Abstract
We performed time-kill studies of antimicrobial combinations that included minocycline, cefotaxime, and ciprofloxacin with Vibrio vulnificus ATCC 27562. Cefotaxime-plus-ciprofloxacin combinations acted synergistically against V. vulnificus in vitro, and this combination regimen can be a good choice as the empirical treatment for suspected necrotizing fasciitis due to V. vulnificus.
Vibrio vulnificus is a halophilic gram-negative bacterium that is one of the most invasive and rapidly fatal known human pathogens. Patients with V. vulnificus bacteremia often have a rapidly progressive, fulminant course (2, 12, 20). The high mortality, the severity of infections, and the rapidity of V. vulnificus infection suggest that early administration of antibiotics and a combination of antibiotics having good activity against V. vulnificus should be required. Most of the V. vulnificus isolates are susceptible in vitro to a variety of antimicrobial agents (3, 7, 15). Thus, the use of a variety of antimicrobials, based on the in vitro susceptibility of the organism, has been reported (11, 13, 14). However, tetracycline has been recommended as the antimicrobial agent of choice for the treatment of V. vulnificus infection based on the results of a single study for the effectiveness of an in vivo test (3, 15). Chuang et al. documented that the combination of cefotaxime and minocycline produced a synergistic inhibitory effect against V. vulnificus (5, 6). More recently, the newer fluoroquinolones have been demonstrated to be as effective as the combination of cefotaxime plus minocycline in vitro and in vivo (21). The aims of this study were to assess the in vitro activities of ciprofloxacin plus cefotaxime against V. vulnificus and to compare the results with those of cefotaxime plus minocycline or ciprofloxacin single therapy, which are the commonly used antibiotics in clinical practice.
V. vulnificus ATCC 27562 was obtained from the American Type Culture Collection, and it was used for the time-kill studies and checkerboard assays. Fourteen clinical isolates of V. vulnificus were also collected from 14 patients who had been admitted to Chosun University Hospital. The following antimicrobial standard powders for susceptibility testing were obtained from their manufacturers: ampicillin (Chong-Kun-Dang, Korea), cefotaxime (Handok-Aventis, Korea), ciprofloxacin (Bayer HealthCare, Korea), minocycline (Wyeth Korea Inc., Korea), moxifloxacin (Bayer HealthCare, Korea), levofloxacin (Ildong Pharmaceutical Co., LTD, Korea), ceftazidime (CJ Corp., Korea), gentamicin (Choongwae Pharmaceutical Co., Korea), and imipenem (Choongwae Pharmaceutical Co., Korea). The MIC of each antibiotic was determined by the agar dilution method with Mueller-Hinton agar with 2% salinity, considering optimal growth of V. vulnificus in salinity of 1 to 3% (9, 10), in accordance with the guidelines of the Clinical and Laboratory Standards Institute (formerly the National Committee for Clinical Laboratory Standards) (16, 17). Time-kill studies were performed for V. vulnificus ATCC 27562 to evaluate synergy as previously described (5). Viability counts were performed at 0, 2, 4, 8, 12, 24, 36, and 48 h on Mueller-Hinton agar. Drug carryover was minimized by dilution as described previously (1, 22). All the experiments were performed at least twice for good confirmation of the results. Synergy was defined as a ≥2 log10 decrease in CFU per milliliter between the combination and its most active constituent after 24 h, and the number of surviving organisms in the presence of the combination must be ≥2 log CFU/ml below the starting inoculum (8, 19). Checkerboard assays were performed as described previously (1). The fractional inhibitory concentrations (FICs) were calculated as (MIC of drug A and B in combination)/(MIC of drug A or B alone), and the FIC index was calculated as the numerical sum of the two FICs for a given combination. The following criteria were used: an FIC index of <0.5 meant synergy, an FIC index of >4 meant antagonism, and an FIC index of >0.5 but ≤4 meant indifferent.
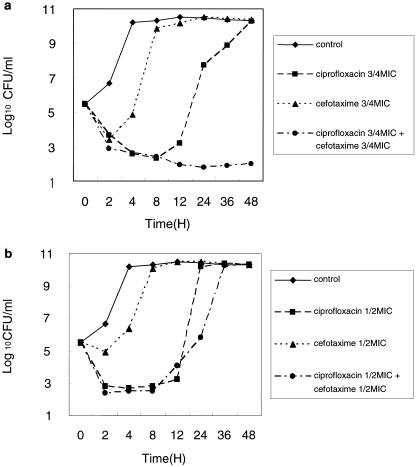
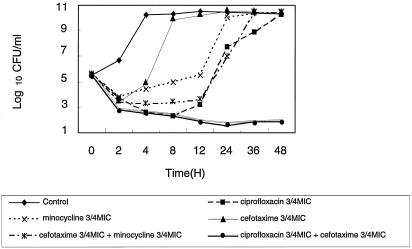
The MICs of 9 antimicrobial agents for the 14 clinical strains and V. vulnificus ATCC 27562 are presented in Table 1. The MICs of cefotaxime, minocycline, and ciprofloxacin for V. vulnificus ATCC 27562 were 0.016, 0.03, and 0.03 μg/ml, respectively. In time-kill studies, combination regimens of cefotaxime plus ciprofloxacin at 3/4 times the MIC resulted in a more significant reduction in bacterial counts of 59 CFU for the starting inoculum of 6.24 × 105 CFU and a more significant reduction at 24 h than that noted with the single-drug regimens of ciprofloxacin (bacterial count of 5.2 × 107 CFU) or cefotaxime (bacterial count of 3.36 × 1010 CFU) (Fig. 1a). The combination therapy with ciprofloxacin plus cefotaxime at 3/4 times the MICs effectively inhibited V. vulnificus ATCC 27562 more than that noted for cefotaxime plus minocycline (Fig. 2). However, the results of the checkerboard assay for V. vulnificus ATCC 27562 were in the indifferent range when the combination of ciprofloxacin and cefotaxime and the combination of cefotaxime and minocycline were tested.
TABLE 1.
Susceptibilities of V. vulnificus ATCC 27562 and 14 clinical isolates of V. vulnificus to 9 antimicrobial agents
| Antimicrobial agent | MIC (μg/ml)
|
|||
|---|---|---|---|---|
| MIC50 | MIC90 | Range | ATCC 27562 | |
| Ampicillin | <0.063 | 32 | <0.063-32 | 1.0 |
| Cefotaxime | <0.063 | 0.5 | <0.063-1.0 | <0.063 |
| Ceftazidime | <0.063 | 0.5 | <0.063-1.0 | <0.063 |
| Ciprofloxacin | <0.063 | <0.063 | <0.063 | <0.063 |
| Minocycline | <0.063 | 0.125 | <0.063-0.125 | <0.063 |
| Moxifloxacin | <0.063 | 0.125 | <0.063-0.125 | <0.063 |
| Levofloxacin | <0.063 | <0.063 | <0.063 | <0.063 |
| Gentamicin | <0.063 | 4.0 | <0.063-8.0 | 0.25 |
| Imipenem | <0.063 | 2 | <0.063-2 | 0.125 |
FIG. 1.
Time-kill curves for V. vulnificus ATCC 27562 after incubation with cefotaxime or ciprofloxacin alone, and with a combination of cefotaxime plus ciprofloxacin at 3/4 times the MIC (a) and at 1/2 times the MIC (b).
FIG. 2.
Time-kill curves for V. vulnificus ATCC 27562 after incubation with cefotaxime, minocycline, or ciprofloxacin alone, with a combination of cefotaxime plus ciprofloxacin, and with a combination of cefotaxime plus minocycline at 3/4 times the MIC.
Currently, the combination of cefotaxime plus minocycline or fluoroquinolone monotherapy is considered to be the first-line therapy (21). Clinical experience for combination therapy with a fluoroquinolone plus a β-lactam to treat V. vulnificus has not been reported. Nevertheless, our clinical experience has suggested the potential clinical usefulness of a ciprofloxacin combination therapy for the treatment of V. vulnificus infections. Thus, we investigated the synergistic activity between ciprofloxacin and cefotaxime. Although synergy was not seen with ciprofloxacin plus cefotaxime and cefotaxime plus minocycline on the checkerboard assays, in our time-kill studies, when cefotaxime at 3/4 times the MIC was combined with minocycline at 3/4 times the MIC or ciprofloxacin at 3/4 times the MIC, the magnitude of the inhibition at 24 h was consistent with the criteria of synergism. The inhibitory effect of ciprofloxacin plus cefotaxime persisted for at least 48 h. This synergistic activity was also observed when the antibiotics were combined at half of the MIC values (Fig. 1b). The in vitro efficacy of ciprofloxacin plus cefotaxime was superior to that of cefotaxime plus minocycline or ciprofloxacin and cefotaxime or minocycline monotherapy. It has been reported that in Escherichia coli, quinolones interact with the outer membrane as chelating agents, raising the permeability of the outer membrane to β-lactam antibiotics. The mechanism by which such combinations achieve synergy is believed to be the facilitation of entry of β-lactam antibiotics into cells after partial disruption of the cell wall through the action of quinolones (4, 18). Despite the synergistic activities of the combination of ciprofloxacin plus cefotaxime against V. vulnificus, the clinical usefulness of this antibiotic combination therapy should be further established. An animal model is being developed to investigate this phenomenon. Clinical studies are required to test the relevance of our findings.
Acknowledgments
This study was supported by research funds from Chosun University, 2004.
REFERENCES
- 1.Bajaksouzian, S., M. A. Visalli, M. R. Jacobs, and P. C. Appelbaum. 1997. Activities of levofloxacin, ofloxacin, and ciprofloxacin, alone and in combination with amikacin, against acinetobacters as determined by checkerboard and time-kill studies. Antimicrob. Agents Chemother. 41:1073-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blake, P. A., M. H. Merson, R. E. Weaver, D. G. Hollis, and P. C. Heublein. 1979. Disease caused by a marine vibrio: clinical characteristics and epidemiology. N. Engl. J. Med. 300:1-5. [DOI] [PubMed] [Google Scholar]
- 3.Bowdre, J. H., J. H. Hull, and D. M. Cocchetto. 1983. Antibiotic efficacy against Vibrio vulnificus in the mouse: superiority of tetracycline. J. Pharmacol. Exp. Ther. 225:595-598. [PubMed] [Google Scholar]
- 4.Chapman, J. S., and N. H. Georgopapadakou. 1988. Routes of quinolone permeation in Escherichia coli. Antimicrob. Agents Chemother. 32:438-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chuang, Y. C., J. W. Liu, W. C. Ko, K. Y. Lin, J. J. Wu, and K. Y. Huang. 1997. In vitro synergism between cefotaxime and minocycline against Vibrio vulnificus. Antimicrob. Agents Chemother. 41:2214-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chuang, Y. C., W. C. Ko, S. T. Wang, J. W. Liu, C. F. Kuo, J. J. Wu, and K. Y. Huang. 1998. Minocycline and cefotaxime in the treatment of experimental murine Vibrio vulnificus infection. Antimicrob. Agents Chemother. 42:1319-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.French, G. L., M. L. Woo, Y. W. Hui, and K. Y. Chan. 1989. Antimicrobial susceptibilities of halophilic vibrios. J. Antimicrob. Chemother. 24:183-194. [DOI] [PubMed] [Google Scholar]
- 8.George, M., R. C. Eliopoulos, and J. R. Moellering. 1996. Antimicrobial combination, p. 338-342. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. Williams & Wilkins, New York, N.Y.
- 9.Howard, R. J., and N. T. Bennett. 1993. Infections caused by halophilic marine Vibrio bacteria. Ann. Surg. 217:525-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly, M. T. 1982. Effect of temperature and salinity on Vibrio (Beneckea) vulnificus occurrence in a Gulf Coast environment. Appl. Environ. Microbiol. 44:820-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly, M. T., and D. M. Avery. 1980. Lactose-positive Vibrio in seawater: a cause of pneumonia and septicemia in a drowning victim. J. Clin. Microbiol. 11:278-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klontz, K. C., S. Lieb, M. Schreiber, H. T. Janowski, L. M. Baldy, and R. A. Gunn. 1988. Syndromes of Vibrio vulnificus infections. Clinical and epidemiologic features in Florida cases, 1981-1987. Ann. Intern. Med. 109:318-323. [DOI] [PubMed] [Google Scholar]
- 13.Kumamoto, K. S., and D. J. Vukich. 1997. Clinical infections of Vibrio vulnificus: a case report and review of the literature. J. Emerg. Med. 16:61-66. [DOI] [PubMed] [Google Scholar]
- 14.Lorian, V. (ed.). 1986. Antibiotics in laboratory medicine, p. 1039. Williams and Wilkins, Baltimore, Md.
- 15.Morris, J. G., Jr., and J. Tenney. 1985. Antibiotic therapy for Vibrio vulnificus infection. JAMA 253:1121-1122. [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 6th ed. Approved standard M7-A6. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 17.National Committee for Clinical Laboratory Standards. 2003. Performance standards for antimicrobial disk susceptibility tests, 8th ed. M2-A8. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 18.Otsuki, M., and T. Nishino. 1996. The synergic effects of quinolones and oral cephem antibiotics on Serratia marcescens. J. Antimicrob. Chemother. 38:771-776. [DOI] [PubMed] [Google Scholar]
- 19.Schaenknecht, F. D., L. D. Sabath, and C. Thornberry. 1985. Susceptibility test: special test, p. 1005-1008. In E. H. Lennette et al. (ed.), Manual of clinical microbiology, 4th ed. American Society for Microbiology, Washington, D.C.
- 20.Tacket, C. O., F. Brenner, and P. A. Blake. 1984. Clinical features and an epidemiological study of Vibrio vulnificus infections. J. Infect. Dis. 149:558-561. [DOI] [PubMed] [Google Scholar]
- 21.Tang, H. J., M. C. Chang, W. C. Ko, K. Y. Huang, C. L. Lee, and Y. C. Chuang. 2002. In vitro and in vivo activities of newer fluoroquinolones against Vibrio vulnificus. Antimicrob. Agents Chemother. 46:3580-3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Visalli, M. A., S. Bajaksouzian, M. R. Jacobs, and P. C. Appelbaum. 1997. Comparative activity of trovafloxacin, alone and in combination with other agents, against gram-negative nonfermentative rods. Antimicrob. Agents Chemother. 41:1475-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]