Abstract
In a previous work, we described the possible relationship between a defect of purine-cytosine permease and the acquisition of a cross-resistance to the antifungal combination flucytosine (5FC) and fluconazole (FLC) in Candida lusitaniae (T. Noël, F. François, P. Paumard, C. Chastin, D. Brethes, and J. Villard, Antimicrob. Agents Chemother. 47:1275-1284, 2003). Using degenerate PCR and chromosome walking, we cloned two FCY2-like genes in C. lusitaniae. Northern blot analysis revealed that only one gene was expressed; it was named FCY2. The other one behaved as a pseudogene and was named FCY21. In order to better characterize the possible role of FCY2 in cross-resistance to 5FC-FLC, disruption experiments with auxotrophic strain 6936 ura3(D95V) FCY2 with an integrative vector carrying the URA3 gene and a partial sequence of the C. lusitaniae FCY2 gene were undertaken. Southern blot analysis revealed that homologous recombination events occurred in all transformants analyzed at rates of 50% at resident locus FCY2 and 50% at resident locus URA3, resulting in the genotypes ura3 fcy2::URA3 and ura3::URA3 FCY2, respectively. It was then demonstrated that only transformants harboring a disrupted fcy2 gene were resistant to 5FC, susceptible to FLC, and resistant to the 5FC-FLC combination. Finally, complementation experiments with a functional FCY2 gene restored 5FC and FLC susceptibilities to the wild-type levels. The results of this study provide molecular evidence that inactivation of the sole FCY2 gene promotes cross-resistance to the antifungal association 5FC-FLC in C. lusitaniae.
Treatment of fungal infections represents a crucial problem for clinicians because of the emergence of resistance to a small number of antifungal drugs available for systemic use (18). In order to minimize the risk of therapeutic failure, the use of bitherapy, which relies on antifungal combinations, is more frequent and is strongly recommended when the treatment includes flucytosine (5FC). Indeed, resistance to 5FC can easily develop as a result of mutations mainly in genes involved in the uptake of 5FC (the FCY2 gene, which encodes purine-cytosine permease [PCP]), in the conversion of intracellular 5FC to 5-fluorouracil (5FU; the FCY1 gene, which encodes cytosine deaminase), or in the transformation of 5FU to 5-fluorouridine monophosphate (the FUR1 gene, which encodes uracil phosphoribosyltransferase) (9, 16, 34).
However, combinations of antifungals may be challenged by the occurrence of cross-resistance, particularly in the commonly used class of azole antifungals, where it can derive from enhanced expression of multidrug resistance genes, such as CDR1 and CDR2 of the ATP-binding cassette transporter family (26, 28, 30), or from point mutations of the genes that encode the lanosterol demethylase and the Δ5,6-sterol desaturase of the ergosterol biosynthesis pathway (17, 29).
Because of the increasing use of antifungal bitherapy, we investigated the possible occurrence of cross-resistance in Candida lusitaniae. Despite its low prevalence as a cause of candidemia (1 to 2%) (19, 21), the emergence of C. lusitaniae has frequently been associated with its propensity to develop antifungal resistance during treatment, mainly to amphotericin B (14, 15, 20, 37), but also to azoles (2, 3, 21) and to 5FC (25). Moreover, C. lusitaniae is a convenient model for the genetic and molecular characterization of antifungal resistance, because it is one of the Candida species known to be haploid with a sexual cycle reproducible in vitro (12) and because we recently developed a simple and efficient transformation system using the URA3 gene as selection marker (13).
In a previous work, an unusual interaction between 5FC and fluconazole (FLC) was observed in four C. lusitaniae 5FC-resistant clinical isolates (23). These strains were specifically cross-resistant to FLC when both antifungals, 5FC and FLC, were used in association, but they were FLC susceptible when FLC was used alone. Genetic analysis and characterization of the biochemical mechanism revealed that 5FC-FLC cross-resistance was monogenic and recessive and that 5FC resistance was due to a defect of PCP, which was unable to transport 5FC. To explain 5FC-FCZ cross-resistance, we hypothesized that extracellular 5FC would behave as a competitive inhibitor of FLC uptake transport. PCP is a plasma membrane protein that is encoded by the FCY2 gene and that is particularly well documented in Saccharomyces cerevisiae. It mediates the active transport of purines (adenine, hypoxanthine, and guanine) and cytosine (7, 32, 36). It has been shown that disruption of FCY2 abolishes the active transport of bases, despite the presence in the yeast genome of FCY21 and FCY22, which belong to the same gene family (35).
We report here on the cloning and characterization of the C. lusitaniae FCY2 and FCY21 genes. We show that, under our experimental conditions, only FCY2 is expressed in C. lusitaniae and that this gene is disrupted by homologous recombination. Molecular and phenotypic analyses of the transformants revealed that disruption of the single FCY2 gene confers not only 5FC resistance but also 5FC-FLC cross-resistance.
MATERIALS AND METHODS
C. lusitaniae strains and growth conditions.
We used the wild-type strain 6936 MATa, obtained from the Centraalbureau voor Schimmelcultures (Utrecht, The Netherlands), for cloning of the FCY2 and FCY21 genes and as a susceptible reference strain for antifungal agents susceptibility tests (5FC MIC ≤ 0.5 μg/ml, FLC MIC ≤ 2 μg/ml). Clinical isolate CL42 MATa fcy2, which has a defective PCP, is resistant to 5FC (MIC ≥ 128 μg/ml), susceptible to FLC (MIC ≤ 2 μg/ml), and cross-resistant to the 5FC-FLC association (23). Auxotrophic strain 6936 [MATa ura3(D95V)] was used for the transformation experiments. This strain harbors a single point mutation at the ura3 locus, which results in the amino acid substitution D95V in a highly conserved domain and in a concomitant EcoRV site polymorphism (13). Auxotrophic transformant TR1 MATa ura3(D95V) fcy2(S122P) was used for complementation experiments.
The yeast cells were routinely cultivated at 35°C in liquid YPD medium (1% yeast extract, 2% peptone, 2% glucose) under constant agitation (250 rpm). YNB medium (0.67% yeast nitrogen base without amino acids [Difco Laboratories] supplemented with 2% glucose) was used for the screening of the auxotrophs and was supplemented with 50 μg/ml uracil and 1 mg/ml 5-fluorootic acid (5-FOA) or with 1 μg/ml cytosine, as required. Solid media were obtained with 2% agar (Sigma).
Antifungal susceptibility tests.
Stock solutions of the antifungal agents were prepared by dissolving FLC (ICN Biomedicals Inc.) and 5FC (Sigma) in water at concentrations of 3.2 mg/ml and 12.8 mg/ml, respectively.
Susceptibility to the antifungal agents was determined with RPMI 1640 medium and by the microdilution method recommended by the guidelines of CLSI (formerly NCCLS) (22), except that inocula of 104 cells/ml were used, as described previously (23). The final antifungal concentrations ranged from 0 to 256 μg/ml for 5FC and 0 to 128 μg/ml for FLC. After 48 h of incubation at 30°C, endpoint readings were recorded with an automated microtiter reader (Molecular Devices) and MICs were defined as the first concentration of drug that inhibited 95% of the growth of the drug-free control growth. All tests were done at least three times in separate experiments. Variations in growth did not exceed ±5%.
DNA and RNA extractions.
C. lusitaniae genomic DNA was extracted by following the protocol described by Scherer and Stevens (31) by using lyticase (Sigma) at a final concentration of 60 U/ml instead of zymolyase for spheroplast production.
Yeast whole-cell RNA was extracted as described by Burke et al. (6) and Ramon et al. (27), with some modifications. C. lusitaniae cells (108 cells/ml), which were grown in 50 ml liquid YPD medium for 16 h, were washed in sterile water and resuspended in 2 ml of LETS buffer (0.1 M LiCl, 0.01 M EDTA, 0.01 M Tris-HCl, pH 7.4, 0.2% sodium dodecyl sulfate). After the addition of glass beads (approximately 3 g) and 2 ml of phenol equilibrated with LETS buffer, cell suspensions were vortexed vigorously for 3 min by alternating 30 s of vortexing with 30 s on ice. After centrifugation, the aqueous phase was collected and extracted twice with an equal volume of phenol-chloroform-isoamyl alcohol (24:24:1). A 1/10 volume of 5 M LiCl was added to the aqueous phase and the RNA was precipitated overnight at −20°C. The RNA precipitate was centrifuged at 10,000 × g for 20 min, washed with 70% ethanol, and dissolved in 200 μl of diethylpyrocarbonate-treated distilled water. The concentrations of DNA and RNA were determined with a spectrophotometer at λ280 and λ260.
PCR amplifications.
Hot-StartTaq DNA polymerase (QIAGEN) was used for PCR to generate DNA fragments used in cloning experiments or as DNA probes. The PCR conditions for amplification were those indicated by the supplier. All the primers were synthesized by Invitrogen and are listed in Table 1, along with the annealing temperatures of the primers used for the PCR amplifications.
TABLE 1.
Oligonucleotides used in this study
| Primer | Sequence (5′ to 3′) | Annealing temp (°C) |
|---|---|---|
| FCaa | TGGGCNGGNTGYYTNGT | 53 |
| FCba | TACCANGTYTGRTTCAT | 53 |
| YPa | TGGAGAAAGGGAAGTTTTGCTTCTTACGAC | 67 |
| YPb | GTCGTAAGAAGCAAAACTTCCCTTTCTCCA | 67 |
| YNa | GGTGTGGTTTTGGGTATGAACCAGACGTGG | 67 |
| YNb | GGCAATCAAGTTCATGAAGTTCTCCATGAC | 67 |
| YP1 | TTCAGAAGAGGATTCAAGGGCTACAATCCG | 67 |
| YP2 | CGGATTGTAGCCCTTGAATCCTCTTCTGAA | 67 |
| YN1 | TGCACGGTTCTTACTATGAACCAAACCTGG | 67 |
| YN2 | ACCGATAATACTCAAGAAGTTCGACATGGT | 67 |
| FC1 | AGCGAAGGGTTGAGTTGC | 64 |
| FC2 | ATGTCGAGTTCCGTTCTC | 64 |
| FC3 | CTGGTAGAATACAACACCG | 60 |
| FC4 | CGGAACGTACGAGAGAGAC | 60 |
| FciSb | TCGAGAACTAGTGGCGAAACAAAGAAGAGA | 60 |
| FciNc | CGAGCTATGCATACCAAACCACCAACAGAC | 60 |
| Fcy2Sb | TCGAGAACTAGTGATGGAAAGGAGAGGCTG | 62 |
| Fcy2Nc | CGAGCTATGCATAGATTTGCTACTCTGATG | 62 |
| 18S1 | TTTCGATGGTAGGATAGAGGC | 62 |
| 18S2 | CATGCTAACAGATTCAAGCGG | 62 |
For degenerate primers FCa and FCb, D is A, G, or T; H is A, C, or T; R is A or G; W is A or T; and Y is C or T.
The SpeI restriction sites located in primers FciS and Fcy2S are indicated in boldface type.
The NsiI restriction sites located in primers FciN and Fcy2N are indicated in boldface type.
Cloning of FCY2 and FCY21 genes from Candida lusitaniae.
The PCR amplification of a first fragment of the PCP-encoding gene in C. lusitaniae was performed with degenerate primers FCa and FCb, designed from conserved regions of the FCY2 gene of S. cerevisiae (GenBank accession no. X51751), FCY2-like genes from Candida tropicalis (partial sequence; GenBank accession no. AL439646), and Pichia angusta (partial sequence; GenBank accession no. AL435993), available in the GenBank database (http://www.ncbi.nlm.nih.gov), as well as FCY2-like genes from Candida albicans: FCY2 (contig6-1838) and FCY22 (contig6-1777) genes, available in the Stanford database (http://www-sequence.stanford.edu:8080/contigs-to-blast6.html).
To obtain the complete sequence overlapping the two distinct FCY2 and FCY21 PCR fragments obtained with primers FCa and FCb, we used the Universal Genome Walker kit (Clontech), according to the supplier's instructions. From the FCY2 fragment, four gene-specific primers were designed for DNA walking. Two primers were designed for walking downstream, YPa for the primary PCR and YNa for the nested PCR, and two others were designed for walking upstream, YPb for the primary PCR and YNb for the nested PCR. In the same way, four gene-specific primers were designed from the FCY21 fragment. Two primers were designed for walking downstream, YP1 for the primary PCR and YN1 for the nested PCR, and two others were designed for walking upstream, YP2 for the primary PCR and YN2 for the nested PCR.
For physical isolation of the complete C. lusitaniae FCY2 and FCY21 genes, two specific pairs of primers, primers FC1 and FC2 and primers FC3 and FC4, respectively, were designed from the nucleotide sequences obtained by DNA walking.
Plasmid constructions.
Plasmid pUF2 was constructed as follows. A 944-bp cassette of the FCY2 gene was amplified by PCR from the genomic DNA of strain 6936 with primers FciS and FciN, thus flanking the cassette with the SpeI and NsiI restriction sites, respectively. The resulting PCR product, named the Fi cassette, was then subcloned into the SpeI-NsiI restriction sites of plasmid pGEM-U, which was derived from pGEM-T easy (Promega) and which contained the C. lusitaniae URA3 gene cloned as a PCR product at the insertion T site (13). The resulting 5.4-kb plasmid was designated pUF2.
To construct plasmid pUFCY2, a 2,117-bp DNA fragment overlapping the full-length FCY2 gene flanked by the SpeI and NsiI restriction sites was obtained by PCR amplification with the primer set Fcy2S and Fcy2N. The resulting PCR product was then subcloned into the SpeI-NsiI restriction sites of plasmid pGEM-U, as described above, to generate plasmid pUFCY2 (6.6 kb).
Yeast transformation.
Auxotroph strains for uracil were transformed by the electroporation procedure described by François et al. (13), except that the plasmids were previously linearized at the unique BamHI restriction site located within the Fi cassette of the FCY2 gene in order to enhance the efficiency of plasmid integration at the homologous locus. Ura+ transformants were selected on YNB selective medium and were incubated for 3 days at 35°C.
Southern and Northern hybridizations.
For Southern analysis, approximately 10 μg of C. lusitaniae DNA was digested with EcoRV and separated by electrophoresis in a 0.8% agarose gel. The DNA was transferred onto a nylon membrane (Hybond N+; Roche Molecular Biochemicals) and hybridized with digoxigenin-labeled DNA probes synthesized with a PCR DIG probe synthesis kit (Roche Molecular Biochemicals), as recommended by the supplier. FCY2 and FCY21 DNA probes, which were homologous to the FCY2 and FCY21 fragments, respectively, were generated by PCR amplification by using both primer FCa and primer FCb. In the same way, the Fi DNA probe, which was homologous to the Fi cassette inserted into plasmid pUF2, was synthesized by using primers FciS and FciN. The URA3 DNA probe, which was homologous to the C. lusitaniae URA3 locus, and the BLA DNA probe, which was specific for the plasmidic ampicillin resistance gene, were also generated with specific primers, as described previously (13).
For Northern analysis, approximately 2 μg of RNA of C. lusitaniae was separated in a 1% agarose gel containing formaldehyde, as described by Brown and Mackey (5). The RNA was transferred onto a nylon membrane (Hybond N+; Roche Molecular Biochemicals) and hybridized with digoxigenin-labeled FCY2 and FCY21 RNA probes, which were antisense to the FCY2 and FCY21 transcripts, respectively, and which were synthesized with a DIG RNA labeling kit (Roche Molecular Biochemicals). For a loading control, membranes were stripped and rehybridized with an RNA probe complementary to a 460-bp fragment derived from the 18S rRNA-encoding gene of C. lusitaniae, isolated as a PCR product with primers 18S1 and 18S2. Probe-target hybrids were visualized by a chemiluminescent assay with the DIG luminescent detection kit (Roche Molecular Biochemicals), according to the manufacturer's instructions, and exposure of the blot to X-ray film for 2 h.
DNA sequencing and sequence analysis.
PCR-generated DNA fragments were cloned into plasmid pGEM-T easy (Promega). Plasmid DNA was purified from JM109 Escherichia coli strains (Promega) by using an anion-exchange column purification system (QIAGEN). Cloned inserts were sequenced by Millegen (Labège, France).
Open reading frames (ORFs) were identified from the nucleotide genomic sequences by using the Multiple Translation Program, available on the Infobiogen website (http://www.infobiogen.fr). The similarity searches in databases were performed with the Basic Local Alignment Search Tool (BLAST) programs (1), available on the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov). Consensus multiple alignments for nucleotide and amino acid sequences was done with the ClustalW program (33), available on the Infobiogen website (http://www.infobiogen.fr).
Nucleotide sequence accession numbers.
The FCY2 and FCY21 sequences determined here have been deposited in the GenBank database (accession nos. AY506866 and AY506867, respectively).
RESULTS
Cloning and characterization of the C. lusitaniae FCY2 and FCY21 genes.
Degenerate FCa and FCb primers (Table 1) were used to amplify a part of the FCY2 gene from the genomic DNA of C. lusitaniae wild-type strain 6936. Sequence analysis revealed that two distinct PCR fragments of 327 bp were amplified. These two fragments were named FCY2 and FCY21, according to the complete sequence analysis and expression study of both genes described below. The FCY2 and FCY21 fragments could each encode a 108-amino-acid sequence that shared 58% and 50% identities with S. cerevisiae Fcy2p, respectively. Moreover, an identity of 54% was found between the partial polypeptides encoded by the FCY2 and FCY21 fragments. These results suggest that we cloned a partial core sequence of two distinct C. lusitaniae FCY2-like genes.
Starting from the 327-bp FCY2 partial sequence, a 2,496-bp nucleotide sequence overlapping the complete FCY2 gene was determined by chromosome walking. The complete C. lusitaniae FCY2 gene was cloned as a 1,968-bp PCR amplification product obtained with the FC1-FC2 set of primers (Table 1). Analysis of this fragment revealed a complete intronless ORF located at nucleotides (nt) 316 to 1857. As expected from the amino acid sequence comparisons with the S. cerevisiae PCPs and as further demonstrated from expression analysis and disruption experiments, this ORF could correspond to the FCY2 gene of C. lusitaniae that encodes a protein, Fcy2p, of 513 amino acid residues. Similarly, a 3,002-bp nucleotide sequence was obtained from the 327-bp FCY21 partial sequence. Cloning of the complete C. lusitaniae FCY21 gene was performed by PCR amplification with the FC3-FC4 pair of specific primers (Table 1), and the resulting 1,950-bp fragment was analyzed. A 1,590-bp complete ORF without an intron located at nt 768 to 2,357 could correspond to the FCY21 gene of C. lusitaniae and could encode a putative protein, Fcy21p, of 529 amino acid residues.
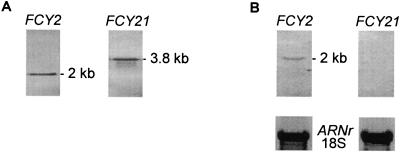
Southern hybridization of EcoRV-digested DNA from wild-type strain 6936 successively with the FCY2 and FCY21 DNA probes allowed detection of a 2-kb fragment that corresponded to FCY2 and of a 3.8-kb fragment that corresponded to FCY21 (Fig. 1A).
FIG. 1.
Molecular characterization of C. lusitaniae FCY2 and FCY21 genes. (A) Southern blot analysis of EcoRV-digested genomic DNA from strain 6936 hybridized with the FCY2 DNA probe and then stripped and hybridized with the FCY21 DNA probe. (B) Northern blot analysis of total RNA from the strain 6936 hybridized with FCY2 and FCY21 RNA probes. The membranes were stripped and hybridized with the 18S RNA probe for RNA loading control.
A homology search revealed high to moderate degrees of identity of C. lusitaniae Fcy2p and Fcy21p with the amino acid sequences of PCPs available in databases. The best scores of identity were found with the PCPs from S. cerevisiae and C. albicans (43% to 67%; Table 2). Moreover, an identity of 45% was found between C. lusitaniae Fcy2p and Fcy21p.
TABLE 2.
Amino acid sequence identities of C. lusitaniae Fcy2p and Fcy21p compared to those of PCPs of S. cerevisiae and C. albicans
| Organism | PCP protein | % C. lusitaniae protein identity
|
|
|---|---|---|---|
| Fcy2p | Fcy21p | ||
| S. cerevisiae | Fcy2pa | 53 | 45 |
| Fcy21pa | 51 | 45 | |
| Fcy22pa | 52 | 45 | |
| C. albicans | Fcy2p/Fcy22pb | 43 | 57 |
| Fcy22p/Fcy21pc | 67 | 45 | |
Fcy2p (GenBank accession no. X51751), Fcy21p (GenBank accession no. AAB64596), and Fcy22p (GenBank accession no. NP010982) of S. cerevisiae were found in the GenBank database.
Fcy2p of C. albicans (contig6-1838; ORF6.1188) was previously found in the Stanford database and was recently deposited in the GenBank database as Fcy22p (accession no. AJ616010).
Fcy22p of C. albicans (contig6-1777; ORF6.1000) was previously found in the Stanford database and was recently deposited in the GenBank database as Fcy21p (accession no. AJ616009).
Analysis of PCP activity of S. cerevisiae strains affected in Fcy2p revealed that a serine residue (S272) located in a transmembrane amphipathic domain (positions 257 to 276) could be part of a hydrophilic pore involved in the base-H+ translocation process (10) and that a segment (positions 371 to 377) located in a hydrophilic loop (positions 367 to 399) was crucial for the uptake activity and specificity (11) (Fig. 2). Multiple-sequence alignment of the C. lusitaniae and C. albicans PCPs with S. cerevisiae Fcy2p showed that the serine residue was conserved in C. lusitaniae Fcy21p and in C. albicans Fcy2p and that it was replaced by a glycine, a weakly conserved residue, in C. lusitaniae Fcy2p and in C. albicans Fcy22p (Fig. 2). Concerning the hydrophilic segment, it was relatively well conserved among the yeast PCPs whose sequences are presented in Fig. 2, with the following consensus motif: (V/I)(A/S)N(N/G)(V/I/L)P(N/G). The conserved proline residue (in boldface type) was thought to be involved in a secondary structure as a beta-turn motif and to play a key role in the translocation process (11). The conserved asparagine residue (in boldface type) was also found in the conserved motif (F/Y/S)X(Q/E/P)NXGXXXXT(K/R/G) located in the same hydrophilic loop of all the members (from humans to bacteria) of a different family of membrane proteins, the nucleobase transporter family (8). It has been shown that this region is a domain especially crucial for the function and specificity of the UapA and UapC proteins, which are involved in purine uptake in Aspergillus nidulans (8).
FIG. 2.
Amino acid sequence alignments of two regions located in a transmembrane amphipathic domain (region 1) and in a hydrophilic loop (region 2) of C. lusitaniae Fcy2p and Fcy21p, C. albicans Fcy2p (Stanford database, contig6-1838; ORF6.1188) and Fcy22p (Stanford database, contig6-1777; ORF6.1000), and S. cerevisiae Fcy2p (GenBank accession no. X51751). The amino acid residues which are thought to be part of a hydrophilic pore involved in the base-H+ translocation process are in boldface. The region essential for the active three-dimensional structure of the transporter is framed. Absolutely conserved residues are marked with asterisks, highly conserved residues are marked with colons, and weakly conserved residues are marked with periods. The amino acid residue number for each protein is indicated at the beginning of each sequence.
In order to study the expression of FCY2 and FCY21 genes in strain 6936, Northern hybridizations were undertaken with FCY2 and FCY21 RNA probes, respectively (Fig. 1B). Under our experimental conditions, FCY21 mRNA could not be detected and only FCY2 was expressed, yielding an mRNA fragment of approximately 2 kb. In S. cerevisiae, it has been shown that PCP is encoded by the FCY2 gene and that disruption of this gene suppresses active transport, despite the presence of the FCY21 and FCY22 pseudogenes (35). Similarly, we hypothesized that Fcy2p, which shares 53% identity with Fcy2p of S. cerevisiae (Table 2), was the only functional PCP in C. lusitaniae.
Disruption of the FCY2 gene in C. lusitaniae.
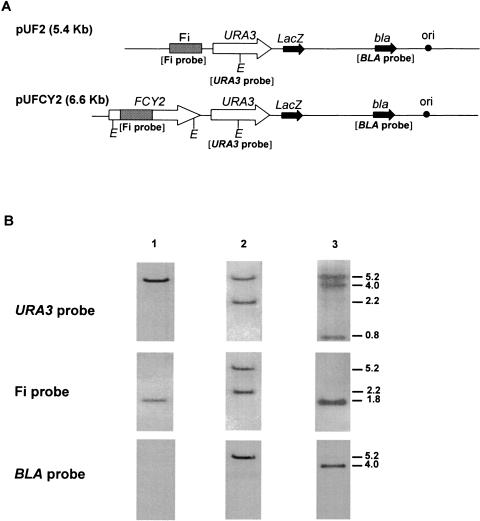
Disruption of the FCY2 gene was undertaken in order to verify its possible involvement in cross-resistance to 5FC and FLC. FCY2 was disrupted by homologous recombination by using the transformation system described previously (13). Plasmid pUF2 (5.5 kb), which contains the entire URA3 gene of C. lusitaniae and a 944-bp partial sequence of the FCY2 gene (Fi cassette), was digested with BamHI, which possesses a single site within the Fi cassette. The linearized plasmid was then used to transform strain 6936 ura3(D95V) to prototrophy.
A gel blot of EcoRV-digested genomic DNA from auxotrophic strain 6936 ura3(D95V) and 10 Ura+ transformants randomly selected on YNB agar plates was successively subjected to hybridization with a URA3 DNA probe, which is homologous to the URA3 locus; with the Fi DNA probe, which is homologous to the FCY2 locus; and with the BLA DNA probe, which is homologous to the bla gene and which is specific to the plasmid sequence. Figure 3A shows a schematic linear genetic map of plasmid pUF2 and the locations of the DNA probes, and Fig. 3B shows the results of Southern blot analysis. The hybridization patterns revealed that homologous integration occurred in the 10 transformants according to two distinct molecular events, which allowed us to classify the transformants into two groups. For group 1, which included five of the transformants, integration of the whole pUF2 plasmid occurred at the FCY2 locus by a single crossing-over event, resulting in disruption of the FCY2 gene and in the genotype ura3 fcy2::URA3. The molecular events were confirmed by hybridization with the BLA DNA probe, which hybridized only to the 5.2-kb DNA fragment, demonstrating that the plasmid had integrated the FCY2 locus. Group 2, which included the remaining five transformants, was derived from gene replacement at the ura3 locus, which resulted in the ura3::URA3 FCY2 genotype (data not shown). Additionally, Southern blot analysis of undigested DNA indicated that no extrachromosomal plasmid DNA could be detected in the transformants (data not shown).
FIG. 3.
Schematic representation of the genetic map of plasmids pUF2 and pFCY2 and Southern blot hybridization. (A) The 5.4-kb pUF2 plasmid, which contains the C. lusitaniae URA3 gene and a 944-bp cassette from the FCY2 gene (Fi cassette), was used to transform strain 6936 ura3(D95V) FCY2 to prototrophy. The 6.6-kb pUFCY2 plasmid, which contains the URA3 and the FCY2 genes of C. lusitaniae, was used to transform strain TR1 ura3(D95V) fcy2(S122P) to prototrophy. The locations of the probes used in this study are indicated in parentheses. (B) Hybridization patterns with URA3, Fi, and BLA DNA probes of EcoRV-digested genomic DNA from 6936 ura3(D95V) FCY2 (lane 1), a representative transformant of group 1 [ura3(D95V) fcy2::URA3] (lane 2), and transformant Rec2 ura3(D95V) fcy2::[URA3 FCY2] (lane 3). The hybridization patterns of EcoRV-digested genomic DNA from the strain TR1 ura3(D95V) fcy2(S122P) were identical to those of 6936 ura3(D95V) (lane 1). Signals revealed by the labeled probes correspond to those expected from the genomic restriction map. Hybridization of the genomic DNA of 6936 ura3(D95V) with the URA3 and Fi probes revealed fragments of 5.2 and 1.8 kb, respectively. For the transformant of group 1, integration of plasmid pUF2 at the FCY2 locus yielded two fragments of 5.2 and 2.2 kb when genomic DNA was hybridized with either the URA3 probe or the Fi probe. For transformant Rec2, integration of plasmid pUFCY2 at the fcy2 locus resulted in the detection of three fragments (5.2, 4.0, and 0.8 kb) when the genomic DNA was hybridized with the URA3 probe and of a single 1.8-kb fragment when the genomic DNA was hybridized with the Fi probe. Hybridization of the genomic DNA of the transformant of group 1 and transformant Rec2 with the BLA probe revealed fragments of 5.2 and 4.0 kb, respectively. DNA fragment sizes are indicated in kilobases.
Phenotypic analysis of the transformants.
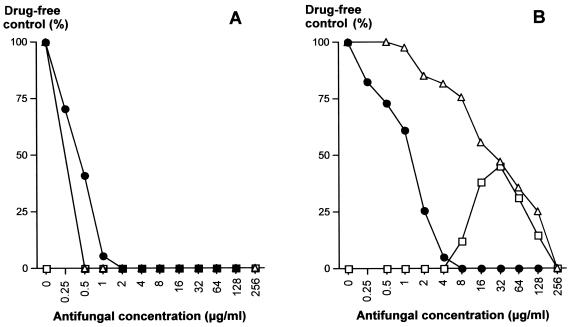
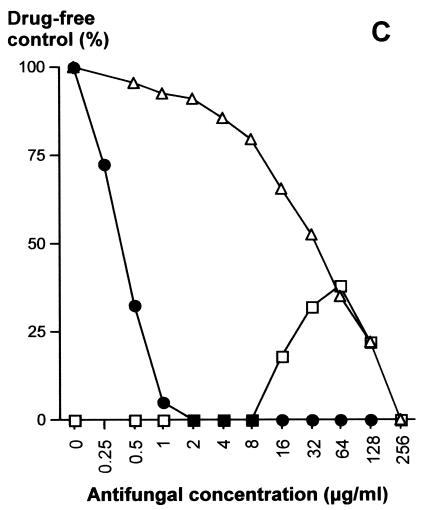
The antifungal susceptibilities of transformants were determined by a microdilution method (Fig. 4). Susceptibility tests were performed in the presence of 5FC alone (concentration gradient, 0 to 256 μg/ml), FLC alone (concentration gradient, 0 to 128 μg/ml), and 5FC (concentration gradient, 0.5 to 256 μg/ml) in combination with a constant concentration of 16 μg/ml of FLC. This corresponds to eightfold the MIC of FLC for the strain used in the transformation experiments. When FLC is associated with 5FC at this concentration, we previously reported that this FLC concentration allows the highest level of cell growth during cross-resistance expression (23). For susceptible strain 6936, as well as for the transformants of group 2 (ura3::URA3 FCY2), the MICs were less than 0.5 μg/ml of 5FC (susceptible) and varied from 2 to 4 μg/ml of FLC (susceptible) (Fig. 4A). When 5FC and FLC were used in combination, the FLC concentration was sufficient to inhibit completely the growth of the strains. For clinical isolate CL42, as well as for transformants of group 1 (ura3 fcy2::URA3), MICs were 256 μg/ml of 5FC (resistant) and varied from 1 to 4 μg/ml of FLC (susceptible) (Fig. 4B and C). When 5FC and FLC were used together, cross-resistance was observed, but not at all 5FC concentrations. Typically, 16 μg/ml of FLC could inhibit the growth of strain CL42 and the transformants in group 1 in the presence of the lowest concentrations of 5FC (up to 4 to 8 μg/ml, respectively). Cross-resistance then developed progressively with increasing concentrations of 5FC, reaching approximately 40% growth for strain CL42 in the presence of 32 μg/ml of 5FC compared to the 100% growth observed for the drug-free control and approximately 30% growth for the transformants in group 1 in the presence of 64 μg/ml of 5FC compared to the 100% growth observed for the drug-free control. In the presence of greater concentrations of 5FC, the growth of the strains decreased progressively, such as that observed with 5FC alone, until complete inhibition of growth was observed in the presence of 256 μg/ml of 5FC.
FIG. 4.
Susceptibilities of control strains and of the strains genetically engineered this study to 5FC and FLC. (A) Typical antifungal susceptibility pattern of strain 6936 URA3 FCY2, of the transformants of group 2 (ura3::URA3 FCY2), and of transformant Rec2 ura3 fcy2::[URA3 FCY2]; (B) typical antifungal susceptibility pattern of clinical isolate CL42 URA3 fcy2; (C) typical antifungal susceptibility pattern of the transformants of group 1 (ura3 fcy2::URA3) and strain TR1 ura3 fcy2. Growth is given as a percentage of the growth level obtained for the drug-free control. ▵, 5FC concentration gradient; •, FLC concentration gradient; □, 5FC concentration gradient with a constant concentration of 16 μg/ml of FLC.
These results demonstrate unambiguously that inactivation of the FCY2 gene confers to C. lusitaniae a 5FC-FLC cross-resistant phenotype strongly similar to that in clinical isolate CL42.
Complementation of the fcy2 gene.
For selection of a fcy2 ura3 mutant usable in complementation experiments, YPD-cultivated cells from one transformant of group 1 (genotype, ura3 fcy2::URA3) were plated at a density of 1.6 × 107 per plate on YNB solid medium supplemented with 50 μg/ml uracil and 1 mg/ml 5-FOA. Resistance to 5-FOA was supposed to be derived from the Ura− auxotrophy generated by excision of plasmid pUF2 containing the URA3 cassette. Ten 5-FOA-resistant Ura− mutants were selected and plated on YNB solid medium supplemented with 1 μg/ml cytosine. One of these strains, designed TR1, was unable to grow with cytosine, indicating that the pyrimidine salvage pathway was inoperative, probably because of the presence of a defective fcy2 allele. Moreover, antifungal susceptibility tests revealed that strain TR1 displayed a 5FC-FLC cross-resistant phenotype identical to that of the transformants in group 1 (ura3 fcy2::URA3) (Fig. 4C).
Nucleotide sequencing of the complete fcy2 allele in strain TR1 and comparison with the wild allele allowed us to identify a single point mutation located at nucleotide 364 which replaced a T by a C. At the protein level, the serine residue at position 122 was then replaced by a proline. Accordingly, the mutation was designated fcy2(S122P). Multiple-sequence alignment of the C. lusitaniae Fcy2p with other Fcy2 and Fcy2-like proteins from C. albicans and S. cerevisiae showed that S122 was strictly conserved (data not shown), suggesting that this serine residue could be important for biological function. A gel blot of EcoRV-digested total DNA from strain TR1 successively hybridized with the Fi, URA3, and BLA DNA probes showed a hybridization pattern identical to that of 6936 ura3(D95V) (Fig. 3B), thus confirming the complete excision of the pUF2 plasmid. Accordingly, the genotype ura3(D95V) fcy2(S122P) was assigned to TR1.
Plasmid pUFCY2 (6.6 kb; Fig. 3A), which contained the URA3 and FCY2 genes of C. lusitaniae, was digested with BamHI, which possesses a single site within the FCY2 gene, and was used to transform strain TR1 to prototrophy. A gel blot of EcoRV-digested genomic DNA from one of the Ura+ transformants obtained, named Rec2, hybridized with the URA3 and FI DNA probes is shown (Fig. 3B). Southern hybridizations revealed that homologous integration of the whole plasmid pUFCY2 had occurred at the fcy2 locus by a single crossing-over event, resulting in the introduction of both the FCY2 and the URA3 genes at the fcy2 locus and, thus, in the genotype ura3 fcy2::[URA3 FCY2]. Only the 4-kb DNA fragment was detected by the BLA DNA probe, providing confirmation that the whole plasmid pUFCY2 had integrated the fcy2 locus. The antifungal susceptibility pattern of transformant Rec2 was identical to those of susceptible reference strain 6936 and the transformants in group 2 (ura3::URA3 FCY2) (Fig. 4A).
This experiment demonstrated that reintroduction of a functional FCY2 gene in a 5FC-FLC cross-resistant fcy2 strain of C. lusitaniae results in restoration of the antifungal susceptibility phenotype.
DISCUSSION
The results of this study provide molecular evidence that disruption of the single FCY2 gene, which encodes PCP, is responsible for both 5FC resistance and 5FC-FLC cross-resistance acquisition in C. lusitaniae. Moreover, we demonstrated that reintroduction of a functional FCY2 gene in a 5FC-FLC cross-resistant fcy2 strain resulted in restoration of the antifungal susceptible phenotype.
Cloning and characterization of the FCY2 gene of C. lusitaniae were made difficult by the occurrence of FCY2-like pseudogenes, as shown in S. cerevisiae. In this species, PCP is encoded by the FCY2 gene, and two pseudogenes, FCY21 and FCY22, which belong to the same gene family, were isolated (35, 36). Unfortunately, few data are available for other yeast species. The situation was particularly confusing for C. albicans because when we started this study, FCY2-like genes, named FCY2 and FCY22, were found only in the Stanford database. Sequences strictly identical to those of the FCY2 and FCY22 genes were thereafter available in the GenBank database but had been renamed the FCY22 and FCY21 genes, respectively. Nevertheless, to our knowledge, no one of the FCY2-like genes isolated from C. albicans can be classified either as a gene encoding a functional PCP or as a pseudogene, because of the lack of information concerning their functional analysis. Using degenerate primers designed from conserved regions from Fcy2-like proteins, followed by chromosome walking, we cloned by PCR two FCY2-like genes in C. lusitaniae. Northern blot analysis revealed that only one gene, named FCY2, was expressed under our experimental conditions. The other one, whose expression level was undetectable in cells cultivated under routine conditions, was named FCY21 and is considered a pseudogene. This finding is in agreement with that previously described in S. cerevisiae (35), except that only one pseudogene could be identified so far in C. lusitaniae. A similar situation occurs in C. albicans because only two FCY2-like genes were annotated in the Stanford database, and only two FCY2-like sequences were recently deposited into the GenBank database by other workers. Multiple-sequence alignment analysis revealed that C. lusitaniae Fcy2p shared the best score of identity (67%) with C. albicans Fcy22p and Fcy21p (Stanford and GenBank databases, respectively), suggesting that this protein corresponds to the functional PCP in C. albicans. In order to confirm this hypothesis, it would be necessary to study the expression of the FCY2-like genes in C. albicans.
The FCY2 gene of C. lusitaniae was disrupted in auxotrophic strain 6936 ura3(D95V) by using plasmid pUF2, which contained a partial sequence of the FCY2 gene (Fi cassette) and the complete URA3 gene of C. lusitaniae. Homologous recombination events occurred in all transformants analyzed at a rate of 50% at the resident locus FCY2 and at a rate of 50% at the resident locus ura3, resulting in the recovery of the genotypes ura3 fcy2::URA3 (transformants of group 1) and ura3::URA3 FCY2 (transformants of group 2), respectively. Testing of the susceptibilities of the transformants to the antifungal agents 5FC and FLC revealed that transformants harboring a wild-type FCY2 gene (group 2) were susceptible to 5FC and to FLC, used separately or in association, whereas transformants harboring a disrupted fcy2 gene (group 1) were resistant to 5FC, susceptible to FLC, and 5FC-FLC cross-resistant when both antifungals were used in combination. We then carried out complementation experiments of the fcy2 mutated allele in 5FC-FLC cross-resistant auxotrophic strain TR1 ura3(D95V) fcy2(S122P), derived from the excision of plasmid pUF2 from one transformant of group 1 (ura3 fcy2::URA3). Reintroduction of the complete FCY2 gene into the genome of TR1 restored drug susceptibility to wild-type levels, whereas the complementation experiment with the FCY21 gene showed no effect (data not shown).
Taken together, these results mean that the C. lusitaniae FCY2 gene really encodes a functional PCP and also that the loss of PCP function is not compensated for by the pseudogene FCY21 under our experimental conditions. Then, it was demonstrated that inactivation of the FCY2 gene not only conferred 5FC resistance but also promoted 5FC-FLC cross-resistance to a level fully comparable to that of clinical isolate CL42. This finding is in agreement with those from our previous work, in which we reported on the occurrence of 5FC-FLC cross-resistance in 4 C. lusitaniae isolates selected from a collection of 60 clinical isolates. It was shown that the cross-resistance phenotype had a monogenic determinism and, by using [14C]5FC uptake measurements, that 5FC resistance was due to a deficit in 5FC uptake. To explain the cross-resistance phenotype, it was hypothesized that 5FC, which accumulates at the extracellular level, behaved as a competitive inhibitor of the uptake of FLC (23). To date, very little is known about the way in which antifungal agents enter the cell. To our knowledge, only one study measured the uptake of [3H]ketoconazole and concluded that an energy-dependent uptake system was present for low concentrations of ketoconazole in C. albicans (4). Moreover, it has recently been shown that the uptake of the echinocandin drug caspofungin is mediated by a high-affinity facilitated-diffusion transporter in C. albicans (24). We now envision cloning and characterization of the mutated fcy2 genes in 5FC-FLC cross-resistant clinical isolates of C. lusitaniae in order to elucidate the molecular mechanisms responsible for the transport defect. Such an analysis could also provide insight into the peptidic regions possibly involved in the recognition and transport of purine and cytosine bases, with a particular attention to the segments already identified in Fcy2p of S. cerevisiae (11). As we have shown that inactivation of the FCY2 gene causes 5FC-FLC cross-resistance in C. lusitaniae, it would be interesting to investigate whether a similar mechanism can occur in clinical isolates of other Candida species.
Acknowledgments
We thank Susan Fox for critical reading of the manuscript.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Grapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behar, S. M., and G. M. Chertow. 1998. Olecranon bursitis caused by infection with Candida lusitaniae. J. Rheumatol. 25:598-600. [PubMed] [Google Scholar]
- 3.Blinkhorn, R. J., D. Adelstein, and P. J. Spagnuolo. 1989. Emergence of a new opportunistic pathogen, Candida lusitaniae. J. Clin. Microbiol. 27:236-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boiron, P., E. Drouhet, B. Dupont, and L. Improvisi. 1987. Entry of ketoconazole into Candida albicans. Antimicrob. Agents Chemother. 31:244-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, T., and K. Mackey. 1997. Analysis of RNA by Northern and slot blot hybridization, p. 4.9.1-4.9.16. In F. M. Ausubel, R. Brendt, R. E. Kingston, et al. (ed.), Currents protocols in molecular biology, vol. 1. John Wiley & Sons, New York, N.Y. [DOI] [PubMed] [Google Scholar]
- 6.Burke, D., D. Dawson, and T. Stearns. 2000. Yeast RNA isolation, p. 117-119. In Methods in yeast genetics. CSHL Press, New York, N.Y.
- 7.Chevallier, M. R., R. Jund, and F. Lacroute. 1975. Characterization of cytosine permeation in Saccharomyces cerevisiae. J. Bacteriol. 122:629-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diallinas, G., J. Valdez, V. Sophianopoulou, A. Rosa, and C. Scazzocchio. 1998. Chimeric purine transporters of Aspergillus nidulans define a domain critical for function and specificity conserved in bacterial, plant and metazoan homologues. EMBO J. 27:3827-3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dodgson, A. R., K. J. Dodgson, C. Pujol, M. A. Pfaller, and D. R. Soll. 2004. Clade-specific flucytosine resistance is due to a single nucleotide change in the FUR1 gene of Candida albicans. Antimicrob. Agents Chemother. 48:2223-2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feirreira, T., J. Chevallier, P. Paumard, C. Napias, and D. Brèthes. 1999. Screening of an intragenic second-site suppressor of purine-cytosine permease from Saccharomyces cerevisiae. Eur. J. Biochem. 260:22-30. [DOI] [PubMed] [Google Scholar]
- 11.Feirrera, T., C. Napias, J. Chevallier, and D. Brèthes. 1999. Evidence for a dynamic role for proline376 in the purine-cytosine permease of Saccharomyces cerevisiae. Eur. J. Biochem. 263:57-64. [DOI] [PubMed] [Google Scholar]
- 12.François, F., T. Noël, R. Pepin, A. Brulfert, C. Chastin, A. Favel, and J. Villard. 2001. Alternative identification test relying upon sexual reproductive abilities of Candida lusitaniae strains isolated from hospitalized patients. J. Clin. Microbiol. 39:3906-3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.François, F., F. Chapeland-Leclerc, J. Villard, and T. Noël. 2004. Development of an integrative transformation system for the opportunistic pathogenic yeast Candida lusitaniae using URA3 as a selection marker. Yeast 21:95-106. [DOI] [PubMed] [Google Scholar]
- 14.Guinet, R., J. Chanas, A. Goullier, G. Bonnefoy, and P. Ambroise-Thomas. 1983. Fatal septicemia due to amphotericin B-resistant Candida lusitaniae. J. Clin. Microbiol. 18:443-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawkins, J. L., and L. M. Baddour. 2003. Candida lusitaniae infections in the era of fluconazole availability. Clin. Infect. Dis. 36:14-18. [DOI] [PubMed] [Google Scholar]
- 16.Hope, W. W., L. Tabernero, D. W. Denning, and M. J. Anderson. 2004. Molecular mechanisms of primary resistance to flucytosine in Candida albicans. Antimicrob. Agents Chemother. 48:4377-4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly, S. L., D. C. Lamb, D. E. Kelly, J. Loeffler, and H. Einsele. 1996. Resistance to fluconazole and amphotericin in Candida albicans from AIDS patients. Lancet 348:1523-1524. [DOI] [PubMed] [Google Scholar]
- 18.Kontoyiannis, D. P., and R. E. Lewis. 2002. Antifungal drug resistance of pathogenic fungi. Lancet 359:1135-1144. [DOI] [PubMed] [Google Scholar]
- 19.Krcmery, V., and A. J. Barnes. 2002. Non-albicans Candida spp. causing fungaemia: pathogenicity and antifungal resistance. J. Hosp. Infect. 50:243-260. [DOI] [PubMed] [Google Scholar]
- 20.Merz, W. G. 1984. Candida lusitaniae: frequency of recovery, colonization, infection, and amphotericin B resistance. J. Clin. Microbiol. 20:1194-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minari, A., R. Hachem, and I. Raad. 2001. Candida lusitaniae: a cause of breakthrough fungemia in cancer patients. Clin. Infect. Dis. 32:186-190. [DOI] [PubMed] [Google Scholar]
- 22.National Committee for Clinical Laboratory Standard. 2003. Reference method for broth dilution antifungal susceptibility testing of yeasts. M-27A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 23.Noël, T., F. François, P. Paumard, C. Chastin, D. Brethes, and J. Villard. 2003. Flucytosine-fluconazole cross-resistance in purine-cytosine permease-deficient Candida lusitaniae clinical isolates: indirect evidence of a fluconazole uptake transporter. Antimicrob. Agents Chemother. 47:1275-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paderu, P., S. Park, and D. S. Perlin. 2004. Caspofungin is mediated by a high-affinity transporter in Candida albicans. Antimicrob. Agents Chemother. 48:3845-3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfaller, M. A., S. A. Messer, and R. J. Hollis. 1994. Strain delineation and antifungal susceptibilities of epidemiologically related and unrelated isolates of Candida lusitaniae. Diagn. Microbiol. Infect. Dis. 20:127-133. [DOI] [PubMed] [Google Scholar]
- 26.Prassad, R., P. De Wergifosse, A. Goffeau, and E. Balzi. 1995. Molecular cloning and characterization of a novel gene of Candida albicans, CDR1, conferring multiple resistance to drugs and antifungals. Curr. Genet. 27:320-329. [DOI] [PubMed] [Google Scholar]
- 27.Ramon, A. M., R. Gil, M. Burgal, R. Sentandreu, and E. Valentin. 1996. A novel cell wall protein specific to the mycelial form of Yarrowia lipolytica. Yeast 12:1535-1548. [DOI] [PubMed] [Google Scholar]
- 28.Sanglard, D., F. Ischer, M. Monod, and J. Bille. 1997. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug ABC transporter gene. Microbiology 143:405-416. [DOI] [PubMed] [Google Scholar]
- 29.Sanglard, D., F. Ischer, L. Koymans, and J. Bille. 1998. Amino acid substitutions in the cytochrome P-450 lanosterol 14α-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob. Agents Chemother. 42:241-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanglard, D., F. Ischer, and J. Bille. 2001. Role of ATP-binding-cassette transporter genes in high-frequency acquisition of resistance to azole antifungals in Candida glabrata. Antimicrob. Agents Chemother. 45:1174-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scherer, S., and D. A. Stevens. 1987. Application of DNA typing methods to epidemiology and taxonomy of Candida species. J. Clin. Microbiol. 25:675-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt, R., M. F. Manolson, and M. R. Chevallier. 1984. Photoaffinity labeling and characterization of the cloned purine-cytosine transport system in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 81:6276-6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vermes, A., H. J. Guchelaar, and J. Dankert. 2000. Flucytosine: a review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J. Antimicrob. Chemother. 46:171-179. [DOI] [PubMed] [Google Scholar]
- 35.Wagner, R., M. L. Straub, J. L. Souciet, S. Potier, and J. De Montigny. 2001. New plasmid system to select for Saccharomyces cerevisiae purine-cytosine permease affinity mutants. J. Bacteriol. 183:4386-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weber, E., C. Rodriguez, M. R. Chevallier, and R. Jund. 1990. The purine-cytosine permease gene of Saccharomyces cerevisiae: primary structure and deduced protein sequence of the FCY2 gene product. Mol. Microbiol. 4:585-596. [DOI] [PubMed] [Google Scholar]
- 37.Yinnon, A. M., K. A. Woodin, and K. R. Powell. 1992. Candida lusitaniae infection in the newborn: case report and review of the literature. Pediatr. Infect. Dis. J. 11:878-880. [DOI] [PubMed] [Google Scholar]