhesion (ica) locus, which plays a role in the synthesis of the polysaccharide intercellular adhesin and biofilm formation (7, 19, 21), is found in the majority of the clinical bovine mastitis strains (10, 32). Another important locus is the biofilm-associated protein (bap) gene, also implicated in biofilm formation and shown to be connected to S. aureus persistence during bovine mastitis (8-10). Cucarella et al. (10) also found that, when S. aureus was grown in conditions that promote biofilm formation, the presence of the ica and bap genetic elements was associated with an increased resistance of the pathogen to antibiotics. As in other infections caused by S. aureus, colonization and biofilm formation during the course of bovine mastitis infection may account for the difficulty in combating infection (with or without antibiotic treatment). Since c-di-GMP can inhibit biofilm formation in vitro (18), we determined whether c-di-GMP treatment could attenuate virulence and prevent infection in vivo caused by biofilm-forming strains of S. aureus using an established mouse model of intramammary infection (4, 5). This is the first report demonstrating the therapeutic efficacy of c-di-GMP in vivo.
MATERIALS AND METHODS
Bacterial strains and c-di-GMP used in this study.
The bacterial strains used were S. aureus Newbould 305 (ATCC 29740), a clinical bovine mastitis isolate previously used for the study of mastitis in mice and cows, and S. aureus STF2004-01044-49 (herein referred to as sa272c), another bovine mastitis strain recently isolated by the public laboratory of the Ministère de l'Agriculture, des Pêcheries et de l'Alimentation du Québec (Québec, Canada). Strain sa272c was selected from a collection of 72 other bovine mastitis isolates that were tested for their relative ability to form biofilms (see below). The c-di-GMP compound used in this study was chemically synthesized and consisted of a pure, high-yield preparation of c-di-GMP diammonium salt as previously described (13, 14), suspended in a sterile 0.9% NaCl solution at 6 mM, and stored at 4°C until used as described previously (18).
c-di-GMP effects on biofilm formation and bacterial growth.
The MIC of c-di-GMP for S. aureus Newbould was measured by a broth microdilution method as recommended by the Clinical and Laboratory Standards Institute, formerly named the NCCLS (22). Experiments designed to measure the effect of c-di-GMP on growth and viability of S. aureus in tryptic soy broth (TSB) were carried out by using an overnight culture diluted to an A600 of 0.1 in broth supplemented with 200 or 400 μM of the cyclic dinucleotide. Cultures were incubated at 35°C (225 rpm). The A600 was recorded at different points in time, and the number of viable CFU was determined. Biofilm formation was evaluated by spectrophotometry in microplates using crystal violet staining, following a method previously described (18). S. aureus Newbould and sa272c were tested for their capacity to form biofilms in TSB (0.25% glucose) in the presence of 0, 20, 200, or 400 μM c-di-GMP. The assays were carried out three times in triplicate, and the data were combined before statistical analysis.
Mouse model of mastitis infection.
The mouse model of mastitis used here was established and optimized for strain S. aureus Newbould in our previous studies (4). Briefly, the 12- to 14-day-old pups were removed from CD-1 lactating mice (Charles River, St.-Constant, Québec, Canada) 1 to 2 h before infection. Animals were anesthetized, and mouse mammary glands were infected with approximately 1 × 102 to 2 × 102 CFU by injection through the lactiferous duct with a syringe and a 32-gauge blunt needle, under binoculars. Previously, using these experimental conditions, a plateau, in terms of CFU per gram of gland, was reached at 12 h postinoculation (4). In the current study, infection was allowed to proceed for 10 h in order to avoid saturation of tissues with bacteria in the nontreated control groups. For each mouse, c-di-GMP was administered twice by intramammary injection in both, from head to tail, the L4 (fourth on the left) and R4 (fourth on the right) glands. At the first administration (concomitant with bacterial inoculation, t = 0 h), c-di-GMP and bacteria were mixed together in 0.9% NaCl just prior to injection into each mammary gland in a final volume of 100 μl. The same amount of c-di-GMP was injected into the mammary gland for the second administration (t = 4 h), in a volume of 50 μl, to anesthetized animals. Control mice were treated in the same way as were c-di-GMP-treated animals but instead using a 0.9% NaCl saline solution. Doses of 5, 50, and 200 nmol (delivered in a 50- or 100-μl volume) were given to c-di-GMP-treated groups composed of three to four mice. The experiments were subsequently repeated, for a total of six to eight mice used per treatment (or 12 to 16 infected glands). Each injection of 5, 50, and 200 nmol of c-di-GMP provided to the mouse corresponded to a dose of 0.1, 1.0, and 4.1 mg of compound per kg of body weight (considering an average weight of 35 g), respectively. Raw bacterial CFU counts obtained after plating serial logarithmic dilutions of mammary gland homogenates were transformed in base-10 logarithm values and grouped. The institutional ethics committee on animal experimentation of the Faculté des sciences of Université de Sherbrooke approved the present study that was carried out following the guidelines of the Canadian Council on Animal Care (website: http://www.ccac.ca).
Statistical analysis.
For the biofilm assays, one-way analysis of variance followed by the Dunnet post test was used for statistical significance analysis whereas bacterial counts in mammary glands were analyzed using the Kruskal-Wallis test combined with the Dunn multiple comparison test (GraphPad Instat, version 3.06).
RESULTS AND DISCUSSION
c-di-GMP inhibition of biofilm formation.
Strains Newbould and sa272c produced more biofilm than the 72 other clinical strains screened (data not shown). Quantification of the biofilm revealed a moderate (A570 of ∼1.0) and a high (A570 of ∼3.2) biofilm production for strains Newbould and sa272c, respectively. For comparison, 90% of all the clinical strains tested showed an A570 lower than 0.5.
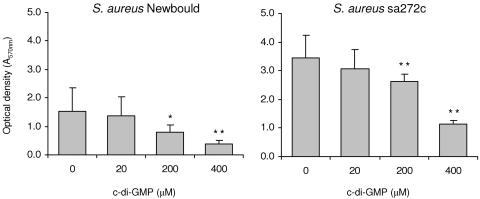
The results in Fig. 2 show that supplementation of the growth medium with c-di-GMP reduced biofilm formation for strain Newbould and sa272c in a dose-dependent manner similar to results previously obtained for other strains of S. aureus including human methicillin-resistant S. aureus and bovine mastitis clinical isolates (18). Note that we also have previously demonstrated that addition of the dinucleotide after biofilm formation also affects its integrity (18). Here, c-di-GMP showed no significant inhibition of growth in vitro against S. aureus Newbould (MIC, >256 μg/ml or 353 μM). Also consistent with that previously reported for other S. aureus strains (18), the growth profile of S. aureus Newbould in TSB was not affected by the presence of cyclic diguanylate any more than was the number of viable bacterial cells found after 12 h of growth in vitro (data not shown).
FIG. 2.
Effect of c-di-GMP on biofilm production. S. aureus biofilm-forming strains Newbould and sa272c were grown in microplates containing TSB supplemented with 0.25% glucose, with or without addition of c-di-GMP. After overnight incubation under static conditions, wells were washed and the remaining bacteria at the bottom of the wells were stained with crystal violet and quantified by spectrophotometry. Averages and standard deviations are shown. The experiment was performed three times in triplicate and data were combined. *, P < 0.05; **, P < 0.01.
c-di-GMP treatment inhibits infection in the mouse.
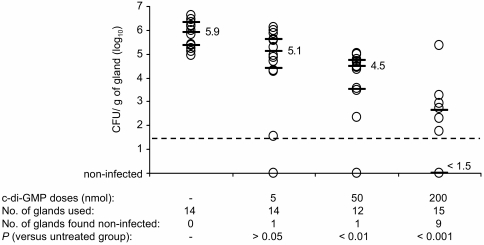
Based on previous in vitro results showing that c-di-GMP can significantly attenuate biofilm formation by S. aureus (18), we determined the ability of c-di-GMP to counteract (inhibit) mastitis infection caused by S. aureus strain Newbould in the mouse model. The administration of two 5-nmol doses of c-di-GMP at 0 h and 4 h postinfection reduced the bacterial burden (median log10 CFU per gram of tissue) by 0.79 log10 compared to the untreated controls (P > 0.05) (Fig. 3). The injection of higher doses of c-di-GMP demonstrated a dose-dependent effect. The 50-nmol doses significantly reduced the infection level by 1.44 log10 (P < 0.01) while the 200-nmol doses reduced infection by more than 4 log10 (P < 0.001). In fact, the median log10 CFU for the 200-nmol-treated group was equivalent to the noninfected level given that 9 of 15 glands were found to be free of bacteria (detection limit, 1.5 log10 CFU/g of gland). To confirm the in vivo antibacterial effect of c-di-GMP on S. aureus, we also tested its ability to inhibit infection by S. aureus strain sa272c. As expected, the intramammary administration of two doses of 200 nmol of c-di-GMP abolished mammary gland colonization for the treated group (no gland was found to be infected) compared to the saline-treated controls (all glands infected, median of CFU per g of gland of 4.1 log10) (data not shown). For comparison, intramammary administrations of 0.5 nmol of the traditional bactericidal β-lactam antibiotic cephapirin were required to achieve an antibacterial effect similar to the one observed for the S. aureus Newbould-infected group treated with 200-nmol doses of c-di-GMP (not shown). No apparent sign of toxicity (alterations in posture, breathing, piloerection, or movement) was observed for any of the animals treated with c-di-GMP.
FIG. 3.
In vivo inhibitory effect of c-di-GMP on S. aureus infection. Lactating mice were infected by intramammary inoculation of 1 × 102 to 2 × 102 CFU of S. aureus Newbould. c-di-GMP was administered into mammary glands at t = 0 and t = 4 h postinoculation. Infection was allowed for 10 h, and mammary glands were aseptically harvested for bacterial CFU determination. Each circle on the graph corresponds to the number of CFU per gram of gland for one gland. Medians (indicated values) and the first and third quartiles are represented by bars, whereas the detection limit is indicated by the dashed line.
Although the dosing schedule used in the present study mainly established the prophylactic property of c-di-GMP, the clearance of S. aureus observed in the mouse suggests an enhanced antibacterial effect in the in vivo environment. It is possible that, in addition to inhibition of biofilm formation, important physiological processes required for microbial growth in vivo may also be affected by c-di-GMP or that inhibition of biofilm formation strongly favors host defenses. Also, a direct effect of c-di-GMP on host cells cannot be excluded at this time (17).
Potential use of c-di-GMP as an antimicrobial agent.
Bacterial genes that are essential to virulence or to survival of a pathogen in vivo represent novel and potential microbial cell targets for the development of alternatives to traditional antibiotics that are currently plagued by bacterial resistance mechanisms. The use of antimicrobial agents targeting virulence factors is a potentially attractive approach. Small therapeutic molecules (such as c-di-GMP) that target specific virulence functions or related genetic transcription mechanisms should not exert the same type of unilateral resistance selection pressure as that created by current treatments that target the metabolic functions that are essential to most cells in all circumstances. Rather, it is possible that the selection and spreading of resistant organisms could be considerably slower than those provoked by conventional antibiotics since “antipathogenic drugs” would be less likely to affect bacteria, such as the microorganisms found in the normal flora or the pathogens and opportunistic bacteria found in the environment, not needing or expressing the targeted pathogenic function. New therapeutic molecules affecting virulence could be used prophylactically before an infection occurs (infection prevention), used directly (for treatment) if suppression of virulence significantly improves host defenses, or possibly in combination with traditional antibiotics during a severe infection for more effective treatment (in vivo synergy). These new molecules would also be protected from most of the bacterial resistance mechanisms generally found in microorganisms, which affect very specific classes of antibiotics (β-lactams, aminoglycosides, macrolides, fluoroquinolones, etc.). We propose that cyclic dinucleotides, such as c-di-GMP, represent an example of a useful new class of antimicrobial molecules.
In this study, we have indeed explored the potential use of c-di-GMP as an antimicrobial agent in vivo and demonstrated that c-di-GMP attenuates the virulence of biofilm-forming clinical isolates of S. aureus (here virulence is defined as the ability of a microorganism to survive and multiply in the tissues of a host). In contrast to other types of S. aureus infections that are usually treated by parenteral administration of antibiotics, bovine mastitis is frequently treated by intramammary administration with a cannula introduced into the duct of the udder. Here, our experimental S. aureus infection was also treated by intramammary administration, clearly establishing the actual potential of c-di-GMP for the prevention of infections such as mastitis. Although we do not yet know the detailed pharmacokinetic and pharmacodynamic properties of c-di-GMP and how such properties compare to those of traditional drugs like cephapirin, it is now quite clear that this molecule can act on S. aureus and attenuate virulence when directly administered at the site of infection.
Conclusion.
In this study, the most unexpected finding was that c-di-GMP has no apparent inhibitory or bactericidal effect on S. aureus in vitro yet it significantly prevented the infection of the mouse by biofilm-forming S. aureus strains in vivo. We do not know the molecular basis of c-di-GMP action on S. aureus cells and its effect on the host immune response. We are currently working on finding hints from transcriptional profiling experiments and classical mode-of-action studies (bacterial physiology). Studies of the efficacy of c-di-GMP in other animal models are also planned and should provide important insights on how to optimize the development of this very promising agent and cyclic dinucleotide analogs to prevent, treat, or control infection.
Acknowledgments
This work was supported by grant no. MOP-57701 to F.M. from the Canadian Institutes for Health Research.
We are grateful to Serge Messier from the Université de Montréal (Montréal, Québec, Canada) for providing the bovine mastitis clinical strains used in this study. We thank Caroline Bergeron for technical assistance with animals.
REFERENCES
- 1.Amikam, D., and M. Benziman. 1989. Cyclic diguanylic acid and cellulose synthesis in Agrobacterium tumefaciens. J. Bacteriol. 171:6649-6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archer, G. L. 1998. Staphylococcus aureus: a well-armed pathogen. Clin. Infect. Dis. 26:1179-1181. [DOI] [PubMed] [Google Scholar]
- 3.Bomchil, N., P. Watnick, and R. Kolter. 2003. Identification and characterization of a Vibrio cholerae gene, mbaA, involved in maintenance of biofilm architecture. J. Bacteriol. 185:1384-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brouillette, E., G. Grondin, C. Lefebvre, B. G. Talbot, and F. Malouin. 2004. Mouse mastitis model of infection for antimicrobial compound efficacy studies against intracellular and extracellular forms of Staphylococcus aureus. Vet. Microbiol. 101:253-262. [DOI] [PubMed] [Google Scholar]
- 5.Brouillette, E., B. G. Talbot, and F. Malouin. 2003. The fibronectin-binding proteins of Staphylococcus aureus may promote mammary gland colonization in a lactating mouse model of mastitis. Infect. Immun. 71:2292-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cosgrove, S. E., G. Sakoulas, E. N. Perencevich, M. J. Schwaber, A. W. Karchmer, and Y. Carmeli. 2003. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin. Infect. Dis. 36:53-59. [DOI] [PubMed] [Google Scholar]
- 7.Cramton, S. E., C. Gerke, N. F. Schnell, W. W. Nichols, and F. Gotz. 1999. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 67:5427-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cucarella, C., C. Solano, J. Valle, B. Amorena, I. Lasa, and J. R. Penades. 2001. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J. Bacteriol. 183:2888-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cucarella, C., M. A. Tormo, E. Knecht, B. Amorena, I. Lasa, T. J. Foster, and J. R. Penades. 2002. Expression of the biofilm-associated protein interferes with host protein receptors of Staphylococcus aureus and alters the infective process. Infect. Immun. 70:3180-3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cucarella, C., M. A. Tormo, C. Ubeda, M. P. Trotonda, M. Monzon, C. Peris, B. Amorena, I. Lasa, and J. R. Penades. 2004. Role of biofilm-associated protein bap in the pathogenesis of bovine Staphylococcus aureus. Infect. Immun. 72:2177-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Argenio, D. A., M. W. Calfee, P. B. Rainey, and E. C. Pesci. 2002. Autolysis and autoaggregation in Pseudomonas aeruginosa colony morphology mutants. J. Bacteriol. 184:6481-6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Argenio, D. A., and S. I. Miller. 2004. Cyclic di-GMP as a bacterial second messenger. Microbiology 150:2497-2502. [DOI] [PubMed] [Google Scholar]
- 13.Hayakawa, Y., R. Nagata, A. Hirata, M. Hyodo, and R. Kawai. 2003. A facile synthesis of cyclic bis(3′-5′)diguanylic acid. Tetrahedron 59:6465-6471. [Google Scholar]
- 14.Hyodo, M., and Y. Hayakawa. 2004. An improved method for synthesizing cyclic bis(3′-5′)diguanylic acid (c-di-GMP). Bull. Chem. Soc. Jpn. 77:2089-2093. [Google Scholar]
- 15.Jenal, U. 2004. Cyclic di-guanosine-monophosphate comes of age: a novel secondary messenger involved in modulating cell surface structures in bacteria? Curr. Opin. Microbiol. 7:185-191. [DOI] [PubMed] [Google Scholar]
- 16.Jones, H. A., J. W. Lillard, Jr., and R. D. Perry. 1999. HmsT, a protein essential for expression of the haemin storage (Hms+) phenotype of Yersinia pestis. Microbiology 145:2117-2128. [DOI] [PubMed] [Google Scholar]
- 17.Karaolis, D. K. R., K. Cheng, M. Lipsky, A. Elnabawi, J. Catalano, M. Hyodo, Y. Hayakawa, and J.-P. Raufman. 2005. 3′,5′-Cyclic diguanylic acid (c-di-GMP) inhibits basal and growth factor-stimulated human colon cancer cell proliferation. Biochem. Biophys. Res. Commun. 329:40-45. [DOI] [PubMed] [Google Scholar]
- 18.Karaolis, D. K. R., M. H. Rashid, R. Chythanya, W. Luo, M. Hyodo, and Y. Hayakawa. 2005. c-di-GMP (3′-5′-cyclic diguanylic acid) inhibits Staphylococcus aureus cell-cell interactions and biofilm formation. Antimicrob. Agents Chemother. 49:1029-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maira-Litrán, T., A. Kropec, C. Abeygunawardana, J. Joyce, G. Mark III, D. A. Goldmann, and G. B. Pier. 2002. Immunochemical properties of the staphylococcal poly-N-acetylglucosamine surface polysaccharide. Infect. Immun. 70:4433-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayer, R., P. Ross, H. Weinhouse, D. Amikam, G. Volman, P. Ohana, R. D. Calhoon, H. C. Wong, A. W. Emerick, and M. Benziman. 1991. Polypeptide composition of bacterial cyclic diguanylic acid-dependent cellulose synthase and the occurrence of immunologically crossreacting proteins in higher plants. Proc. Natl. Acad. Sci. USA 88:5472-5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKenney, D., K. L. Pouliot, Y. Wang, V. Murthy, M. Ulrich, G. Doring, J. C. Lee, D. A. Goldmann, and G. B. Pier. 1999. Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science 284:1523-1527. [DOI] [PubMed] [Google Scholar]
- 22.NCCLS. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard M7-A6. NCCLS, Wayne, Pa.
- 23.Rashid, M. H., C. Rajanna, A. Ali, and D. K. R. Karaolis. 2003. Identification of genes involved in the switch between the smooth and rugose phenotypes of Vibrio cholerae. FEMS Microbiol. Lett. 227:113-119. [DOI] [PubMed] [Google Scholar]
- 24.Römling, U., M. Rohde, A. Olsen, S. Normark, and J. Reinkoster. 2000. AgfD, the checkpoint of multicellular and aggregative behaviour in Salmonella typhimurium regulates at least two independent pathways. Mol. Microbiol. 36:10-23. [DOI] [PubMed] [Google Scholar]
- 25.Ross, P., R. Mayer, and M. Benziman. 1991. Cellulose biosynthesis and function in bacteria. Microbiol. Rev. 55:35-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross, P., R. Mayer, H. Weinhouse, D. Amikam, Y. Huggirat, M. Benziman, E. de Vroom, A. Fidder, P. de Paus, L. A. Sliedregt, G. A. van der Marel, and J. H. van Boom. 1990. The cyclic diguanylic acid regulatory system of cellulose synthesis in Acetobacter xylinum. Chemical synthesis and biological activity of cyclic nucleotide dimer, trimer, and phosphothioate derivatives. J. Biol. Chem. 265:18933-18943. [PubMed] [Google Scholar]
- 27.Ross, P., H. Weinhouse, Y. Aloni, D. Michaeli, P. Weinberger-Ohana, R. Mayer, S. Braun, E. de Vroom, G. A. van der Marel, J. H. van Boom, and M. Benziman. 1987. Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid. Nature 325:279-281. [DOI] [PubMed] [Google Scholar]
- 28.Sears, P. M., and K. K. McCarthy. 2003. Management and treatment of staphylococcal mastitis. Vet. Clin. N. Am. Food Anim. Pract. 19:171-185, vii. [DOI] [PubMed] [Google Scholar]
- 29.Sutra, L., and B. Poutrel. 1994. Virulence factors involved in the pathogenesis of bovine intramammary infections due to Staphylococcus aureus. J. Med. Microbiol. 40:79-89. [DOI] [PubMed] [Google Scholar]
- 30.Tal, R., H. C. Wong, R. Calhoon, D. Gelfand, A. L. Fear, G. Volman, R.Mayer, P. Ross, D. Amikam, H. Weinhouse, A. Cohen, S. Sapir, P. Ohana, and M. Benziman. 1998. Three cdg operons control cellular turnover of cyclic di-GMP in Acetobacter xylinum: genetic organization and occurrence of conserved domains in isoenzymes. J. Bacteriol. 180:4416-4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tatusov, R. L., D. A. Natale, I. V. Garkavtsev, T. A. Tatusova, U. T. Shankavaram, B. S. Rao, B. Kiryutin, M. Y. Galperin, N. D. Fedorova, and E. V. Koonin. 2001. The COG database: new developments in phylogenetic classification of proteins from complete genomes. Nucleic Acids Res. 29:22-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vasudevan, P., M. K. Nair, T. Annamalai, and K. S. Venkitanarayanan. 2003. Phenotypic and genotypic characterization of bovine mastitis isolates of Staphylococcus aureus for biofilm formation. Vet. Microbiol. 92:179-185. [DOI] [PubMed] [Google Scholar]